Abstract
The present study has demystified the first and single step prunosynthesis of the spherical silver nanoparticles (AgNPs) from aqueous fruit extract of angiospermic plant, Prunus cerasifera, which has remarkable fusion of reducing cum stabilizing bioactive components (phenols, anthocyanins, carotenoids, flavonoids, organic acids, tannins and vitamins). Highly stable prunosynthetic AgNPs with 2.04 nm average crystallite size were synthesized in dark and in sunlight at optimized condition of temperature, time and P. cerasifera concentration. Synthesized nanoparticles were characterized through UV–Vis, FTIR, XRD, TGA, SEM and GCMS. Photocatalytic activity of prunosynthetic AgNPs was evaluated for methyl red, erichrome black, methyl blue, bromophenol blue and bromocresol green via UV–Vis. Degradation was achieved (<15 minutes) and expressed as pseudo-first-order kinetics. Prunosynthetic AgNPs demonstrated broad spectrum dose-dependent inhibition (in vitro) in comparison to standard antimicrobial drugs against pathogenic strains X. citri, P. syringae, A. niger, A. flavus, A. fumigatus, A. terreus, P. chrysogenum, F. solani and L. theobromae. Photocatalytic degradation results show the nanobioremediation potential of prunosynthetic AgNPs in indemnifying the persistent environmental pollutants. From the inherently higher inhibition rates for biomimetic prunosynthetic AgNPs, it is envisioned that these can be commercialized as future “green” nanobactericide and nanofungicide at industrial scale economically from nontoxic phytoconstituents.
Introduction
Materials at nanoscale (1–100 nm) of variable dimensions and morphological configurations have been scrupulously investigated for their remarkable attributes in terms of electrical and physicochemical properties in comparison to bulk materials. Among all the nanometallic materials, silver nanoparticles (AgNPs) have been heavily utilized in a wide range of health care and commercial products owing to the inherent toxicity of silver against a wide assemblage of microbial pathogens. Silver nanoparticles have been predominantly synthesized via chemical and physical methods. Ag NPs of unvarying dimension and morphology have been obtained via chemically driven wet reduction of silver salt with synthetic compounds like NaBH4, C6H8O7 and C6H8O6 [Citation1]. Nonetheless, the augmented ecological insalubrity and biological unconformity have significantly given rise to the need for greener and environmentally sustainable synthetic methods for AgNPs fabrication [Citation2,Citation3]. Bioinspired synthetic routes, i.e. fungal, microbial, algal and plant based have been adopted as the ecofriendly substitute for biogenic silver nanoparticles (BSNPs) [Citation4]. Biogenic synthesis of AgNPs with plants is favoured in terms of sustainability for its alacrity, ecological conformity, non-pathogenicity and cost effectiveness by utilizing the reducing plant biochemicals in single stage reactions. AgNPs have been successfully synthesized recently with different plants instead of using toxic chemical reducing agents [Citation5–9].
Plant based biomimetic techniques with BSNPs are preferred for photocatalytic waste degradation over myriad of physical and chemical processes posing ecological and economical challenges. In addition to photocatalytic efficiency, the inherent biocidal property of BSNPs is enhanced when capped with plant metabolites showing outstanding antimicrobial activity. Currently, BSNPs are favourable candidates for nanobiomedicine for their consummate disinfectant potential against wide range of microbial communities [Citation10]. Effectiveness of BSNPs against resistant pathogenic bacterial and fungal strains involved in damaging valuable crops makes them future green pesticides. Biogenic synthesis can be carried out at industrial scale for the commercial scale production of nanofungicidal and nanobactericidal BSNPs for wiping out resistant phytopathogens [Citation10].
Prunus cerasifera Ehrh, cherry plum is an angiospermic plant belonging to family Rosaceae, bears edible fruits widely used in making jams compotes, cognac and champagne [Citation11]. Whole plant is also used as cure for various ailments. All prune fruits are reservoirs of phytochemicals of antioxidizing nature [Citation12]. The present study has prunosynthesized the BSNPs from reducing agents found in P. cerasifera fruit extract (PCFE) for the first time. Prunosynthesis has been carried out in direct sunlight as well as dark. Novel prunosynthetic BSNPs were applied for the photocatalytic dye degradation (PDD). Foreseen potential of BSNPs for conversion into a “green” bactericide and fungicide was tested against pathogenic bacteria and fungi. Furthermore, prunosynthesized BSNPs were analysed via UV–vis, FTIR, XRD, TGA, SEM and GCMS.
Materials and methods
Materials
Silver nitrate (AgNO3), potassium bromide (KBr) and nutrient agar (NA) culture media were purchased from Merck (Germany). Dyes were purchased from BDH (Poole, UK). Potato dextrose agar (PDA) culture media was purchased from Liofilchem (Roseto degli Abruzzi , Italy). All chemicals used in investigation were of analytical grade and have been used without further purification.
Fruit extract preparation
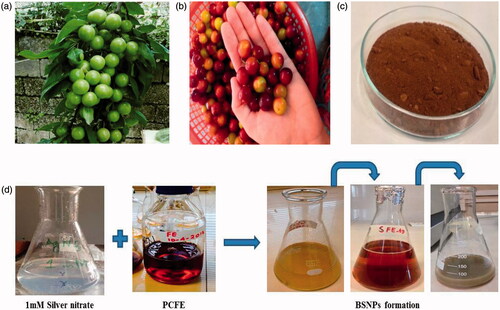
P. cerasifera ripened fruits collected from Parachinar (Pakistan) were ground into fine powder with the help of pestle and mortar, sieved and stored in sealed polyethene bags. For aqueous filtrate preparation, 10 g of fruit powder was weighed and extracted with 1000 ml deionized water and heated at 30 °C for 10 min. It was followed by double filtration with Whatman No. 1 filter paper (pore size: 11 µm) and refrigerated at 4 °C for further use ().
Prunosynthesis of biogenic silver nanoparticles
Batches were conducted in two sets of sunlight enticed biogenic silver nanoparticles (SLEBSNPs) and dark incubation biogenic silver nanoparticles (DIBSNPs). SLEBSNPs and DIBSNPs were optimized for time duration of 30, 60, 90, 120 and 150 min with 80 ml of PCFE in 400 ml of 1 mM AgNO3, second for temperature variation with 80 ml of PCFE in 400 ml of 1 mM AgNO3 magnetically stirred on for 30 min at 40, 60, 80, 100 and 120 °C and optimization was also done for PCFE concentration variation with 80, 100, 120, 140 and 160 ml of PCFE in 400 ml of AgNO3. Exposure to sun light and dark incubation for both sets were keenly monitored. They exhibited a colour change from yellowish brown to dark brown and finally greyish colour confirmed the BSNPS formation regularly monitored via UV–vis Spectrophotometer (1602, Biomedical Services, Madrid, Spain).
Characterization
Prunosynthetic nanoparticles were analysed via UV–vis Spectrophotometer (1602, Biomedical Services, Madrid, Spain) in range of 200–800 nm. The interplay of such functional groups was checked by FTIR Spectrophotometer (8400, Shimadzu, Kyoto, Japan). Surface morphology and particle size ranges were analysed by scanning electron microscopy (SEM JEOL JSM-6490, Freising, Germany). XRD patterns were recorded with Bruker AXS D-8 powder X-ray diffractometer (Shimadzu, Kyoto, Japan), operated at 40 kV, 20 mA, with CuKα radiation (λ = 1.5406 A°). Thermal stability was analysed by TGA-7 (Perkin-Elmer, San Diego, CA, USA) in temperature range of 50–700 °C at heating rate of 10 °C/min with inert N2 atmosphere (50 ml/min of N2 purge flow). The presence of biomolecules in PCFE was further confirmed via GCMS (QP5050, Shimadzu, Kyoto, Japan).
Photocatalytic dye degradation
PDD was carried out in two sets comprising of first set where the absorbance of dye solution without NP addition was checked and it was kept in sunlight for 30 min from 12:00 to 12:30 PM on a sunny day with an average intensity 68–73 klux (LT300, Extech, Leeds, UK). The second set of dyes was mixed with 1 ml of BSNPs solutions and was kept under same conditions. Prior to UV–vis analysis, these sets were exposed to UV-Lamp (SN500712, Kohler, Lahr, Germany) and then spectra were noted from 350–650 nm. Spectra were noted at specific times intervals in addition to the discolouration detected and the values were compared with dyes’ lambda maximum.
Antibacterial and antifungal assay
Antibacterial potential of BSNPs was tested against Xanthomonas axonopodus pv. citri and Pseudomonas syringae by standard Kirby–Bauer disc diffusion assay. Prior to inoculation with BSNPs, the bacterial test strains were grown in NA broth for 24 h in incubator at 37 °C. The NA plates were prepared followed by sterilization and solidification. The bacterial cultures grown overnight were spread on the solidified plates with help of sterile loop for obtaining bacterial lawns. The autoclaved and dried filter paper discs were picked up with help of a sterile forcipes and inserted on NA plates. Discs on control set were loaded with 10 μL of AgNO3 salt solution, 10 μL of standard antibacterial drug Ampicillin and 10 μL PCFE as a control while the discs on another set of NA plates were loaded with 2, 4, 6 and 10 μL of BSNPs (both SLEBSNPs and DIBSNPs separately) with 24 h incubation time at 37 °C in incubator (Sanyo MR-153, GeminiBV, Etten-Leur, Netherlands). On the next day, the zones of inhibition were measured. BSNPs were also tested for their fungicidal activity against pathogens like Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus, Aspergillus terreus, Penicillium chrysogeum, Fusarium solani and Lasiodiplodia theobromae by standard Kirby–Bauer disc diffusion assay. The fungal organisms were grown on PDA media for 72 h. Two hundred microlitres of each fungal culture was poured onto the PDA with help of sterile spreader for obtaining fungal lawns. Discs on control set were loaded with 10 μL of AgNO3 salt solution, 10 μL of standard antibiotic amphotericin B and 10 μL PCFE as a control while the discs on another set of PDA plates were loaded with 5, 10, 15 and 20 μL of BSNPs (both SLEBSNPs and DIBSNPs) and were incubated for 72 h [Citation13].
Statistical analyses
Antimicrobial assays were performed in triplicates, all values have been expressed as means with standard deviation. Assays were statistically evaluated by two tailed Student's t test (MS Excel 2013) assuming equal variance and one-way analysis of variance (ANOVA) test in addition to Tukey’s test (Origin 8.1). p Values lower than .05 were considered statistically significant.
Results
Prunosynthesis of AgNPs was done by extraction of reducing agents from Prunus cerasifera. Optimized BSNPs have been synthesized in two sets, i.e. SLEBSNPs and DIBSNPs. For all the nanoparticles synthesized, the progress of the chemical reaction initially was determined from physical attribute, i.e. colour change of the reaction mixture. With an augmentation in incubation time, the intensity of colour enhanced turning to dark brown and finally greyish colour signified the development of BSNPs. The physical colour change can be attributed to surface plasmon vibrations in BSNPs [Citation14].
) represents increase in SPR as the time is increased from 30 min to 150 min. The lower absorbance and light colour at lower incubation times are indicative of reaction proceeding slowly. While the remarkable increase becoming stable at 150 min shows the formation of DIBSNPs at longer durations. PCFE phytochemicals reduced and stabilized the DIBSNPs and SLEBSNPs maximally at longer incubation period of 150 min. The augmented absorbance and intense dark brown colour turning to grey indicated the formation of greater [Citation15,Citation16]. Both the reactions in dark as well in sunlight exposed generated 442 and 410 nm absorption maxima respectively thus pointing towards the possibility of prunosynthesis of BSNPs in sunlight where no additional magnetic stirring and heat were provided as in case of DIBSNPs. ) illustrates the UV–vis absorption spectra for DIBSNPs and SLEBSNPs when the PCFE concentration was varied from 80 ml to 160 ml with 400 ml of 1 mM AgNO3. The absorption maxima were observed at 442 nm and 417 nm. The absorbance for initial concentrations is considerably low as compared to highest PCFE concentration, thus suggests higher PCFE provides greater quantity for reduction of prunosynthetic BSNPs. Highest PCFE concentration is terminating the reaction after reduction cum stabilization.
Figure 2. UV–Visible spectroscopy of prunosynthetic silver nanoparticles synthesized with reducing agents from P. cerasifera. (a) DIBSNPs with time variation, (b) SLEBSNPs with time variation, (c) DIBSNPs with PCFE conc. Variation, (d) SLEBSNPs with PCFE conc. Variation, (e) DIBSNPs with temperature variation, (f) SLEBSNPs with temperature variation, (g) P. cerasifera aqueous fruit extract and (h) post-synthetic stability of BSNPs.
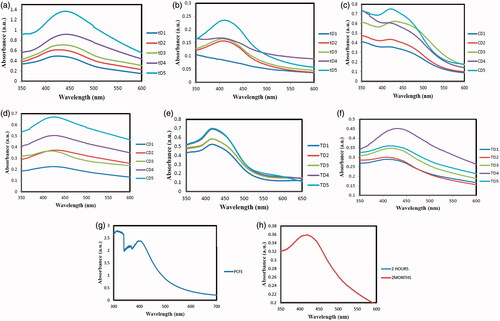
Temperature significantly affects the formation of BSNPs. Upon exposure to 40, 60, 80, 100 and 120 °C, the lower temperatures gave rise to DIBSPs but with lower absorbance. While nearly same SPR were detected for 60, 80 and 100, 120 °C, it shows that maximum DIBSNPs synthesis can be achieved at both 100 and 120 °C at which PCFE phytochemicals carry out maximum reduction and stabilization at 422 nm. The highest SPR for SLEBSNPs was detected at 430 nm for 100 °C. Increasing temperature beyond this range expressed alleviation in absorbance showing that maximum SLEBSNPs have been capped and reduced at 100 °C. The spectral analysis for PCFE exhibited the maximum absorbance at 398 nm. Prunosynthetic BSNPs exhibited remarkable stability at ambient condition (25 ± 2 °C) with no agglomeration and produced SPR at 420 nm shown in .
FTIR spectra were recorded between the wavenumber spanning over a range of 4000–400 cm−1 as shown in ). Prominent IR peaks of PCFE were 3342.75, 3342.75, 2929.97, 1732.13, 1627.97, 1400.37, 1261.49, 1078.24, 891.14, 800.49 and 611.45 cm−1 which correspond to alcohol or phenol, carboxylic acid, ketones, amines, aromatics, aromatic amines, aliphatic amines, aromatics, alkyl halides and alkynes. However, the common trend in terms of prominent IR peaks 3736.24 cm−1 and 1616.40 cm−1 (DIBSNPs), 3448.84 cm−1 and 1629.53 cm−1 (SLEBSNPs) and 3443.05 cm−1 indicates the presence of phenolic components and proteins that are actively involved in bioreduction of Ag+ to Ag0.
Figure 3. FTIR overlay spectra PCFE with (a) prunosynthetic silver nanoparticles in dark incubation (DIBSNPs), (b) prunosynthetic silver nanoparticles exposed in sunlight (SLEBSNPs), (c) XRD pattern of prunosynthetic silver nanoparticles prepared with 80 ml PCFE and (d) TGA curve for prunosynthetic silver nanoparticles synthesized in dark with P. cerasifera extract at a heating rate of 10 °C/min under N2 atmosphere.
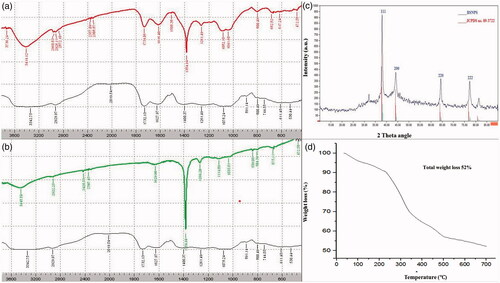
XRD spectra revealed prominent peaks at 2θ = 37.89° (111), 44.00° (200), 64.39° (220) and 77.36° (222) for BSNPs (JCPDS card no. 89–3722) indicative of face centred cubic geometry () [Citation17]. Spectra expressed some smaller peaks which can be assigned to the PCFE phytochemicals having crystalline nature. BSNPs average crystallite size obtained from XRD patterns was 2.04 nm calculated from Scherer’s equation. Thermal stability of prunosynthetic BSNPs in N2 at a heating rate of 10 °C/min has been demonstrated in . BSNPs expressed regular weight loss at temperature ranging from 50 to 700 °C. Weight loss happened in four successive stages showing multistage decomposition. Remaining weight losses occurred at 245 °C at 10.20 min, 350 °C at 15.35 min and final stage weight loss was observed from 505 to 700 °C initiating at 23.30 min.
Representative SEM micrographs () illustrated the morphology of both DIBSNPs and SLEBSNPs synthesized at highest variation of time, concentration and temperature. expresses the size ranges for the prepared nanoparticles. Prunosynthetic BSNPs were found to be spherical in shape with higher degree of polydispersity exhibiting nonuniform particles sizes. GC–MS chromatogram for PCFE exhibited 17 peaks of secondary metabolites which were followed by identification of compounds from MS spectra by matching with Wiley and NIST libraries (). Seventeen peaks present in GC–MS chromatogram are all significantly involved in biogenic synthesis with highest peaks for 5-(hydroxymethyl)furfural, 3-furaldehyde, hexadecanoic acid, 1,3-dioxane, 4,4-dimethyl- and heptanoic acid, 3-hydroxy-, methyl ester.
Figure 4. SEM micrographs of prunosynthetic silver nanoparticles synthesized with reducing agents from P. cerasifera, time variation (a) DIBSNPs, (b) SLEBSNPs, PCFE conc. Variation, (c) DIBSNPs, (d) SLEBSNPs, temperature variation, (e) DIBSNPs and (f) SLEBSNPs.
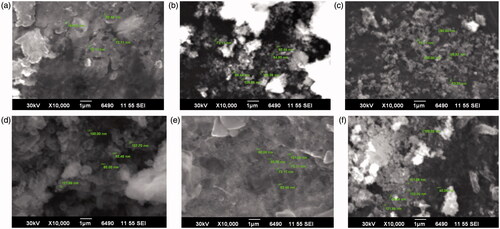
Table 1. SEM size ranges of prunosynthetic silver nanoparticles.
Table 2. GC–MS peaks of secondary metabolites in P. cerasifera fruit extract.
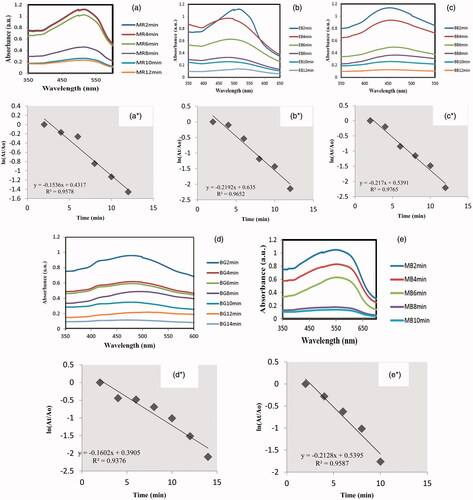
BSNPs were employed for degradation of five environmentally persistent dyes (). Pure dye solution expressed no changes in absorption upon exposure to sunlight followed by UV-lamp while the test solution having BSNPs + dye in all five experiments expressed a gradual decrease in absorbance. Photocatalytic degradation was also monitored optically as the colour of dyes faded with incubation time. Wavelength for each decreased maxima was recorded and final overlays were formed upon the discolouration. UV–vis overlay spectra show this gradual absorbance reduction corresponds to the increase in degradation efficiency. BSNPs succeeded in degrading 88.58% of MR only in 12 min, 88.26% of EB in 12 min, 88.99% of BB in 12 min, 80% of BG in 14 min and 86.83% of MB in 10 min. The order of degradation of dyes is MB > BB > MR > EB > MB. illustrates the reaction kinetics (ln (At/Ao) vs. time) for determining the order of photocatalytic reaction for all dyes. For all the dyes, the reduction caused by BSNPs was found to be pseudo first-order kinetics. There was a linear relationship between ln (At/Ao) vs. time with R2=0.95, 0.97, 0.98, 0.94 and 0.96 for MR, EB, BB, BG and MB, respectively.
Figure 5. Alleviating UV–vis spectra expressing photocatalytic dye degradation potential of BSNPs and ln (At/Ao) vs. time plot of (a) methyl red, (b) erichrome black T, (c) bromophenol blue, (d) bromocresol green and (e) methyl blue.
The present study is the foremost investigation of P. cerasifera fruit driven AgNPs potency against the pathogenic strains. BSNPs exhibited comparatively superior effectiveness against both bacterial strains in comparison to AgNO3 salt solution, standard antibiotic ampicillin and PCFE. No zone formation () for both strains in case of AgNO3 salt solution exhibits their resistance. BSNPs surpassed the antibiotics tested consistent with biogenic AgNPs expressing potent antimicrobial activity towards different antibiotics [Citation18]. Moreover, pronounced augmentation in ZOI was observed with an increase in BSNPs conc. from 2 to 10 μL expressing enhanced antibacterial activity at higher BSNPs dose. Causative agent of citrus canker Xanthomonas axonopodis pv. citri expressed higher susceptibility than Pseudomonas syringae towards BSNPs with higher clearance ratio. Both BSNPs and PCFE expressed variable zones of inhibition for all the pathogenic fungal strains. Resistance was also observed in all fungal cultures towards AgNO3 salt solution with no clearance zone. However, PCFE expressed good antifungal activity thus expressing its favourability for bioprospecting in development of drugs. Commendable augmentation in ZOI was achieved by increasing BSNPs conc. from 5 to 20 μL. BSNPs produced highest clearance zone against Aspergillus flavus while the minimum susceptibility was shown by Aspergillus terreus. The BSNPs concentrations for all fungal cultures exceeded the standard antifungal drug amphotericin B.
Table 3. In vitro antimicrobial efficacy of prunosynthetic silver nanoparticles against pathogens (zone of inhibition (mm ± SD)).
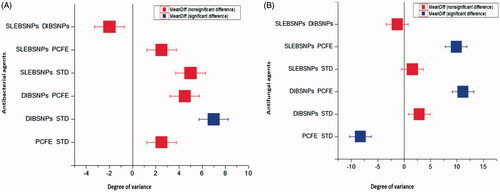
Antimicrobial activity of BSNPs was statistically evaluated by two-tailed Student’s t test assuming equal variance and one-way ANOVA test with Tukey’s test. t Test applied signifies the variation in activity of standard, PCFE, DIBSNPs and SLEBSNPs for triplicates (n = 3) having a dose level of 10 μL. shows the variance between standard and BSNPs was unequal. T-Stat value was negative for all nine microbes as t < 0, P(T≤t) with p < .05 showing significant variations. Significance of variability was further confirmed via ANOVA test expressing the potential of BSNPs as superior quality antibacterial and antifungal drug as a green alternative to presently manufactured antibiotics. ANOVA test () compared this potential harmoniously and p values confirmed the significant difference in the degree of inhibition for tested material. Provided that F value (11.36) with p>F (0.02) for antibacterial tests and F value (11.98) with p>F (0.02) for antifungal tests, it is suggestive of the significant difference between the dose and response of all microbes when disc loaded with 10 μL of BSNPs. Lower p values are indicative of significance and reliability of data.
Table 4. Student’s t test for mean diameter of antimicrobial activity against tested microorganisms.
Table 5. One-way ANOVA and Tukey’s test parameters for statistical variance in nine microbes towards prunosynthetic silver nanoparticle.
Tukey’s test () was performed for specifying the significance with which the means varied for the susceptibility of microbes towards the tested material. The Box and Whisker plots () were used to show general pattern of response of microbes towards the tested material. Shape of box plots revealed the symmetrical nature of data being tested against same number of microbes though with considerable intersubject variability. BSNPs expressed highest variation, however, the variation between SLEBSNPs and standard in both made shafts in medium of all groups. Microbial strains expressed higher susceptibility towards DIBSNPs in comparison to all data with highest (7.75) q level and p < .03 and (7.69) q level and p < .02 for bacterial and fungal strains.
Discussion
Engineered nanomaterials have been developed through advanced techniques, e.g. ultraviolet irradiation, aerosol assisted techniques, photochemical routes, etc. The exorbitant nature and utilization of environmentally perilous raw materials and requirement for elevated temperatures make these techniques a serious presage to biota [Citation19]. However, the prunosynthesis of AgNPs serves as an effective and sustainable alternate for it is carried out with abundantly Prunus cerasifera fruits through ecologically benign pathway. Phytoconstituents not only caused reduction but are also involved in stabilization of AgNPs for extended periods (). Furthermore, the effectiveness of prunosynthesis is reflected from its one pot fabrication in contrary to physicochemical modes employing tedious multistep synthesis of AgNPs. Thus, prunosynthesis being a biomimetic route, not only makes nanobiotechnology to be used for biomedical applications but also enhances its role in environmental protection. Unlike physicochemical routes, prunosynthesis is in complete conformity with principles of green chemistry having an elevated efficiencies at room temperature.
Nano silver particle morphology, dimensions, distribution and chemistry are highly dependent upon the SPR. For all the synthesized BSNPs, the UV–vis peaks obtained were broad denotative of the greater extent of polydispersity which was further confirmed through other analytical tools [Citation20,Citation21]. Broader peaks obtained in current investigation spanning over a range of 400–442 nm can be attributed to not only non-uniform particle sizes but also the anisotropic nature of BSNPs [Citation22]. Furthermore, phytoconstituents of stabilizing nature present in PCFE also gives rise to broader peaks, upon increasing the PCFE concentration, there is an increase in absorbance, however, beyond a certain limit the aggregate formation starts making the peak broader [Citation23]. The colloidal BSNPs are absorbed at approximately 400 nm (visible region) due to their interplay with solar light and the presence of free electrons is signified as surface plasmon resonance (SPR). Postsynthetic stability test for BSNPs exhibited no change in absorbance when the UV–vis spectrum was compared for 2 h and 2 months. Post synthetic stability of prunosynthetic BSNPs can be attributed to the reducing cum stabilizing role of PCFE phytoconstituents which prevented any aggregation in BSNPs and thus keeping them intact. PCFE phytochemicals not only served as catalysts for fabrication of BSNPs but also expressed surface attachment to nanoparticle [Citation24].
The common trend in terms of prominent IR peaks 3736.24 cm−1 and 1616.40 cm−1 (DIBSNPs), 3448.84 cm−1 and 1629.53 cm−1 (SLEBSNPs) and 3443.05 cm−1 is indicative of the presence of phenolic components and proteins that are actively involved in bioreduction of Ag+ to Ag0. The results signified the presence of distinctive functional groups in PCFE owing to the presence of different phytoconstituents. Upon reduction, there have been changes in the characteristic IR absorbance peaks. Additionally, an exsurgence of new IR peaks due to association of PCFE biomolecules with silver metal has also been observed. There have been numerous IR peak shifts rendering changes in shape and intensity of bands due to the chemical changes inducing reduction and stabilization of BSNPs. Biomolecules develops interactions with metallic salts via functional groups and participates in the reduction cum stabilization of BSNPs. Comparatively alleviated intensities seen for all synthesized NPs were due to the capping of these NPs with organic portions of P. cerasifera. FTIR spectra for synthesized materials divulge amine linkages which are known for their potential in binding with Ag for stabilization of Ag+ to Ag0 through formation of a covering. The lowest intensities and comparatively indistinct peaks were obtained for SLEBSNPs due to the interplay of sunlight which has photo activating effect on the silver reduction. Furthermore, the IR spectra transitions are demonstrative of structural changes in protein component of PCFE for its involvement in bioreduction. Metallic ion reduction is caused due to the flavonoid containing ketonic and terpenoids containing aldehydic group may convert to carboxylic acids. Additionally, there is strong binding between metal ions and functional groups present in PCFE thus causing reduction cum stabilization [Citation25].
Prunus cerasifera fruit extract reduced BSNPs produced conspicuous and sharp XRD peaks indicative of phytochemicals role in reducing silver cations to metallic BSNPs. Relatively smaller and unassigned peaks refer to the phytochemicals in PCFE of crystalline nature. The higher intensities of such peaks are due to metalloproteins found in cherry plum having stronger X-ray scattering over the crystalline phase [Citation26]. Peaks obtained are similar as that of metallic silver. BSNPs exhibited commendable thermal stability enhancing its suitability to be utilized at higher temperatures. The decomposition pattern expresses the loss of organic compounds present in PCFE which reduced and stabilized the BSNPs. Total weight loss for BSNPs was 52% indicative of the plant biochemical involvement in reducing and stabilizing the BSNPs [Citation27]. BSNPs degradation in multiple steps shows remarkable thermal stability at elevated temperatures [Citation28].
Operating mechanism for no or negligible agglomeration can be attributed to the extemporaneous nucleation and isotropic growth of nanoparticles with PCFE. The polydispersity extent has been predicted earlier as well by broader peaks in UV–vis spectra. The present work is in complete conformity with earlier reports where sizes up to 287.5–293.2 nm and 106.67 to 147.27 nm have been mentioned [Citation29,Citation30]. Thin layer of phytoconstituents can also be seen in the SEM micrographs which are covering the BSNPs. Highest GCMS peak 5-(hydroxymethyl)furfural having aromatic ringed structure is possibly involved reducing the nanoparticles. 3-Furaldehyde, another aromatic compound accounts for the characteristic sweet and slightly sour taste of P. cerasifera fruit and is plausibly involved in reducing the synthesized NPs. Hexadecanoic acid was detected in P. cerasifera fruit which is responsible for oily portion imparting glossy colour to the fruit. The long C-backbone with OH in hexadecanoic acid can readily reduce BSNPs. The antimicrobial potential of BSNPs is enhanced due to the presence of such secondary metabolites which possess natural ability of inhibiting plant pathogens in addition to their role in BSNPs fabrication.
Due to promising structural and appreciable size of the presently prepared prunosynthetic BSNPs, they were further employed for degradation of five dyes. BSNPs produced good photocatalytic efficiencies for all dyes. Significantly high degradation efficiencies expressed the extra ordinary photocatalytic potential of synthesized BSNPS. These percentages are suggestive of degrading the dyes under principles of green chemistry with prunosynthetic BSNPs and under renewable source of energy. Antimicrobial activity of BSNPs was tested against nine microbes for investigating its biomedical significance. The microbes selected for investigation are responsible for various human and plant diseases and have not been explored with Prunus cerasifera reduced AgNPs, such microbes also have elevated growth rates, multidrug resistance and thus cause a large scale damage to agricultural crops and human population by causing vicious diseases, e.g. Xanthomonas axonopodis (causative agent of citrus canker) and Pseudomonas syringae (blight in beans, cherries and tomatoes) resistant against streptomycin; Aspergillus spp. (vegetal and fruit blights, bones and joints diseases, respiratory infections, i.e. pneumonitis, aspergillosis) resistant against fluconazole and ketoconazole; Penicillium (ulcers) resistant against benzimidazole; Fusarium solani (dermal and optical disease) resistant to aminoglycoside and Lasiodiplodia theobromae (mango dieback disease) shows resistance to benomyl fungicide. Control of these microbes by physicochemical means has ranged in millions of dollars on global scale yet satisfactory inhibition has not been obtained and such microbes are growing remarkably. The higher toxicities obtained by green BSNPs for all nine microbes synthesized via facile and economically favourable route can help in annihilation of such microbes. Large-scale destruction caused by these microbes can be controlled sustainably by BSNPs. BSNPs as a novel source have been employed to combat this resistance via enhanced antibiotic impact. Furthermore, in case of agricultural protection, the environmentally obnoxious fungicides and bactericides can be efficiently replaced with BSNPs. BSNPs’ vanquishing of multidrug resistance makes them a suitable candidate for biomedical and agricultural applications. Thus, the toxic potential in addition to solution of drug resistance, BSNPs are advantageous over commercially available antibiotics with no disadvantages for human or physical environment.
Prunosynthetic BSNPs possess inherent toxicity towards bacterial and fungal strains, however, the phytochemicals capping of BSNPs further augment their perniciousness. Results expressed the effectiveness of BSNPs against all tested bacterial and fungal strains at lowest doses even producing clearance zones that varied in diameter in all cases. BSNPs’ antimicrobial potential is in agreement with the particle size distribution as the smaller sizes have effective influence in DNA damage [Citation31], since DIBBSNPs exhibited smaller size ranges than SLEBSNPs thus for all microbes DIBSNPs excelled except Fusarium solani for which equal clearance zones were produced by both NPs. Thus, showing that multifariousness agricultural crops being targeted by Fusarium solani can be effectively and efficiently controlled by both BSNPs. Fusarium solani spore germination inhibited equally despite larger size range of SLEBSNPs can attribute to the fact that SLEBSNPs possessed specialized and effective secondary metabolites which in highest dose induced the linear reduction in growth of pathogen. However, major inhibition in all other cases was size governed. The differences in zone of inhibition are due to varied sensitivities towards BSNPs.
Nanoparticle driven alterations induced in microbial cells can be attributed to various mechanisms, i.e. (a) surface attachment with microbial membrane and inducing cellular permeability and respiration; (b) direct cellular damage via microbial cell penetration and reacting with S or P compounds, e.g. DNA; (c) direct release of Ag+ thus augmenting the antimicrobial impact [Citation1]. Bacterial cell metabolic pathways are interrupted particularly in cell wall and membranes due to conformational alterations as a consequence of interaction between Ag+ and oppositely charged phytoconstituents, ultimately causing cell death [Citation32,Citation33]. BSNPs are known for releasing Ag+ which interferes with bacterial cell membrane permeability and induce structural disruption [Citation34]. Though the accurate antimicrobial mechanism is not comprehended fully yet can be attributed to operation of either of these mechanisms, or it can be due to BSNPs’ involvement in injuring mitochondria, cell membrane, DNA, oxidative stress or apoptotic initiation [Citation35,Citation36]. Weaker peptidoglycan layer in bacteria and effective electrostatic bonding between BSNPs and test microbes inducing physicochemical changes in bacterial cells elevated the inhibition. AgNPs antibacterial efficacy is also related to its power of releasing reactive oxygen species (ROS) [Citation37]. Such enhanced zones of inhibitions are indicative of BSNPs conversion to effective antifungal as well as antibacterial products in medicinal paradigms. However, the fungicidal potential of BSNPs is expressed by their inherent ability in deactivation of sulfhydryl groups found in fungal cell wall and forming the insoluble products, this is followed by alterations in enzymes and lipid content associated with cell membrane ultimately leading to cellular lysis [Citation38].
The maximum obliteration and enhanced fungicidal potential of BSNPs can be related to its ability of inducing higher mechanical injuries to cellular membrane as compared to the bulk AgNO3. The accurate mechanism behind fungal obliterative functioning of AgNPs is yet to be explored as in case of antibacterial mechanism. However, some studies are of the notion that the cationic Ag+ plays a significant role in development of electrostatic bonding between negatively charged cellular membrane and cationic Ag+. Bacterial strains have shown maximum susceptibility than fungal cultures as reported earlier [Citation13,Citation39,Citation40]. Such differences arise due to structural and morphological alterations found in bacteria and fungi. Fungal cell walls are made up of complex polysaccharide containing N-based chitin which is not easily degradable than gram negative bacterial cell walls having thinner morphology easily available to be attacked by BSNPs. Antimicrobial actions of BSNPs exhibited dose dependency and all clearance zones can be attributed to phenols, flavonoid and alkaloids present in PCFE that capped the BSNPs [Citation41]. Effective clearance zones for bacterial and fungal strains obtained with BSNPs make them versatile nanobactericide and fungicides [Citation42].
Prunosynthesis of BSNPs showed its aptness for use in biomedical applications and environmental remediation. The present work confirmed the biogenic synthesis with unrevealed cherry plum fruit extract via analytical techniques. BSNPs efficiently photodegraded the persistent dyes, showed antimicrobial inhibition against nine strains of public health and agricultural importance that possess higher multi drug resistance.
Conclusions
Green and facile AgNPs having an average crystallite size of 2.04 nm were synthesized from P. cerasifera fruit in one step, fast, economical and free of consuming any hazardous substances. Post synthetic stability up to 2 months without any aggregation revealed its favourability in pollution control and biomedical applications. Secondary metabolites found in PCFE exerted significant impacts on reduction cum stabilization of AgNPs. Prunosynthetic AgNPs were nanocrystalline with face centred cubic geometry, higher polydispersity extent demonstrated rough surface morphology and multistep thermal decomposition. Persistent dyes were photo catalytically degraded in less than 15 min having pseudo-first-order kinetics. Thus, prunosynthetic AgNPs can be efficiently used in nanobioremediation of water pollution. Furthermore, significantly higher antibacterial and antifungal activity against pathogenic strains causing large scale harm to human health and crops on annual basis expresses the potential of prunosynthetic AgNPs as nanobactericides and nanofungicides in conformity with principles of green chemistry.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Panáček A, Kvítek L, Prucek R, et al. Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem B. 2006;110:16248–16253.
- Ramanathan R, O’Mullane AP, Parikh RY, et al. Bacterial kinetics-controlled shape-directed biosynthesis of silver nanoplates using Morganella psychrotolerans. Langmuir. 2010;27:714–719.
- Aziz N, Faraz M, Pandey R, et al. Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir. 2015;31:11605–11612.
- Faramarzi MA, Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv Colloid Interface Sci. 2013;189:1–20.
- Lalitha P. Apoptotic efficacy of biogenic silver nanoparticles on human breast cancer MCF-7 cell lines. Prog Biomater. 2015;4:113–121.
- Fatimah I. Green synthesis of silver nanoparticles using extract of Parkia speciosa Hassk pods assisted by microwave irradiation. J Adv Res. 2016;7:961–969.
- Kumar VA, Ammani K, Jobina R, et al. Photo-induced and phytomediated synthesis of silver nanoparticles using Derris trifoliata leaf extract and its larvicidal activity against Aedes aegypti. J Photochem Photobiol. 2017;171:1–8.
- Buhroo AA, Nisa G, Asrafuzzaman S, et al. Biogenic silver nanoparticles from Trichodesma indicum aqueous leaf extract against Mythimna separata and evaluation of its larvicidal efficacy. J Plant Prot Res. 2017;57:194–200.
- Bagherzade G, Tavakoli MM, Namaei MH. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac J Trop Biomed. 2017;7:227–233.
- Jyoti K, Baunthiyal M, Singh A. Characterization of silver nanoparticles synthesized using Urtica dioica Linn. leaves and their synergistic effects with antibiotics. J Rad Res Appl Sci. 2016;9:217–227.
- Morarita SV, Hossu AM, Georgescu AA. The study of analysed biochemical parameters on fruits of Prunus cerasifera Ehrh. Biotypes Rev Chim. 2017;68:121–124.
- Birwal P, Deshmukh G, Saurabh SP. Plums: a brief introduction. J Food Nutr Popul Health. 2017;1:1–5.
- Ajitha B, Reddy YA, Reddy PS. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:164–172.
- Ahmed S, Shah A. Synthesis of silver nanoparticles using Hedichium spicatum extract and their characterization. J Adv Mater. 2017;1:1–5.
- Govarthanan M, Seo YS, Lee KJ, et al. Low-cost and eco-friendly synthesis of silver nanoparticles using coconut (Cocos nucifera) oil cake extract and its antibacterial activity. Artif Cells Nanomed Biotechnol. 2016;44:1878–1882.
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol. 2017;31:1–9.
- Saravanakumar A, Peng MM, Ganesh M, et al. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif Cells Nanomed Biotechnol. 2017;45:1165–1171.
- Soshnikova V, Kim YJ, Singh P, et al. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif Cells Nanomed Biotechnol. 2017;13:1–10.
- Osibe DA, Chiejina NV, Ogawa K, et al. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif Cells Nanomed Biotechnol. 2017;23:1–8.
- Noguez C. Surface plasmons on metal nanoparticles: the influence of shape and physical environment. J Phys Chem C. 2007;111:3806–3819.
- Amendola V, Bakr OM, Stellacci F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: effect of shape, size, structure, and assembly. Plasmonics. 2010;5:85–97.
- Shah M, Poinern GE, Fawcett D. Biosynthesis of silver nanoparticles using indigenous Xanthorrhoea glauca leaf extract and their antibacterial activity against Escherichia coli and Staphylococcus epidermis. Int J Res Med Sci. 2017;4:2886–2892.
- Ahmed Q, Gupta N, Kumar A, et al. Antibacterial efficacy of silver nanoparticles synthesized employing Terminalia arjuna bark extract. Artif Cells Nanomed Biotechnol. 2017;45:1192–1200. 1
- Johnson P, Krishnan V, Loganathan C, et al. Rapid biosynthesis of Bauhinia variegata flower extract-mediated silver nanoparticles: an effective antioxidant scavenger and α-amylase inhibitor. Artif Cells Nanomed Biotechnol. 2017;7:1–7.
- Mahitha B, Raju BD, Dillip GR, et al. Biosynthesis, characterization and antimicrobial studies of AgNPs extract from Bacopa monniera whole plant. Dig J Nanomater Biostruct. 2011;6:135–142.
- Dauthal P, Mukhopadhyay M. In-vitro free radical scavenging activity of biosynthesized gold and silver nanoparticles using Prunus armeniaca (apricot) fruit extract. J Nanopart Res. 2013;15:2–11.
- Mittal AK, Bhaumik J, Kumar S, et al. Biosynthesis of silver nanoparticles: elucidation of prospective mechanism and therapeutic potential. J Colloid Interface Sci. 2014;415:39–47.
- Patra JK, Baek KH. Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 2016;10:326–333.
- Medda S, Hajra A, Dey U, et al. Biosynthesis of silver nanoparticles from Aloe vera leaf extract and antifungal activity against Rhizopus sp. and Aspergillus sp. Appl Nanosci. 2015;5:875–880.
- Durgawale PP, Phatak RS, Hendre AS. Biosynthesis of silver nanoparticles using latex of Syandenium grantii hook f and its assessment of antibacterial activities. Dig J Nanomater Biostruct. 2015;10:847–853.
- Velusamy P, Das J, Pachaiappan R, et al. Greener approach for synthesis of antibacterial silver nanoparticles using aqueous solution of neem gum (Azadirachta indica L.). Ind Crops Prod. 2015;66:103–109.
- Jadhav K, Dhamecha D, Bhattacharya D, et al. Green and ecofriendly synthesis of silver nanoparticles: characterization, biocompatibility studies and gel formulation for treatment of infections in burns. J Photochem Photobiol. 2016;155:109–115.
- Nirmala R, Sheikh FA, Kanjwal MA, et al. Synthesis and characterization of bovine femur bone hydroxyapatite containing silver nanoparticles for the biomedical applications. J Nanopart Res. 2011;13:1917–1927.
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182.
- Sukirtha R, Priyanka KM, Antony JJ, et al. Cytotoxic effect of Green synthesized silver nanoparticles using Melia azedarach against in vitro HeLa cell lines and lymphoma mice model. Process Biochem. 2012;47:273–279.
- Velmurugan P, Lee SM, Iydroose M, et al. Pine cone-mediated green synthesis of silver nanoparticles and their antibacterial activity against agricultural pathogens. Appl Microbiol Biotechnol. 2013;97:361–368.
- Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008;112:13608–13619.
- Jaidev LR, Narasimha G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. J Colloid Interface Sci B. 2010;81:430–433.
- Kumar CM, Yugandhar P, Savithramma N. Biological synthesis of silver nanoparticles from Adansonia digitata L. fruit pulp extract, characterization, and its antimicrobial properties. J Intercult Ethnopharmacol. 2016;5:79–85.
- Yugandhar P, Savithramma N. Biosynthesis, characterization and antimicrobial studies of green synthesized silver nanoparticles from fruit extract of Syzygium alternifolium (Wt.) Walp. an endemic, endangered medicinal tree taxon. Appl Nanosci. 2016;6:223–233.
- Francis S, Joseph S, Koshy EP, et al. Microwave assisted green synthesis of silver nanoparticles using leaf extract of Elephantopus scaber and its environmental and biological applications. Artif Cells Nanomed Biotechnol. 2017;5:1–10.
- Al-Shmgani HS, Mohammed WH, Sulaiman GM, et al. Biosynthesis of silver nanoparticles from Catharanthus roseus leaf extract and assessing their antioxidant, antimicrobial, and wound-healing activities. Artif Cells Nanomed Biotechnol. 2017;45:1234–1240.