?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
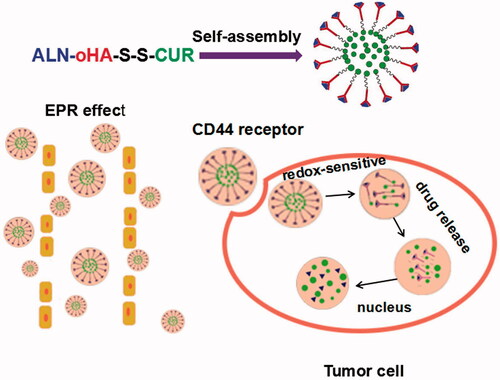
The traditional therapy of cancer has systemic side effects, and many cancers, such as human breast cancer and lung cancer easily metastasize to bones, leading to the formation of secondary tumours. This study was aimed at enhancing the anti-tumour effect of curcumin (CUR) and preventing tumour spread to the bone. A novel multifunctional redox-responsive and CD44 receptor targeting polymer-drug, poly alendronate-hyaluronan-S-S-curcumin copolymer (ALN-oHA-S-S-CUR) based CUR and alendronate (ALN) were synthesized successfully with the disulphide bond linker. The structure of ALN-oHA-S-S-CUR was characterized by 1H-NMR. The nanomedicine had natural anti-tumour drugs (CUR) as the hydrophobic kernel, and targeting CD44 receptor oligosaccharides of hyaluronan (oHA) and other anti-tumour drugs (ALN) as hydrophilic shell, named ALN-oHA-S-S-CUR conjugates, which could self-assemble into micelle-like nano-spheres in water via a dialysis method with hydrodynamic diameters of 179 ± 23 nm. Interestingly, the cur-loaded ALN-oHA-S-S-CUR micelles were stable in PBS but were capable of releasing the drug under the reducing environment. The rate of drug release was proportional to the GSH concentration. The uptake and cytotoxicity of micelles were higher in MDA-MB-231 cells than in MCF-7 cells because of a higher expression of the CD44 receptor in the former cell line. And compared to the cur-loaded oHA-CUR micelles, the cur-loaded ALN-oHA-S-S-CUR micelles had a good cellular uptake in 2D cancer cell and penetrability in 3D cancer cell spheroids. These results indicated the active targeting redox-sensitive micelles were promising as intracellular drug delivery systems for cancer treatment.
Introduction
Curcumin (CUR), is a therapeutic cancer drug obtained from curcuma longa plant, but its low aqueous solubility in medium and poor absorption extremely limit its application in clinical [Citation1,Citation2]. Polymer-drug conjugates are consisted by water soluble and biocompatible polymers that can enhance the solubility and stability of hydrophobic drugs [Citation3]. So making use of hydrophilic polysaccharide to form polymer-drug conjugates is a promising method to enhance the solubility of hydrophobic drug. Oligosaccharides of hyaluronan (oHA) is a non-toxic, biocompatible hydrophilic polysaccharide, thus its own characteristics that can specific binding with CD44 receptors that tumours cells overexpression, many reports made it as tumour targeting capacity [Citation3,Citation4]. In this study, we combined oHA with curcumin to gain polymer-drug conjugates to enhance curcumin solubility and also be used as a fluorescence marker.
Because the physiological microenvironment of bone was very suitable for cancer cells adhere and proliferate, many cancers, such as human breast cancer, lung cancer were easily metastasize to bone, leading to a series of bone disease, such as bone metastases tumours, secondary bone tumours [Citation5]. On the one hand, alendronate (ALN) was an effective therapeutic drug for bone metastases; it also had been proven to possess antitumour and anti-angiogenic activity [Citation6,Citation7]. Its strong hydrophobicity could enhance hydrophobic drug solubility and penetrability. So in this work, we designed a drug delivery system that could co-delivery ALN and curcumin to enhance antitumour activity and also prevented tumour metastasize to bone.
Tumour microenvironment-responsive drug delivery system could minimalize undesired toxicity and maximum desired effect [Citation8]. There was a research that the reducing substances (7–10-folds) higher in tumour cells than normal cells [Citation9,Citation10]. Thus, we could make use of tumour microenvironment designing a redox-responsive drug delivery system with the disulphide bond to achieve tumour targeting [Citation11]. In this work, we made disulphide bond as connection arm that combined the hydrophobic curcumin with hydrophilic oHA and ALN to be an amphiphilic material that could self-assemble into micelles in water. When the micelles entered into tumour cells by CD44 receptors active targeting and EPR effect, micelles were broken that triggered by the disulphide bond fractured under the reducing environment, causing the drug release and inhibited the tumour growth.
Compared to 2D cancer cell culture models, the multicellular 3D cancer cell models could accurately mimic tumour features, such as drug penetration, their spatial architecture and physiological response [Citation12]. And many results had proven the ability of 3D cancer cell spheroid as an effective in vitro model to evaluate antitumour effect [Citation13]. So in this work, we choose the 3D theoretical models as a good way to research the penetration of micelles into solid tumour.
In order to enhance the solubility of curcumin, the Gum arabic-curcumin conjugates were prepared by Sarika. This method was enhancing the solubility of curcumin, but its systemic side effects and non-targeting in tumour tissues that limited its application in vivo [Citation14]. So in this work, in order to enhance the DL/EE of curcumin and achieve the tumour targeting (shown in ), we successfully synthesized ALN-oHA-S-S-CUR, oHA-CUR conjugates to selectively response to reducing environment in tumour cells and prepared the drug-loaded micelles. Compared to ordinary nanomedicine and oHA-CUR micelles, ALN-oHA-S-S-CUR micelles showed higher DL/EE, more redox-responsive characteristics in vitro and desired uptake effect, higher cytotoxicity in 2D tumour cells. We had also built the multicellular 3D cancer cell spheroid to research the penetration of ALN-oHA-S-S-CUR micelles. Finally, we had proven the deeper penetration of micelles combination treatment of ALN and CUR, implying that the ALN-oHA-S-S-CUR micelles might have a promising future in cancer therapy.
Materials and methods
Materials
Oligosaccharides of oHA (Mn = 10 KDa) were purchased from Shandong Freda Co. Ltd (Shandong, China). Curcumin was purchased from Aladdin Reagent Net (Shanghai, China). ALN, Dimethylamino pyridine (DMAP), L-Gutathione (GSH), 3,3-dithiodipropionic acid and hochest33342 were purchased from Sigma-Aldrich (Shanghai, PR China). Foetal bovine serum (FBS), DMEM and MTT were purchased from Saiersi Biotechnology Co. Ltd (Shangdong, Yantai, China). All reagents were commercially available grade. The water used in experiments was deionized water.
HPLC analysis
The concentration of curcumin was determined by high-pressure liquid chromatography (HPLC, Agilent 1260GB12C) with a Phenomenex C18 column (250 × 4.6 mm2, 5 um). The mobile phases were 0.5% glacial acetic acid and acetonitrile (40:60 v/v) with a flow rate of 1.0 ml·min−1. And the column temperature was 25 °C. And the injection volume was 20 ul. The detection wavelength was 425 nm [Citation15].
Methods
Synthesis and characterization of ALN-oHA-S-S-CUR
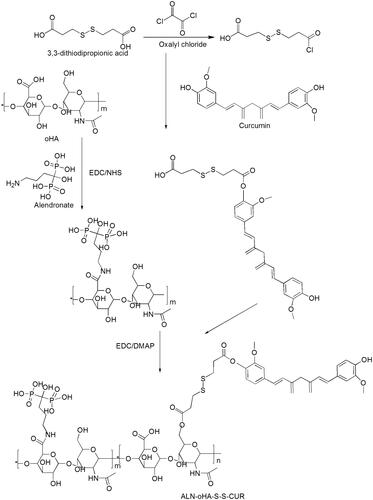
The polymer was synthesis via a three-step reaction and the synthetic routes are shown in .
Step 1: First, 3,3-dithiodipropionic acid was activated by oxalyl chloride in THF, then the product was reacted with curcumin catalysed by TFA. The reaction product was separation by column chromatography to gain pure product HOOC-S-S-CUR.
Step 2: ALN was combined with oHA via amino bond. First, oHA, EDC and NHS were dispersed in water and DMSO (1:1 v/v) to react 2 h, then ALN was dissolved in water and dropped the above reaction into it for 24 h at 55 °C [Citation16]. The reaction mixture was dialysed (MWCO:2000) in deionized water for 48 h to remove the unreacted ALN, and lyophilized to gain ALN-oHA polymer.
Step 3: Briefly, HOOC-S-S-CUR was activated by EDCI and DMAP in DMSO for 2 h. Then ALN-oHA was dissolved in water, and the above reaction was dropped into it and stirred for 24 h at 55 °C. The reaction mixture was dialysed (MWCO:2000) in deionized water for 48 h and centrifuge at 2000 rpm for 10 min to remove the unreacted HOOC-S-S-CUR, lyophilized to gain ALN-oHA-S-S-CUR conjugates.
Structure characterization of polymers
The structure of HOOC-S-S-CUR, ALN-oHA and ALN-oHA-S-S-CUR were verified by 1H-NMR spectra. For 1H-NMR spectra, the hydrophilic materials (ALN-oHA) were dissolved in D2O, the hydrophobic materials (HOOC-S-S-CUR) were dissolved in DMSO-d6 and the amphiphilic materials were dissolved in D2O:DMSO-d6 (1:1 v/v) to measure.
Preparation of self-assembly micelles
ALN-oHA-S-S-CUR micelles and oHA-CUR micelles were both prepared by the dialysis method [Citation17,Citation18]. Briefly, 15 mg ALN-oHA-S-S-CUR and 1 mg cur in 6 ml formamide, ultrasound to dissolved the mixture solution, then the mixture solution was dialysed against deionized water for 24 h in darkness and the dialysed solution was centrifuged at 3000 rpm for 15 min further to remove unloaded cur. Finally, the solution was filtered through Millipore membrane (Millipore, Billerica, MA) of 800 nm and the filtrate was lyophilized to gain cur-loaded micelles. The cur-loaded oHA-CUR micelles were gained by the same ways.
Determination of drug loading and entrapment efficiency of micelles
We used membrane filtration method to measure the entrapment efficiency and drug loading content of the freeze-dried micelles [Citation19]. First, 5 mg cur-loaded micelles and 5 mg cur-loaded micelles after 450 nm filter membrane were dispersed in 3 ml deionized water. Then put them into 10 ml volumetric flask and dilute with methanol to 10 ml, respectively. And the samples containing cur were measured by HPLC (Agilent Technologies, Santa Clara, CA). The mobile phase consisted of acetonitrile/0.5% acetic acid (60:40 v/v) with a flow rate of 1.0 ml/min. The equations that calculated entrapment efficiency (EE) and drug loading content (DL) are as follows:
Observation of particle size, zeta potential, polydispersity index and morphology
The particle size, zeta potential and polydispersity index (PI) of micelles were measured by Beckman Coulter Particle Analyzer (Beckman Coulter, Inc., Brea, CA) at room temperature. The morphology of micelles was observed by transmission electron microscope (TEM).
In vitro GSH-triggered drug release study
A dialysis method was used to investigate the redox-responsive characteristic of cur-loaded ALN-oHA-S-S-CUR micelles [Citation20]. Briefly, 5 ml solution of micelles was put in dialysis bag (MWCO:2000) and immersed in PBS containing 0.5% Tween 80 with GSH (0, 0.1, 1 and 10 mM) [Citation21]. Then put them in water bath and shook at 37 °C. Of, 2 ml of release medium was removed and replaced with the same fresh medium to keep accordance with total volume at various time intervals. The samples containing cur were analysed by HPLC.
Cell culture
The human breast cancer cell lines (MDA-MB-231 and MCF-7) were easy to metastasize to bone, and CD44 receptors were high-expression in MDA-MB-231 cells, normal-expression in MCF-7 cells [Citation4,Citation22]. So we selected the two cells to culture at 37 °C in a humidified atmosphere containing 5% CO2 in DMEM with 10% foetal bovine serum.
Cell uptake study
Compared to cur-loaded oHA-CUR micelles, the cur-loaded ALN-oHA-S-S-CUR micelles had tumour cells targeting, so it could be more uptake by tumour cells. In order to have a qualitative analysis about the results of cellular uptake, we observed the cellular uptake in cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles. Briefly, the MDA-MB-231, MCF-7 cells were seeded into 24-well plate at a density of 1 × 104 cells per well and cultured for 24 h. Then, the medium was replaced with fresh medium containing cur-loaded ALN-oHA-S-S-CUR micelles or cur-loaded oHA-CUR micelles (cur concentration: 20 μg/ml) and was cultured for 2 and 4 h at 37 °C in a humidified atmosphere containing 5% CO2. Finally, the cells were rinsed thrice with PBS (pH: 7.4) and fixed with 4% paraformaldehyde for 15 min, rinsed again with PBS [Citation23]. The uptake was visualized by inverted fluorescence microscope.
In vitro cytotoxicity study
In order to evaluate the antitumour activity of free cur, oHA-CUR and ALN-oHA-S-S-CUR conjugates, MTT assay was used on MDA-MB-231 and MCF-7 cells. First, MDA-MB-231 and MCF-7 cells were plated onto a 96-well plate with a density of 5000 cells/well and cultured for 24 h at 37 °C. Then, samples including cur were diluted with fresh culture medium to gain different concentrations of cur (1.25, 2.5, 5, 10, 20 and 40 μg/ml) to replace the old culture medium and incubated for 48 h at 37 °C. Finally, 20 ul of MTT reagent (5 mg/ml) was added to every well and incubated for another 4 h at 37 °C. MTT reagent was replaced by 200 ul DMSO and put them in water bath and shook to make formazan product solubilized sufficiently, and the absorbance of each well was measured using a multiwell plate reader (Thermo Fisher Scientific Co., Waltham, MA) at 570 nm [Citation24].
The flow cytometry
The flow cytometry was used to evaluate the quantitative result of the cellular uptake. First, MDA-MB-231 and MCF-7 cells were plated onto a 6-well plate with a density of 1 × 105 cells/well and cultured with 10% FBS for 24 h at 37 °C. Then, cells were treated with different formulations with the final concentration of 10 μg/ml and cultured 5, 30, 90 min, respectively. After incubation, cells were put into 1.5 ml EP tube, centrifuged at 1500 rpm for 5 min and re-suspended with 600 ul PBS. After washed thrice with PBS, the cells were suspended and entered into flow cytometry analysis.
Spheroid culture and micelles distribution in spheroids
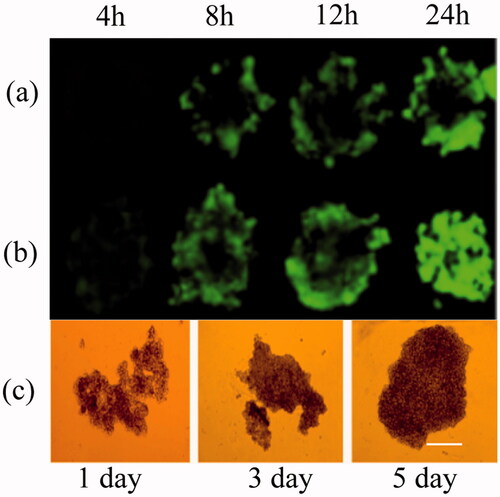
Multicellular 3D cancer cell spheroids of MDA-MB-231 were cultured using liquid overlay method as document reported [Citation25]. Briefly, 60-ul agarose solution (2% W/V) was added into each well of 96-well plate, cooling to room temperature under ultraviolet radiation. Then, MDA-MB-231 cells were seeded in each well with a density of 1 × 103 cells/well and cultured at 37 °C, 5% CO2. Finally, changed the culture medium every 24 h and observed the growth status of spheroids. After 5 d, the spheroids were formed and treated with different formulations to observe its uptake status.
The fluorescence intensity of coumarin-6 is higher than curcumin and its cytotoxicity is lower than curcumin. In this study, in order to have a clear and long-time observation about the penetration of ALN-oHA-S-S-CUR micelles, we selected coumarin-6 as a higher intensity fluorescence marker.
Statistical analysis
All data are reported as mean ± standard deviation (SD) of results from at least three independent runs conducted at three different times. Student’s two-tailed t-test assuming equal variance was performed to determine the p values for assessing statistical significance.
Results and discussion
The design of the targeted delivery system
The traditional therapy of cancer has systemic side effects, non-targeting in tumour tissues, so in order to enhance the tumour targeting ability; we made use of the tumour microenvironment designing a novel multifunctional redox-responsive and CD44 receptor targeting polymer-drug ALN-oHA-S-S-CUR. We hypothesized that ALN-oHA-S-S-CUR material would self-assemble into cur-loaded micelles. First, the micelles would accumulate in tumour tissue by EPR effect. Then, the micelles delivered the drug to tumour cells by CD44 receptor targeting. Finally, the release of the drug would be caused by the breakage of the disulphide bond under the reducing environment. And ALN not only an antitumour drug, but also prevent the tumour metastasize to bones. So, the cur-loaded ALN-oHA-S-S-CUR micelles constitute a promising drug delivery system for tumour therapy.
Characterization of ALN-oHA-S-S-CUR
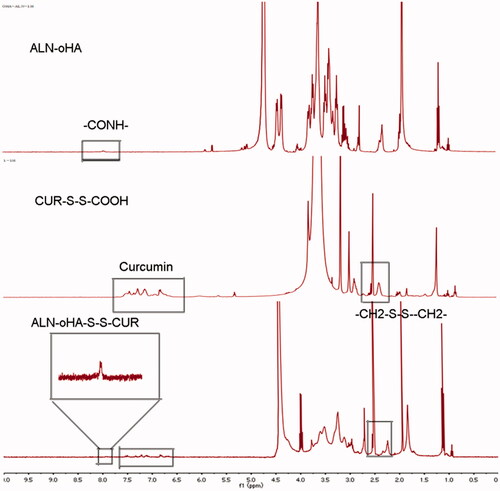
The 1H-NMR spectrum of HOOC-S-S-CUR, ALN-oHA and ALN-oHA-S-S-CUR are shown in . The cur in ALN-oHA-S-S-CUR was seen in the region between 6.5 and 8 ppm [Citation26], and the appearance of signal at peak 7.8 ppm was verified the amide bond (–CONH–) connection between ALN and oHA [Citation16]. In addition, the –CH2– proton peak of 3,3-dithiodipropionic acid was seen at 2.8 ppm that accordance with its standard substance. These result indicated that the amphiphilic materials ALN-oHA-S-S-CUR were successfully synthesis.
Characterization of micelles
The particle size, PI, zeta potential, DL (%) and EE (%) of cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles are seen in . The average size of cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles were 258 ± 12 and 179 ± 23 nm, respectively. May be because ALN changed the physiochemical properties of surface of cur-loaded ALN-oHA-S-S-CUR micelles that leading to the differences of two micelles [Citation27]. And the DL and EE were higher than the ordinary micelles that synthesized by oHA-SA. The zeta potential is an important parameter to measure the micelles stability. In this research, the zeta potential of cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles were −23.6 and −25.7 mV, respectively. These might be also because the presence of the ALN that reduced the aggregation of the micelles. These results were in accord with what was reported in the document [Citation6]. Such as, it was reported that the size of ALN modified DOX-hyd-PEG-ALN micelles (114 ± 17 nm) was smaller than DOX-hyd-PEG micelles (278 ± 20 nm).
Table 1. The physiochemical properties of different micelles.
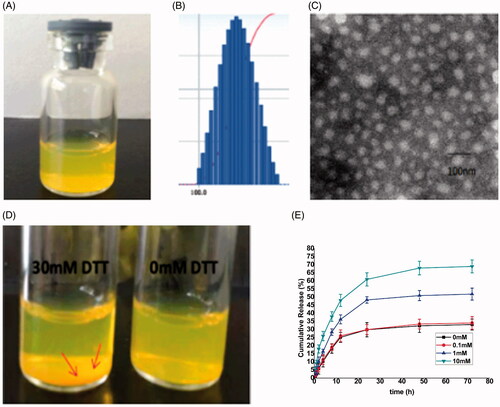
The transmission electron microscopy (TEM) was used to observe the morphology of the micelles. As illustrated in , the cur-loaded ALN-oHA-S-S-CUR micelles possessed a spherical shape and the particle size was 80 nm, these results were smaller than the Beckman Coulter Particle Analyzer, these probably because the micelles were dehydrated that made the size contraction, when using the TEM to observe the micelles morphology [Citation28]. On the other, the PI of the cur-loaded ALN-oHA-S-S-CUR micelles was 0.14 smaller than 0.2, showed the uniform of the micelles size, and these results were consistent with TEM.
Figure 4. (A) The redissolved cur-loaded ALN-oHA-S-S-CUR micelles with PBS; (B) The size distribution of cur-loaded ALN-oHA-S-S-CUR micelles; (C) The TEM images of cur-loaded ALN-oHA-S-S-CUR micelles; (D) Photograph of ALN-oHA-S-S-CUR micelles dissolved by 30 and 0 mM DTT after 12 h. (E) In vitro release profiles of cur- loaded ALN-oHA-S-S-CUR micelles at different GSH concentration.
Reduction response of ALN-oHA-S-S-CUR
The reduction response ability of ALN-oHA-S-S-CUR was studied by micelles in different GSH concentration, as illustrated in . Compared to 0 and 0.1 mM GSH, 1 and 10 mM GSH showed significant difference in release profile. This was verified that the drug release in response to the GSH concentration.
In addition, in order to observe the stability of the micelles under the reduction environment, we immersed the cur-loaded ALN-oHA-S-S-CUR micelles in PBS containing 30 mM DTT at 37 °C for 12 h and made the no DTT group as a control, the depositing cur was more than no DTT after 12 h, this further showed that the cur-loaded ALN-oHA-S-S-CUR micelles had a reduction property [Citation29].
Uptake study
The cellular uptake of the cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles were studied by inverted fluorescence microscope. In this study, Cur itself not only was a green fluorescence probe but also an anticancer drug, different CD44 receptors expression cells, such as MCF-7 and MDA-MB-231 cells were selected to research the cur-loaded ALN-oHA-S-S-CUR micelles had an active targeting redox-sensitive ability. As illustrated in , both cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles showed cellular uptake in both cell lines and the uptake efficiency was in direct proportion to time, the fluorescence signals were higher at 4 h. In addition, because of the CD44 receptors high expression in MDA-MB-231 cells, the cur fluorescence efficiency of uptake was higher than MCF-7 cells.
Figure 5. The uptake analysis of two micelles. (A) The uptake of two micelles at 2 and 4. a: oHA-CUR micelles. b: ALN-oHA-S-S-CUR micelles. (B) Internalization imaging of two micelles in MDA-MB-231 cells after 2 h incubation. Cell-nucleus was stained by Hoechst 33342 with blue fluorescence. (C), (D) The cytotoxicity of different formulations at 48 h in MCF-7 cells and MDA-MB-231 cells. n = 6; *indicates p < .05.
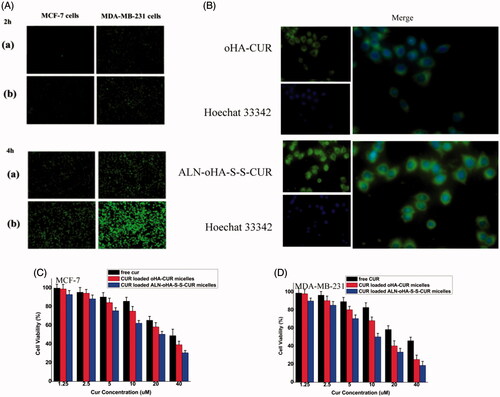
At the same time, because of the redox-sensitive ability of cur-loaded ALN-oHA-S-S-CUR micelles, its fluorescence signals were higher than cur-loaded oHA-CUR micelles. And after stained by Hoechst 33342 (Thermo Fisher Scientific Co., Waltham, MA), most green fluorescence was in the cytoplasm as illustrated in , this result demonstrated cellular uptake through caveolae-mediated endocytosis. By enhancing the cur accumulation, it would be induced the cytotoxicity and apoptotic of cancer cells.
Cytotoxicity studies
The cytotoxicity of free cur, cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles in MCF-7 and MDA-MB-231 were studied by MTT assay. As illustrated in , different cur concentration cells had a different cell viability at 48 h. With the concentration of cur ranging from 1.25 to 5 μg/ml, the cell viability of the same formulations had no obvious difference. But when the concentration of cur higher to 5 μg/ml, the cell viability of the same and different formulations had a significant difference, and 40 μg/ml cur-loaded ALN-oHA-S-S-CUR micelles had the lowest cell viability after 48 h. These result could be caused by the factor which the ALN-oHA-S-S-CUR micelles had a good biocompatibility, it was easy to enter into the cells and release the drug. Meanwhile, the cell viability of MDA-MB-231 was lower than MCF-7 in the same formulations and cur concentration. This result was in line with uptake result.
In addition, because the cur was grafted to polymer by a chemical method, so the blank micelles had included cur. And the blank micelles also had cytotoxicity, so we did not study the cytotoxicity of blank micelles.
Flow cytometry
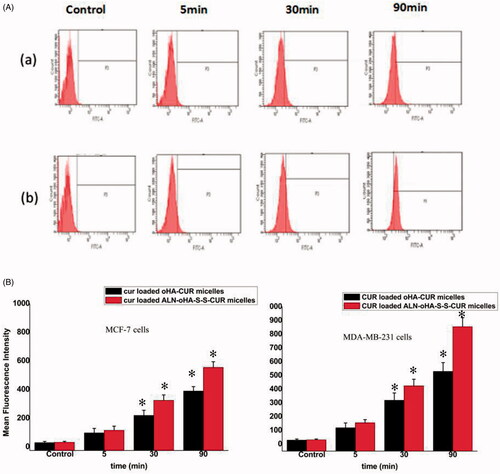
The mean fluorescent intensity (MFI) of cellular uptake was measured by the flow cytometry. As illustrated in , the MFI was directly proportional to the administration time. In addition, the MFI of cur-loaded ALN-oHA-S-S-CUR micelles in MDA-MB-231 cells was the highest among all formulations. This result was consistent with the inverted fluorescence microscope.
Figure 6. The flow cytometry images. (A) MDA-MB-231 cells incubated with cur-loaded oHA-CUR micelles and cur-loaded ALN-oHA-S-S-CUR micelles for 5, 30 and 90 min at 37 °C. Quantitative analysis of flow cytometry images. (B) The quantitative analysis of MCF-7cells. (C) The quantitative analysis of MDA-MB-231 cells, respectively. n = 6; *indicates p < .05; CUR concentration is 20 μg/ml.
ALN-oHA-S-S-CUR micelles enhanced the penetration of drugs
Spheroid models had been applied as a model to mimic the tumours in vivo and also used as a tool to evaluate the penetration efficiency of the nano-formulations in tumour [Citation30]. In this work, we cultured the multicellular 3D cancer cell spheroid to evaluate the penetration of ALN-oHA-S-S-CUR micelles in vitro. As illustrated in , compared to oHA-CUR micelles, ALN-oHA-S-S-CUR micelles had a deeper penetration. These were probably because ALN changed the physiochemical properties of the micelles, thus the redox-sensitivity and CD44-targeted ability of the micelles that made it aggregation in multicellular 3D cancer cell spheroid, and in favour of the penetration of micelles. These were consistent with the result that document reported.
Conclusions
In this work, in order to enhance the treatment effect of cur and ALN on tumour tissue, reduce the systemic side effects of traditional therapy and increase the tumour targeting. Based on the microenvironment of tumour cells, we made disulphide bond as a connection arm and successful prepared a reduction-responsive, CD44 receptor-targeting polymer-drug conjugates (ALN-oHA-S-S-CUR) to enhance the DL/EE of cur and achieve tumour targeting. Due to the connection between hydrophilic oHA and the hydrophobic cur, ALN-oHA-S-S-CUR could self-assemble to cur-loaded micelles in water. The cellular uptake study showed that the cur-loaded ALN-oHA-S-S-CUR micelles could successfully transport cur entering into cytoplasm. The results of cellular uptake and cytotoxicity showed the cur-loaded ALN-oHA-S-S-CUR micelles had a reduction-responsive and CD44 receptor-targeting ability. And the 3D penetration experiments showed the cur-loaded ALN-oHA-S-S-CUR micelles could enter the tumour tissue. Next step, we will evaluate the antitumour activity of cur-loaded ALN-oHA-S-S-CUR micelles in vivo.
In addition, we designed a drug delivery system which called polymer-drug conjugates to trap curcumin. By a chemical method and a physical method entrapping curcumin, not only improve its encapsulation efficiency, but also increase its stability and blood circulation time. This method also offered a new idea for enhancing the solubility and stability of hydrophobic drug.
All these results showed we could make use of the microenvironment of tumour cells designing a tumour-targeted drug delivery system. This method provided a new idea for tumour treatment.
Acknowledgements
All the authors contributed equally to this work.
Disclosure statement
The authors report no declarations of interest.
Additional information
Funding
References
- Namdari M, Eatemadi A. Cardioprotective effects of curcumin-loaded magnetic hydrogel nanocomposite (nanocurcumin) against doxorubicin-induced cardiac toxicity in rat cardiomyocyte cell lines. Artif Cells Nanomed Biotechnol. 2017;45:731–739.
- Wang L, Chen X, Du Z, et al. Curcumin suppresses gastric tumor cell growth via ROS-mediated DNA polymerase γ depletion disrupting cellular bioenergetics. J Exp Clin Cancer Res. 2017;36:47.
- Kesharwani P, Xie L, Banerjee S, et al. Hyaluronic acid-conjugated polyamidoamine dendrimers for targeted delivery of 3,4-difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf B Biointerfaces. 2015;136:413–423.
- Yan H, Song J, Jia X, et al. Hyaluronic acid-modified didecyldimethylammonium bromide/ d-a-tocopheryl polyethylene glycol succinate mixed micelles for delivery of baohuoside I against non-small cell lung cancer: in vitro and in vivo evaluation. Drug Deliv. 2017;24:30–39.
- Huang Y, Chu T, Liao T, et al. Downregulation of lysosomal and further gene expression characterization in lung cancer patients with bone metastasis. Artif Cells Nanomed Biotechnol. 2017;45:758–764.
- Miller K, Clementi C, Polyak D, et al. Poly(ethylene glycol)-paclitaxel-alendronate self-assembled micelles for the targeted treatment of breast cancer bone metastases. Biomaterials. 2013;34:3795–3806.
- Ryu TK, Kang RH, Jeong KY, et al. Bone-targeted delivery of nanodiamond-based drug carriers conjugated with alendronate for potential osteoporosis treatment. J Control Release. 2016;232:152–160.
- Chen WL, Yang SD, Li F, et al. Tumor microenvironment-responsive micelles for pinpointed intracellular release of doxorubicin and enhanced anti-cancer efficiency. Int J Pharm. 2016;511:728–740.
- Jiang M, Zhang R, Wang Y, et al. Reduction-sensitive paclitaxel prodrug self-assembled nanoparticles with tetrandrine effectively promote synergistic therapy against drug-sensitive and multidrug-resistant breast cancer. Mol Pharm. 2017;14:3628–3635.
- Yang X, Cai X, Yu A, et al. Redox-sensitive self-assembled nanoparticles based on alpha-tocopherol succinate-modified heparin for intracellular delivery of paclitaxel. J Colloid Interface Sci. 2017;496:311–326.
- Liu X, Wang J, Xu W, et al. Glutathione-degradable drug-loaded nanogel effectively and securely suppresses hepatoma in mouse model. Int J Nanomedicine. 2015;10:6587–6602.
- Klimkiewicz K, Weglarczyk K, Collet G, et al. A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 2017;396:10–20.
- Sarisozen C, Dhokai S, Tsikudo EG, et al. Nanomedicine based curcumin and doxorubicin combination treatment of glioblastoma with scFv-targeted micelles: in vitro evaluation on 2D and 3D tumor models. Eur J Pharm Biopharm. 2016;108:54–67.
- Sarika PR, James NR, Kumar PR, et al. Gum arabic-curcumin conjugate micelles with enhanced loading for curcumin delivery to hepatocarcinoma cells. Carbohydr Polym. 2015;134:167–174.
- Hong J, Liu Y, Xiao Y, et al. High drug payload curcumin nanosuspensions stabilized by mPEG-DSPE and SPC: in vitro and in vivo evaluation. Drug Deliv. 2017;24:109–120.
- Ye WL, Zhao YP, Li HQ, et al. Doxorubicin-poly (ethylene glycol)-alendronate self-assembled micelles for targeted therapy of bone metastatic cancer. Sci Rep. 2015;5:14614.
- Cui X, Guan X, Zhong S, et al. Multi-stimuli responsive smart chitosan-based microcapsules for targeted drug delivery and triggered drug release. Ultrason Sonochem. 2017;38:145–153.
- Fang XB, Zhang JM, Xie X, et al. pH-sensitive micelles based on acid-labile pluronic F68-curcumin conjugates for improved tumor intracellular drug delivery. Int J Pharm. 2016;502:28–37.
- Yin J, Lang T, Cun D, et al. pH-sensitive nano-complexes overcome drug resistance and inhibit metastasis of breast cancer by silencing Akt expression. Theranostics. 2017;7:4204–4216.
- Zhao YP, Ye WL, Liu DZ, et al. Redox and pH dual sensitive bone targeting nanoparticles to treat breast cancer bone metastases and inhibit bone resorption. Nanoscale. 2017;9:6264–6277.
- Dey S, Ambattu LA, Hari PR, et al. Glutathione-bearing fluorescent polymer-curcumin conjugate enables simultaneous drug delivery and label-free cellular imaging. Polymer. 2015;75:25–33.
- Zhang Z, Xu S, Wang Y, et al. Near-infrared triggered co-delivery of doxorubicin and quercetin by using gold nanocages with tetradecanol to maximize anti-tumor effects on MCF-7/ADR cells. J Colloid Interface Sci. 2018;509:47–57.
- Li Z, Cai Y, Zhao Y, et al. Polymeric mixed micelles loaded mitoxantrone for overcoming multidrug resistance in breast cancer via photodynamic therapy. Int J Nanomedicine. 2017;12:6595–6604.
- Ding Y, Chen W, Hu J, et al. Polymerizable disulfide paclitaxel prodrug for controlled drug delivery. Mater Sci Eng C Mater Biol Appl. 2014;44:386–390.
- Costa EC, Moreira AF, de Melo-Diogo D, et al. 3D tumor spheroids: an overview on the tools and techniques used for their analysis. Biotechnol Adv. 2016;34:1427–1441.
- Raveendran R, Bhuvaneshwar GS, Sharma CP. Hemocompatible curcumin-dextran micelles as pH sensitive pro-drugs for enhanced therapeutic efficacy in cancer cells. Carbohydr Polym. 2016;137:497–507.
- Ge P, Sheng F, Jin Y, et al. Magnetic resonance imaging of osteosarcoma using a bis(alendronate)-based bone-targeted contrast agent. Biomed Pharmacother. 2016;84:423–429.
- Wu S, Zheng L, Li C, et al. Grafted copolymer micelles with pH triggered charge reversibility for efficient doxorubicin delivery. J Polym Sci Part A. 2017;55:2036–2046.
- Chi Y, Yin X, Sun K, et al. Redox-sensitive and hyaluronic acid functionalized liposomes for cytoplasmic drug delivery to osteosarcoma in animal models. J Control Release. 2017;261:113–125.
- Khakpour S, Di Renzo A, Curcio E, et al. Oxygen transport in hollow fibre membrane bioreactors for hepatic 3D cell culture: a parametric study. J Membr Sci. 2017;544:312–322.