?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study chitosan nanoparticles (CS NPs) and mannosylated chitosan nanoparticles (MCH NPs) loaded with recombinant hepatitis B surface antigen (rHBsAg) was synthesized as a vaccine delivery system and assessed toxically and immunologically. The physicochemical properties of the nanoparticles (NPs) were determined by methods including scanning electron microscope (SEM) and dynamic light scattering (DLS). The morphology of the NPs was semi spherical and the average diameter of the loaded CS and MCH NPs was found to be 189 and 239 nm, respectively. The release studies showed that after the initial burst, both of the loaded NPs provided a continuous and slow release of the antigens. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay showed concentration and time dependent toxicity profile for both formulations, but rHBsAg loaded CS nanoparticle showed higher toxicity due to smaller particle size and larger zeta potential. Abnormal toxicity test (ATT) results showed no signs of toxicity in mice and guinea-pigs treated with loaded MCHNPs. Stability test for six months showed acceptable changes in size, surface charge, and antigenicity for loaded MCH nanoparticles. Finally, in vivo immunogenicity study revealed greater adjuvant capability of MCH nanoparticles than others formulations. Our results showed MCH NPs can be used as a controlled and targeted vaccine delivery system.
Introduction
The adjuvant is an ingredient of vaccine formulation that helps create a stronger immune response in the patient’s body by a variety of mechanisms [Citation1,Citation2]. Aluminum salts (alum), such as aluminum hydroxide, aluminum phosphate, and aluminum potassium sulfate have been used safely in vaccines for more than 70 years [Citation3,Citation4]. The problem with pure recombinant or synthetic antigens used in modern day vaccines is that they are generally far less immunogenic than older style live or killed whole organism vaccines. This has created a major need for improved and more powerful adjuvants for use in these vaccines [Citation5,Citation6].
Nowadays, recombinant hepatitis B vaccine is administered in three doses over a period of six months, though some vaccine recipients fail to return for all doses, thereby limiting the potential vaccine efficacy. Also sometimes there is a need to complete the immunization program in a shorter duration, for example, in healthcare workers, travelers to endemic areas, or prisoners who are not immune [Citation7,Citation8]. Like many other vaccines, aluminum hydroxide is used as the adjuvant in the hepatitis B vaccine formulation. The alum is able to trigger humoral immune response (Th2), but it is inefficient in inducing cellular immune response (Th1) which is important for the immune responses against many viral and intracellular pathogens [Citation9].
In recent years, nanotechnology has played a significant role in various biomedical fields specially drug delivery, cancer research, regenerative medicine and vaccine development [Citation10–14]. Using conjugated nanoparticles (NPs) with pathogen-associated molecular patterns (PAMPs) that have receptors on the antigen-presenting cells (APCs) can be a superior strategy for development of new generation vaccines that generate strong and sustained immunity following a single dose [Citation7]. In fact, stimulating innate immune responses directly with PAMPs may lead to increased vaccine potency [Citation15]. chitosan (CS) has some unique properties like polycationic, nontoxic, biodegradable, and biocompatible that has attracted much attention and has wide applications in pharmaceutical and biomedical applications [Citation16,Citation17]. CS is a unique biopolymer that exhibits outstanding properties. Most of these peculiar properties arise from the presence of primary amines along the CS structure which can be conjugated to other molecules [Citation18,Citation19]. Todays some PAMPs have been investigated as immune cells targeted delivery system. Among the various PAMPs, mannose is the most promising for vaccine targeting because they exhibit high affinity and are rapidly internalized by immune cells [Citation20].
In the present study, recombinant hepatitis B surface antigen (rHBsAg) loaded CS and CS NPs conjugated with mannose (as a PAMPs) were synthesized. Physico-chemical characteristics, in vitro and in vivo toxicity, and immunogenicity of both above mentioned formulations and also stability of loaded MCH NPs as a new targeted vaccine delivery vehicle were analyzed.
Materials and methods
Materials
Low molecular weight chitosan (CS) was purchased from Primex, Iceland (deacetylation degree ≥95% and viscosity <20 cps), which was obtained from fresh North Atlantic shrimp (Pandalus Borealis) shells. Coomassie Blue G250, Tripolyphosphate (TPP), dimethyl sulfoxide (DMSO) and mannopyranosylphenyl isothiocyanate were purchased from Sigma Aldrich, St. Louis, MO. The rHBsAg and hepatitis B surface antibody (HBsAb) were supplied by Pasteur Institute of Iran. Dulbecco's Modified Eagle Medium (DMEM) was purchased from Gibco (Carlsbad, CA). Mouse Anti-Hepatitis B Surface Antigen (anti-HBsAg) IgG ELISA kit and HBsAg (native or recombinant) ELISA Kit, quantitative were obtained from Alpha Diagnostic (San Antonio, TX). All other chemicals and reagents were of the highest grade commercially available.
Methods
Preparation of mannosylated chitosan (MCH)
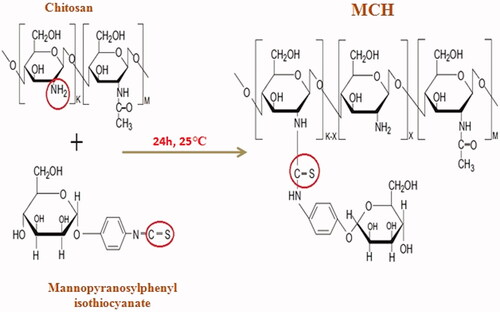
MCH was prepared by some modified methods described in other papers [Citation20]. Briefly, 100 mg of CS was dissolved in 2 ml of double distilled water, pH was adjusted to 5.5 and mixed with solution of mannopyranosyl phenyl isothiocyanate in 1 ml of DMSO. After stirring for 24 h at room temperature, the MCH was precipitated by adding 10 volumes of isopropanol and centrifuged at 15,000 RCF for 20 min. After repeating this process for four times, the pellets were dried in a vacuum oven.
Characterization of polymers
Fourier Transform Infrared spectroscopy (FTIR), CHNS elemental analysis, thermal gravimetric analysis (TGA), and differential thermal analysis (DTA) were used for the chemical analysis of conjugated CS.
FTIR measurements were recorded on a Jasco FTIR-410 instrument using KBr pellets at room temperature and scanned against a blank KBr pellet background. The elemental analyses for carbon, hydrogen, nitrogen, and sulfur were performed using an ECS 4010 CHNSO Analyzer and the percentages of nitrogen, sulfur, carbon, and hydrogen were estimatedusing BÄHR-Thermoanalyse GmbH – Simultaneous Thermal Analyzer STA 503 for TGA and DTA analyses. Samples containing CS and MCH were heated at a scanning rate of 10 °C/min in N2 atmosphere over a temperature range of 0–1000 °C.
Preparation of MCH NPs containing rHBsAg
The formulation of NPs were done by ionic gelation technique using TPP as a cross linking agent [Citation21]. Briefly, 19 mg of MCH was dissolved in 10 ml deionized water, and then pH of solution was adjusted to 3.5 with acetic acid. Later, 4 ml of TPP (0.93 mg/ml) was slowly added dropwise to the MCH solution under stirring (60 min and 1300 rpm) at room temperature. MCH NPs were produced by the interaction between the negative groups of TPP and the positively charged amino groups of CS. Antigen loaded NPs were prepared through addition of rHBsAg (100 µg/ml) to TPP solution before incorporation of MCH solution. The NPs were separated by centrifugation at 18,000 RCF at 10 °C for 30 min and supernatants were analyzed for amount of free rHBsAg.
Physico-chemical characterization of NPs
Size, zeta potential, and morphology of the NPs were performed by the methods reported previously [Citation22,Citation23]. For scanning electron microscope (SEM) imaging of NPs, one drop of NPs was placed on an aluminum substrate. After air-drying at room temperature, the samples were coated with a gold film using a sputter coater and NPs were observed using a SEM (Hitachi H-600, Japan). The mean diameter and zeta potential of the NPs were determined by dynamic light scattering (DLS) using a Zetasizer Nano ZS (Malvern, UK).
Antigen encapsulation efficiency (EE) and loading capacity (LC)
The EE and LC of the antigen were calculated by difference between the total amount of rHBsAg added to the loading solution and the amount of non-entrapped rHBsAg remaining in the supernatant. Free amount of antigens in the supernatants were measured by the Bradford assay. The suspension of NPs was centrifuged for 30 min at 18,000 RCF and 5 °C and the supernatants were then separated from the NPs. The amount of non-entrapped protein remaining in the supernatant was measured by the Bradford assay at 595 nm. A supernatant of non-loaded NPs suspension was used as a blank. The EE and LC of rHBsAg loaded NPs were calculated from the following EquationEquations (1,2) [Citation24]:
(1)
(1)
(2)
(2)
Antigen release kinetics
Release of rHBsAg from the nanoparticle was measured in PBS (pH 7.4) containing 0.02% of thimerosal. Different vials containing 1 mg of loaded NPs and 1 ml of PBS were shaker incubated at 37 °C under constant shaking (100 rpm) for 49 days. At different time intervals, one sample was taken and centrifuged (20,000 RCF, 20 min). The antigen concentration in the supernatant was estimated by Bradford protein assay. The obtained data was then fitted into different release kinetics models, such as zero order, first order, and Higuchi release model [Citation25,Citation26].
Cell viability analysis
Human embryonic kidney cells 293 (HEK293) cells were used for cytotoxicity studies. Cells were cultured for 48 h in DMEM) at 37 °C with 10% FBS (humidified atmosphere and 5% CO2/air). The cytotoxicity of antigen-loaded CS NPs was evaluated using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. When the cells were confluent, they were seeded into 96-well culture plates with cell density of 2 × 104 cells/well and the plates were maintained under the conditions previously mentioned for 24 h. The old medium was carefully aspirated and the cells were incubated in 100 μl of antigen containing NPs at different concentrations for another 24 and 48 h. Antigen-loaded NPs were used in 12.5, 25, 50, 100, 200, 400, 800, and 1600 μg/ml concentrations. Then, 20 μl of MTT dye solution (5 mg/ml) was added to each well and the plate was then incubated for 4 h at 37 °C allowing viable cells to reduce the MTT into purple colored formazan crystal. Subsequently, the culture medium containing MTT solution was removed and the formazan crystals were dissolved in 150 μl of DMSO. Then, the optical density (OD) was measured at 570 nm. The cell viability was expressed as percentage compared to a control that had not been treated with NPs suspension, using the EquationEquation (3)(3)
(3) :
(3)
(3)
Abnormal toxicity test (ATT)
ATT (also known as the general safety test) has been used to detect non-specific toxicity and approved by the WHO as a safety test for vaccines quality [Citation27]. This test was done according to British pharmacopoeia. In this study 0.5 ml of samples with different concentration of CS and MCH loaded NPs (200, 800, and 1600 µg/ml) was injected intraperitoneally into five healthy mices (weighing 17 to 24 g) and two healthy guinea-pigs (weighing 250 to 400 g). Later, the animals were screened for seven days for any toxicity signs and symptoms. The preparation passed the test if none of the animals shows signs of ill health.
In vivo immunogenicity study
The in vivo potency assay was carried out according to WHO Technical Series, 1989 [Citation27]. In brief, 10 mices (female BALB/c), 5 weeks of age, or in the weight range of 17–22 g, are immunized intraperitoneally with a series of at least three dilutions (1/8, 1/32, and 1/128) of commercial hepatitis b vaccine as a reference, MCH, and CS NPs containing rHBsAg (20 µg/ml), using a normal saline as diluent. Forty two days after the inoculation, the mice were bled by the retroorbital route and blood samples from each animal were collected separately. The samples were centrifuged to extract the sera, which were evaluated to determine the presence of antibodies against HBsAg through ELISA.
Stability of rHBsAg loaded MCH NPs
The stability of the loaded MCH NPs was analyzed by using accelerated condition (T: 25 °C ± 2 °C/60% ± 5% RH) for six months. The samples were taken in specified intervals to analyze for zeta potential and morphology of the loaded NPs and also antigenicity of rHBsAg released from MCH NPs.
Statistical analysis
Potency of the formulations was analyzed by CombiStats version 4. All statistical analyses were carried out using SigmaPlot 12 software. The statistical significance of the differences between IgG levels was tested by the analysis of variance (ANOVA) followed by post hoc Tukey–Kramer test. Differences were considered to be significant at a level of p < .05. All data were expressed as mean ± standard deviation (n = 3).
Results and discussion
FT-IR spectroscopy
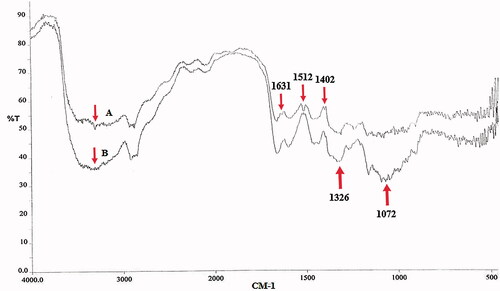
MCH was synthesized by the reaction between amine groups of CS and isothiocyanate groups of α-D-mannopyranosylphenyl isothiocyanate, as shown in . The results of FTIR spectra of CS and MCH are presented in . In the CS and MCH spectra, the strong and wide peak in the 3500–3300 cm−1 area is attributed to stretching vibration of hydrogen-bonded hydroxyl groups. The peaks attributed to the N-H stretching vibration of primary amine and type ІІ amide are overlapped in the same region. The peak for asymmetric stretching vibration of C-O-C was found at around 1072 cm−1 and the peak at 1326 cm−1 belongs to the C-N stretching vibration of type І amine. The MCH showed the characteristic band of COO asymmetric stretching vibration at 1631 cm−1 and COO symmetric stretching at 1402 cm−1. The peak at 1512 cm−1 could be attributed to the S = C-NH in MCH which confirmed the attachment of α-D-mannopyranosylphenyl isothiocyanate with amine groups of CS [Citation20,Citation28,Citation29].
Thermal analysis
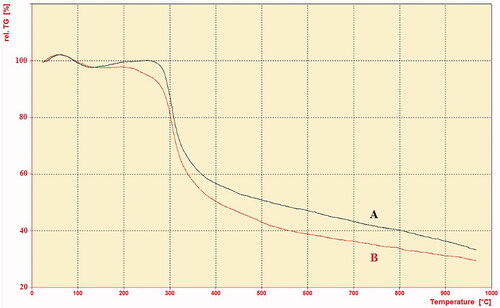
The TGA/DTA curve for the CS and MCH are presented in and , respectively. The TGA curves of CS and MCH showed a weight loss in two stages and two decomposition phases. The first stage of weight loss for CS and MCH at the temperature of less than 150 °C was related to the evaporation of water. The second weight loss of CS started at temperature of about 215 °C and continued to 900 °C which ascribe to the degradation of main chain of chitosan (about 60% weight loss). But, the weight loss of 63% was observed for MCH at the same condition. This difference may be attributed to the amount of mannose residue attached to the CS. According to the TGA result, the substitution value of α-D-mannopyranosylphenyl isothiocyanate coupled with CS was about 3%.
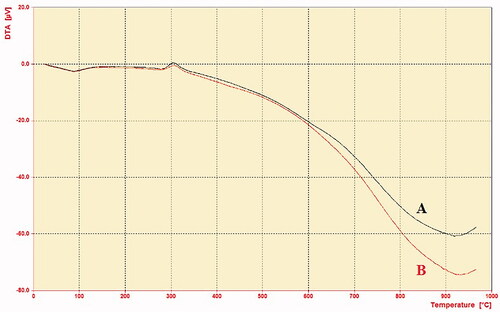
The DTA study showed that the decomposition of CS and MCH are an exothermic event that occurs in the temperature range of 280–340 °C. It can be assigned to the thermal decomposition of amino and N-acetylglucosamine residues [Citation30]. The result showed that CS was slightly more exothermic than MCH. These results indicated that, in some extent, the thermal stability of MCH was slightly decreased compared with CS, due to the introduction of the mannose residue and change in the crystallinity of CS [Citation31].
Elemental analysis
CHNS elemental analysis results of CS and MCH are shown in . Elemental analysis of CS show carbon, hydrogen, and nitrogen peaks. On the other hand, elemental analysis of MCH show carbon, hydrogen, nitrogen, and sulfur peaks. These results confirm the successfully conjugation of α-D-mannopyranosylphenyl isothiocyanate to CS. The degree of substitution was determined to be about 2%. which is the rough elemental analysis according to the percentages of sulfur in MCH.
Physico-chemical properties of CS and MCH-based NPs containing rHBsAg
The NPs size and zeta potential results were reported in . Zeta potential of rHBsAg loaded MCH NPs was lower than loaded CS NPs, possibly due to conjugation of mannose with amine groups of CS and also covering and interaction of antigen with CS reducing the positive charge of the polymer [Citation20]. The results indicated that the size of the rHBsAg loaded MCH increases, likely due to the loading of antigen within the NPs. Mannosylated NPs have also exhibited a larger particle size than CS, probably due to the reduced surface charge, aggregation, and particle growth. The SEM images are shown in . For both formulation, the NPs were nearly oval in shape and have a smooth surface. The NPs size, zeta potential, and enhanced antigen encapsulation efficiency might play a major role in induction of the immune response [Citation20,Citation32–34]. Some studies have shown that particles prepared in this study can be easily up taken and can stimulate the immune system [Citation20,Citation35,Citation36]. According to the , the EE of MCH nanoparticle has decreased compared to CS nanoparticle, which could be due to the involvement of amine groups of CS with mannose and a weaker reaction with the antigen. The LC of MCH nanoparticle has notably decreased compared to CS nanoparticle, due to the poor reaction of the antigen with the polymer, as mentioned above in MCH nanoparticle and the reduction of surface charge, which leads to the precipitation of NPs and increasing particle size as well as decreased surface-to-volume ratio [Citation37].
Figure 6. SEM images of free CS NPs (A), Loaded CS NPs (B), unloaded MCH NPs (C), and Loaded MCH NPs (D).
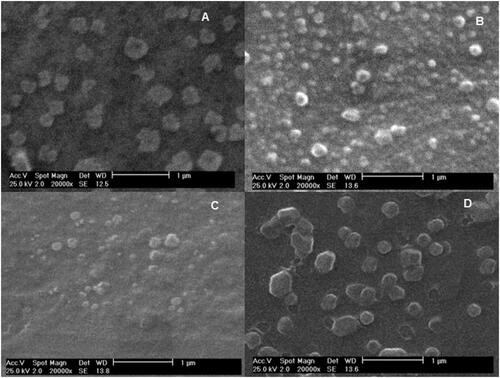
Table 1. The characterization of CS and MCH NPs.
Release profile study
Release kinetic analysis of rHBsAg from CS and MCH NPs was carried out in various models (). The release of antigen from the NPs showed an initial burst release within initial days of incubation followed by constant release for up to 49 days. The initial burst release possibly occurred due to release of antigen from the surface of nanoparticle. The second constant release could be due to the release of antigen from the core of NPs as a consequence of CS hydration and swelling [Citation38,Citation39]. The data of release showed that MCH NPs released antigen faster than CS NPs. This is may be due to the occupation of amine groups of CS with mannose and the weakness of the network structure as well as the greater solubility of MCH in water media [Citation20].
Table 2. Release parameters of rHBsAg sustained release from NPs.
The kinetics of the antigen release from the NPs was also investigated by some mathematical models such as Korsmeyer-Peppas, Hixson-Crowell, Higuchi, first order, and zero order. The correlation value (R2) of Korsmeyer-Peppas and Higuchi’s model were better and higher than others for both formulations. In model Korsmeyer-Peppas the value of n characterizes the release mechanism of antigen. For CS and MCH NPS n ≤ 0.5 corresponds to a Fickian diffusion mechanism, 0.5 < n < 1.0 to non-Fickian transport and n = 1 for zero order release. When the release data were analyzed using the Korsmeyer-Peppas equation, the “n” values indicated that the mechanism of antigen release from the NPs was Fickian diffusion or diffusion controlled for both formulations [Citation25,Citation38].
In vitro cytotoxicity (MTT assay)
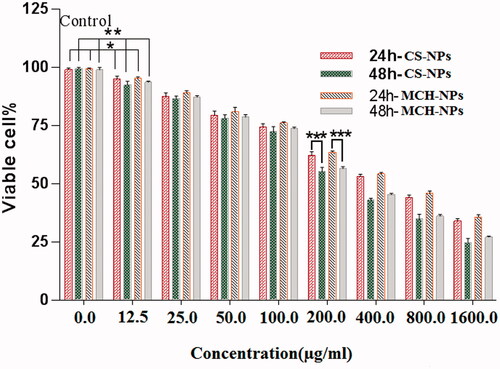
In this study the toxicity of CS and MCH loaded NPs were analyzed on HEK293 cells. Toxicity of all NPs concentration for both formulations showed a dose dependent effect on cell viability, but only the concentrations above 200 µg/ml showed the time-dependent effect. These cytotoxic effects may be occurred due to interaction of highly positive-charged NPs with negatively charged cell membranes [Citation40,Citation41]. The results in clearly indicate higher toxic effects of the CS NPs on HEK293 cells than the MCH NPs. This is probably due to the lower surface charge and larger particle size of the loaded MCH NPs as well as steric hindrance and the charge shielding effects offered by mannose moiety [Citation20].
In vivo toxicity test (ATT)
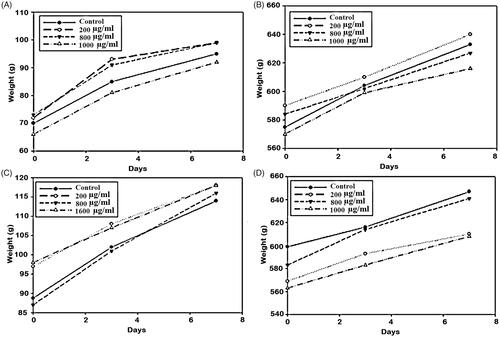
The in vivo toxicity results were obtained by monitoring illness, death, and weight loss of subsequent injection of the samples in mice and guinea-pigs (). The results showed body weight increase after the administration of three different doses of CS and MCH loaded NPs during the seven day period. In the other hand, no symptom of illness and death was observed during this period.
Immunogenicity study
In order to evaluate the potential of rHBsAg loaded CS and MCH NPs to induce the generation of protective IgG titers, immunogenicity studies were performed by intraperitoneally administration of nanoparticle based vaccines and commercial hepatitis B vaccine as a comparator.
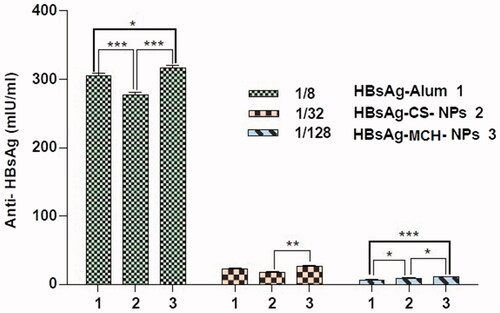
The parallel line analysis curves of the IgG titers created by the rHBsAg entrapped CS and MCH NPs relative to the standard vaccine in mice have been depicted in . The order of immunogenicity for the examined samples is found to be rHBsAg entrapped MCH-NPs > alum-adsorbed rHBsAg > rHBsAg entrapped CS-NPs.
Figure 9. CombiStats analysis of commercial vaccine (standard), CS nanoparticle (sample 1), and MCH NPs (sample 2) IgG titer results after intraperitoneally injection in mice.
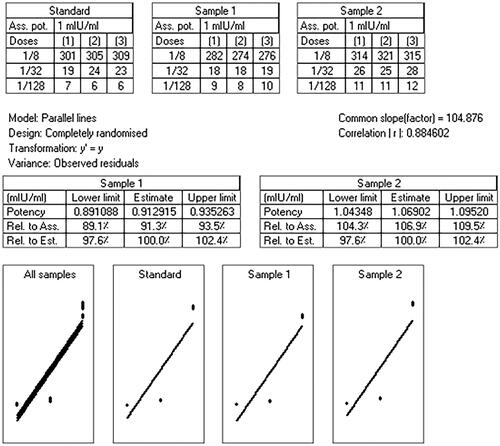
The results of IgG titers induced by different doses of samples are shown in . The statistical analysis of outcomes clearly indicates significant improvement of immune response in mice immunized with rHBsAg MCH NPs. It was suggested that loaded MCH NPs are bound with mannose receptors on immune cells and induce higher IgG immune responses than other formulations upon [Citation42]. In the dose of 1/128 CS-NPs vaccine exhibited higher IgG titers than the commercial vaccine, probably due to the better protection of antigen in chitosan NPs against degeneration and its slow delivery to the immune cells.
Stability test
In this work the stability of MCH NPs was followed up to for six months. The antigenicity of the released rHBsAg indicated small reduction after this period of time. Morphology, zeta potential, and size of loaded MCH-NPs was evaluated at two, four and six months during the stability test (). It is noticed that the shape of the NPs did not change after two and four months as compared with zero time, but significant changes were observed after six months (). The size of the particles increased after two and four months and aggregated particles time were observed in the third sampling. Also the zeta potential of the NPs showed a reduction after six months from ( ∼ 13 +µV) at a zero time to (∼ 25 +µV). Zeta potential of the MCH loaded NPs can greatly influence their stability in media through electrostatic repulsion between the particles. The stability of antigen during encapsulation and storage of antigen loaded NPs is a very important technical concept. Therefore, in this study at zero time and six months during stability study, samples were taken from released antigen in in vitro condition and evaluated for antigenicity. No significant changes was obtained for antigenicity of the antigen after entrapment process and only approximately 16% reduction of activity was observed after six month storage under accelerated stability condition. Reduction in antigenicity of the released antigen and zeta potential may be due to change in antigen 3D structure, growth of NPs (reducing the surface area to volume ratio) which result to aggregation, and decreasing zeta potential and also morphological changes of NPs [Citation26].
Figure 11. SEM images of rHBsAg loaded MCH NPs incubation (A) two months, (B) four months, and (C) six months in stability condition.

Table 3. Characterization of MCH NPs during stability test.
Conclusion
In the present work, mannosylated CS was successfully prepared and used as a suitable targeting vaccine delivery carrier. This study represents a comparison of some parameters of CS and MCH NPs, to be used as immunity enhancing adjuvants. Both formulation exhibited sustain release manner and no in vivo toxicity effect. Loaded MCH-NPs showed much higher induction of immune system than other formulation. Our results strongly support the efficacy of MCH NPs as an adjuvant-carrier system for the effective induction of a specific immune response and targeted vaccine delivery system.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Jafari M, Moghaddam Pour M, Taghizadeh M, et al. Comparative assessment of humoral immune responses of aluminum hydroxide and oil-emulsion adjuvants in Influenza (H9N2) and Newcastle inactive vaccines to chickens. Artif Cells Nanomed Biotechnol. 2017;45:84–89.
- Chen Z, Zhang S, Li Z, et al. Construction of a stable w/o nano-emulsion as a potential adjuvant for foot and mouth disease virus vaccine. Artif Cells Nanomed Biotechnol. 2017;45:897–906.
- Amini Y, Moradi B, Fasihi-Ramandi M. Aluminum hydroxide nanoparticles show strong activity to stimulate Th-1 immune response against tuberculosis. Artif Cells Nanomed Biotechnol. 2017;45:1331–1335.
- Mangal S, Pawar D, Agrawal U, et al. Evaluation of mucoadhesive carrier adjuvant: toward an oral anthrax vaccine. Artif Cells Nanomed Biotechnol. 2014;42:47–57.
- Reed SG, Bertholet S, Coler RN, et al. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32.
- Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488.
- Lugade AA, Bharali DJ, Pradhan V, et al. Single low-dose un-adjuvanted HBsAg nanoparticle vaccine elicits robust, durable immunity. Nanomedicine. 2013;9:923–934.
- Dewangan HK, Pandey T, Singh S. Nanovaccine for immunotherapy and reduced hepatitis-B virus in humanized model. Artif Cells Nanomed Biotechnol. 2017 [1–10]. doi: https://doi.org/10.1080/21691401.2017.1408118
- Fakharzadeh S, Kalanaky S, Hafizi M, et al. The new nano-complex, Hep-c, improves the immunogenicity of the hepatitis B vaccine. Vaccine. 2013;31:2591–2597.
- Nejati-Koshki K, Mortazavi Y, Pilehvar-Soltanahmadi Y, et al. An update on application of nanotechnology and stem cells in spinal cord injury regeneration. Biomed Pharmacother. 2017;90:85–92.
- Mohammadian F, Abhari A, Dariushnejad H, et al. Effects of chrysin-PLGA-PEG nanoparticles on proliferation and gene expression of miRNAs in gastric cancer cell line. Iran J Cancer Prev. 2016;9:1–8.
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif Cells Nanomed Biotechnol. 2017;45:1201–1206.
- Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif Cells Nanomed Biotechnol. 2017 [1–11]. doi: https://doi.org/10.1080/21691401.2017.1337022
- Farajzadeh R, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. Nano-encapsulated metformin-curcumin in PLGA/PEG inhibits synergistically growth and hTERT gene expression in human breast cancer cells. Artif Cells Nanomed Biotechnol. 2017 [1–9]. doi: https://doi.org/10.1080/21691401.2017.1347879
- Vasou A, Sultanoglu N, Goodbourn S, et al. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: from synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses. 2017;9:186.
- Khanmohammadi M, Elmizadeh H, Ghasemi K. Investigation of size and morphology of chitosan nanoparticles used in drug delivery system employing chemometric technique. Iran J Pharm Res. 2015;14:665.
- Hembram KC, Prabha S, Chandra R, et al. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif Cells Nanomedicine Biotechnol. 2016;44:305–314.
- Al-So'od K. Interaction between low molecular weight chitosan and some types of drugs and insulin. Asian J Chem. 2012;24:3785–3790.
- Başak E, Aydemir T. Immobilization of catalase on chitosan and amino acid-modified chitosan beads. Artif Cells Nanomedicine Biotechnol. 2013;41:269–275.
- Shilakari Asthana G, Asthana A, Kohli DV, et al. Mannosylated chitosan nanoparticles for delivery of antisense oligonucleotides for macrophage targeting. BioMed Res Int. 2014;2014:1–17.
- Gomathi T, Sudha P, Florence JAK, et al. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int J Biol Macromol. 2017;104:1820–1832.
- Amirsaadat S, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Silibinin-loaded magnetic nanoparticles inhibit hTERT gene expression and proliferation of lung cancer cells. Artif Cells Nanomedicine Biotechnol. 2017;45:1649–1656.
- Farajzadeh R, Zarghami N, Serati-Nouri H, et al. Macrophage repolarization using CD44-targeting hyaluronic acid–polylactide nanoparticles containing curcumin. Artif Cells Nanomed Biotechnol. 2017 [1–9]. doi: https://doi.org/10.1080/21691401.2017.1408116
- Esfandiarpour-Boroujeni S, Bagheri-Khoulenjani S, Mirzadeh H, et al. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr Polym. 2017;168:14–21.
- Raja Azalea D, Mohambed M, Joji S, et al. Design and evaluation of chitosan nanoparticles as novel drug carriers for the delivery of donepezil. Iran J Pharm Res. 2012;8:155–164.
- Rezayat M. Preparation, characterization and stability investigation of chitosan nanoparticles loaded with the Echis carinatus snake venom as a novel delivery system. Arch Razi Institute 2015;70:269–277.
- Mizukami T, Masumi A, Momose H, et al. An improved abnormal toxicity test by using reference vaccine-specific body weight curves and histopathological data for monitoring vaccine quality and safety in Japan. Biologicals. 2009;37:8–17.
- Bhumkar DR, Pokharkar VB. Studies on effect of pH on cross-linking of chitosan with sodium tripolyphosphate: a technical note. AAPS PharmSciTech. 2006;7:E138–EE43.
- Li P, Dai YN, Zhang JP, et al. Chitosan-alginate nanoparticles as a novel drug delivery system for nifedipine. Int J Biomed Sci. 2008;4:221.
- Arora S, Lal S, Kumar S, et al. Comparative degradation kinetic studies of three biopolymers: chitin, chitosan and cellulose. Arch Appl Sci Res. 2011;3:188–201.
- Yao W, Jiao Y, Luo J, et al. Practical synthesis and characterization of mannose-modified chitosan. Int J Biol Macromol. 2012;50:821–825.
- Ahmed TA, Aljaeid BM. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des Devel Ther. 2016;10:483.
- Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target. 2012;20:291–300.
- Shukla S, Myers JT, Woods SE, et al. Plant viral nanoparticles-based HER2 vaccine: immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials. 2017;121:15–27.
- Ho HN, Tran TH, Tran TB, et al. Optimization and characterization of artesunate-loaded chitosan-decorated poly (D, L-lactide-co-glycolide) acid nanoparticles. J Nanomater. 2015;16:383.
- Kim TH, Jin H, Kim HW, et al. Mannosylated chitosan nanoparticle–based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther. 2006;5:1723–1732.
- Bhattarai N, Ramay HR, Chou SH, et al. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int J Nanomedicine. 2006;1:181.
- Aydin R, Pulat M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: evaluation of controlled release kinetics. J Nanomater. 2012;2012:42.
- Xue M, Hu S, Lu Y, et al. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int J Pharm. 2015;495:771–782.
- Mobasseri R, Karimi M, Tian L, et al. Hydrophobic lapatinib encapsulated dextran-chitosan nanoparticles using a toxic solvent free method: fabrication, release property & in vitro anti-cancer activity. Mater Sci Eng C. 2017;74:413–421.
- Parida UK, Rout N, Bindhani BK. In vitro properties of chitosan nanoparticles induce apoptosis in human lymphoma SUDHL-4 cell line. Adv Biosci Biotechnol. 2013;4:1118.
- Peng Y, Yao W, Wang B, et al. Mannosylated chitosan nanoparticles based macrophage-targeting gene delivery system enhanced cellular uptake and improved transfection efficiency. J Nanosci Nanotechnol. 2015;15:2619–2627.