Abstract
Many of the therapeutics used for the treatment of brain disorders are not effective and not delivered to the brain due to the complex structure and its barriers. In recent years, many advanced approaches have emerged for the brain drug delivery. Intranasal drug delivery is one of non-invasive approach has gained interest because of direct transport of drugs circumventing the brain barriers through olfactory and trigeminal nerve pathways. Eventhough through these pathways the therapeutics have direct access to the brain, the main limitations of this approach are only limited drug absorption, and nasal permeability. To overcome the issues related to the brain targeting via nasal drug delivery encourage the development of novel drug delivery by combining with nanotechnology. This article will discuss pathways of drug transport form nose to brain, toxicity of nanoparticles role and need of nanostructured lipid carriers (NLCs) and recent advance in combination of NLCs with intranasal drug delivery for targeting the brain.
Introduction
Delivery of drug to the brain is the most challenging task due to its anatomy and physiological barriers such as blood–brain barrier (BBB). BBB acts as a major obstacle for the entry of active molecules into the central nervous system (CNS). More than 98% of CNS active drugs not able to cross the barrier because of its physiochemical properties do not match the criteria for the entry of molecules into the CNS. Lipophilic molecules, the drug that has a partition coefficient (Log P) between 1.5–2.7 and with the molecular weight less than 600 Dalton (Da) may be permeable to the BBB [Citation1,Citation2]. The BBB that separates the brain interstitial fluid from the circulating blood acts an efficient barrier for the diffusion of most drugs from the blood to receptors in the central nervous system (CNS). Furthermore, BBB contain more number of transporters, mainly P-glycoprotein (P-gp) efflux transporter limits the entry of drug to the CNS [Citation3]. Due to these limitations, researchers facing many difficulties for achieving therapeutic efficacy to treat many of the CNS diseases, such as Parkinson's and Alzheimer's diseases. Many invasive and non-invasive strategies have been utilized to target CNS. But in recent years, interest has been expressed in the use of the nasal route (non-invasive) to deliver drugs to the brain, exploiting the olfactory pathway/trigeminal pathway.
Many researchers have been tried nose to brain drug delivery to overcome the BBB [Citation1,Citation4–7]. The merits of nose to brain drug delivery reported that safety, avoidance of hepatic first pass metabolism, non-invasive, convenience of administration and patient compliance [Citation8]. Olfactory region due to the presence of olfactory receptors, neurons and its axons end in the olfactory bulb which has a direct contact with the CNS. Olfactory region is the only part in the whole body where CNS is in contact with the peripheral environment. The olfactory region is the prominent site from where the active moieties can be absorbed directly into the brain by olfactory and trigeminal nerve pathways [Citation1]. When the drug or formulation was administered it comes in contact with mucosa and it is directly transported into the brain bypassing the BBB, thereby achieving excellent bioavailability, reduction of dose and also reducing the side effects. Though nasal drug delivery achieves interest in the research, but there are some limitations of nasal drug delivery includes limited dose of administration (25–200 μl), mucociliary clearance and nasal enzymatic barriers [Citation9]. These attenuate the researchers to develop an advanced drug delivery system to overcome these limitations. Recently, nanotechnology-based approach has gained interest to overcome the limitations of nasal drug delivery [Citation10]. Though, applications of nanoparticles in various fields like pharmaceutics, medicine and cosmetics. However, due to its special properties nanoparticles, it can bypass the BBB, but the toxicity of nanoparticles in CNS is still lacking. After entering into brain, the nanoparticles may cause neurotoxicity. However, nanoparticles through nasal route may reach the brain but after entering into the brain some nanoparticles may not be cleared by systemic circulation it can accumulate in brain cause toxicity [Citation11,Citation12]. Here, we summarized the transport pathways of nanoparticles, toxicity and recent development in nanotechnology in brain targeting.
Anatomy of nose
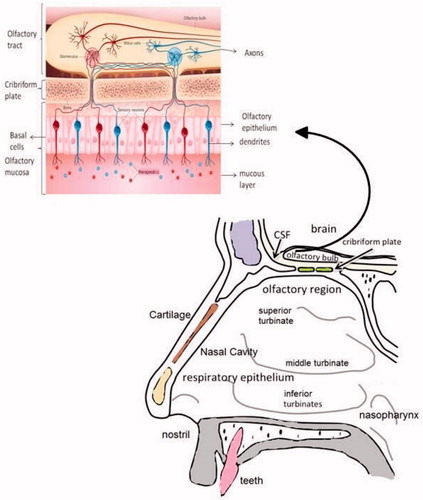
The nose is a complex structure. The nasal cavity is divided into three regions namely vestibule, respiratory and olfactory region. Vestibule region is not involved in the absorption functions and it is the anterior external region opening to the nasal cavity. The respiratory epithelium consists of ciliated and non-ciliated columnar cells, mucus secreting goblet cells and basal cells. The respiratory region is mainly involved in the drug absorption and the surface area of respiratory region is approximately 160 cm2 in humans. The third region is olfactory region consists of olfactory receptor cells, basal and sustentacular cells. The olfactory region has a surface area of 10 cm2. Olfactory receptor neurons are bipolar neurons involves in the transduction of information from epithelium to olfactory bulb [Citation13]. The olfactory pathway is explained in the following section.
Transport of active moieties/nanocarriers from nose to brain
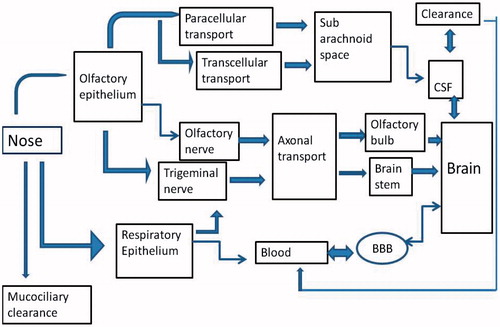
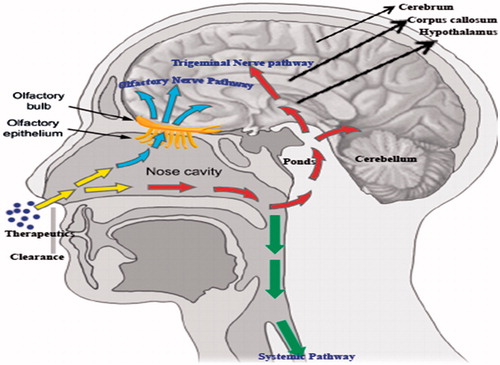
Based on the recent research, nose to brain pathway is the potential route for the transport of therapeutic modalities/nanocarriers directly into the brain by non-invasive route bypassing the BBB. The transport mechanism involves three different ways such as olfactory pathway, Trigeminal nerve pathway, and systemic pathway an overview represented in the . Transport pathways represented in the .
Olfactory pathway
Therapeutic modalities once administered via nose, it travels to the olfactory mucosa (also known as olfactory epithelium) represented in the . Olfactory mucosa contains olfactory receptor neurons that are responsible for the transduction. Transduction happens in olfactory receptors on the cilia which is the end of the olfactory receptor neurons. Molecules reach the olfactory receptor neurons by paracellullar or transcellular mechanism. The integrity of nasal epithelium, along with the tight junctions, desmosomes, adherent junctions and space between the epithelial cells allows the entry of compounds by paracellular transport [Citation14]. The neuronal pathway considered to be determining step of the nose to brain route. Drug moieties travels along axon and via nerve bundle cross the cribriform plate and reach the olfactory bulb which is actually appear on the surface of the brain. From the olfactory nerves, the therapeutic moiety can enter the cerebrospinal fluid (CSF) and olfactory bulb [Citation15]. The drug can be distributed from the CSF to brain by mixing with interstitial fluid in the brain. After a nasal administration of drug it takes only few minutes to reach brain via olfactory transport. Intra-neuronal pathway and extra-neuronal pathway are the two different pathways of the olfactory neuronal pathway into the brain. Intra-neuronal pathway involves axonal transport and it requires hours to days for active moiety to reach different regions of the brain. Incase of extra-neuronal pathway which involves transport through perineural channels; it takes only few minutes to reach active moiety directly to brain [Citation16–18]. The olfactory neuronal pathway innervate to the deeper areas of brain such as cortex, cerebrum and cerebellum.
Trigeminal pathway
Trigeminal nerve pathway connecting to the tail part of the brain such as spinal cord, the medulla and the pons. Drug transported through nose via trigeminal nerve pathway by intracellular transport (axonal transport) or by endocytosis. The trigeminal nerve is the largest and fifth cranial nerve and it composed of three branches such as ophthalmic, maxillary and mandibular. Out of these three mainly ophthalmic and maxillary branches plays an important role in nose to brain drug delivery, the neurons from these branches pass directly through the nasal mucosa. Some segment of trigeminal nerve also ends in the olfactory bulbs [Citation19]. Branches from the ophthalmic part of the trigeminal nerve innervate to the dorsal part of the nasal mucosa and the anterior nose but considering maxillary branch innervate to the turbinates of the nasal mucosa. Once the compounds diffuse through the mucosa of the nasal cavity, it reaches the branches of trigeminal nerves in olfactory and respiratory regions, and via brain stem transported to the axonal route. A part of the trigeminal nerve that passes through the cribriform plate that may also involved in the delivery of therapeutics from nasal cavity to the forebrain [Citation20]. Thorne et al. [Citation21] reported after an intranasal administration of insulin-like growth factor-I (IGF-I) rapidly reached brain via trigeminal neuronal pathway. Intranasally administered drug/nanoparticles absorbed from nasal cavity is passage through the mucus, this is the first step involved in absorption. After passing through the mucus, there are several mechanisms involved in the transportation through mucosa. There are paracellullar, transcellular, carrier-mediated transport, receptor-mediated transport and transcytosis [Citation22].
Paracellular route is the transport of molecules between the cells. Transcellular route refers to the transport of drug across the cells this may occur by carrier-mediated transport or by endocytosis. In transcellular route, adsorptive transcytosis mechanism involves transport of macromolecules. This process involves interaction between the ligand in blood stream and cell surface. This type of interaction may be due to electrostatic interaction between the positively charged ligand such as protein or macromolecules and negatively charged membrane. Nanoparticles and some compounds undergo transcytosis for the permeation [Citation23].
Kimura et al. [Citation24] suggested that mechanism of carrier-mediated absorption takes place by the organic cation transporters, P-glycoprotein, aminoacid transporters, dopamine transporter acts as a carrier of molecules in nasal mucosa.
Systemic pathway
Drug uptake into the brain from nasal cavity occurs also through blood circulation. Due to the rich vasculature of the respiratory epithelium than olfactory mucosa fraction of the drug was absorbed into the systemic circulation [Citation25]. The respiratory segment comprises of combination of the continuous and fenestrated endothelium which allows the passage of both small and large molecules into the blood circulation subsequently transport across the BBB to the CNS. Small lipophilic molecules easily enter into the blood and cross the BBB compared to the high molecular weight and hydrophilic molecules. The active moiety was distributed throughout the systemic circulation and it enters into the nasal blood vessels and they were rapidly transferred to the carotid arterial blood supply to the brain and spinal cord, this process is called counter current exchange. Some of Marketed products available in the market was shown in .
Table 1. List of marketed nasal products for CNS targeting.
Potential of nanocarriers for nose to brain targeting
In recent years, nanotechnology becomes very interesting field in which particles of less than 100 nm which has more advantage in the pharmaceutical and biomedical field over conventional drug therapy. There are different types of nanocarriers such as polymeric, lipid and inorganic nanoparticles. Nanocarriers become exciting field and gained more interest in brain drug delivery because of its special characteristic includes overcoming the issues related to BBB. Various biological macromolecules that can be used for brain disorder crossing the BBB by the use of nanotechnology [Citation37–39] so that dose required to treat the brain disorders can be minimized, thereby toxicity and adverse effects also can be subsequently reduced. Nanocarriers because of their unique properties they become a promising carriers in brain drug delivery. There are some colloidal carriers commonly used for brain targeting such as polymeric and metallic nanoparticles and lipid-based nanocarriers.
Chen et al. [Citation40] appropriately discussed polymeric nanoparticles as suitable drug delivery system for brain targeting. But, there are various limitations associated with the polymeric nanoparticulate systems are contamination from the production process by the use of organic solvents, polymer aggregates, toxic monomers, polymerization initiation, toxic degradation production methods, production methods are expensive, large-scale production was not possible, sterilization process, consideration of toxicity and stability. Polymeric nanoparticles have limited permeation to cross the BBB without the surface modification.
Metallic nanoparticles are called hard nanomaterials also suitable carriers in drug delivery. Kao et al. [Citation41] detected that the translocation of zinc oxide (ZnO) nanoparticles into the brain following in vivo nasal administration in a Sprague Dawley rat model. Raghnaill et al. [Citation42] iron oxide (IO) nanoparticles, ZnO nanoparticles and titanium dioxide (TiO2) nanoparticles have also been found to be translocated to the brain in various animal models [Citation43–45]. Though, nanoparticles helps to cross the BBB for the treatment of CNS disorders, but the safety of nanoparticles in biological systems in more important in terms of neurotoxicity. Considering the neurotoxicity, researchers developed the lipid-based nanocarriers using physiological lipids such as solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), liposomes, nanoemulsion, nanoliposomes.
Among the lipid-based carriers, NLCs has gained more interest in research due low toxicity and stability by the use of physiological lipids such as mono-, di-, triglycerides, fatty acids, and waxes [Citation46]. This NLCs reduce the toxicity and allows controlled or sustained release of the drug [Citation47], they can encapsulate both hydrophilic and lipophilic therapeutic agents and they are highly stable when compare to the other nanocarriers. Due to their lipophilic nature, they can cross the BBB and also NLCs can accommodate more amount of the drug due to its imperfect shape. To date, a wide range of NLCs have been efficiently designed and administered through nose to transport the biological and pharmaceutical agents to the CNS. Eventhough intranasal route of administration is a non-invasive mode to overcome the BBB and also avoids the first-pass metabolism but the major limitation of this route is only limited drug can be absorbed [Citation48,Citation49]. To resolve the problem researchers made an effort to combine nanotechnology with nasal drug delivery [Citation50–52]. The nanocarriers improve the retention time and nasal permeation of the therapeutic moiety also they act as a carrier for the transport of encapsulated drug across the membrane. Recent advancement in nanostructured lipid carriers and their efficiency in nose to brain were explained by the examples discussed in the further section.
Current development of NLCs in brain targeting through nasal route
Eskandari et al. [Citation50] developed the valproic acid-NLCs for the brain delivery via intranasal route for the treatment of epilepsy. The valproic acid-NLCs were prepared by solvent diffusion followed by ultrasonication. The NLCs were characterized for the particle size, zeta potential, drug loading and entrapment efficiency. The valproic acid–loaded NLCs with the particle size of 154 ± 16 nm and drug loading percentage 47 ± 0.8% was observed. In vivo pharmacodynamics study was done via administering the valproic acid-NLCs and drug solution by intra-peritoneal or intranasal route on rats by maximal electroshock method. The study results observed that brain: plasma concentration ratio was higher in case of intranasal administration of valproic acid-NLCs compared to the intra-peritoneal administered control group rats. From the study suggests that intranasal administration provide a better protection for the seizure therapy.
Alam et al. [Citation51] evaluated the duloxetine-NLCs for the treatment of depression through intranasal route. The duloxetine-NLCs were prepared by homogenization followed ultrasonication. The in vivo nasal infusion study was performed on rat to estimate the amount of duloxetine permeated into the brain. The absorption rate of nanocarriers and drug solution was assessed for nose to brain and blood permeation. Duloxetine-NLCs were found to be more permeable through nose to brain as compared to the drug solution. The duloxetine-NLCs were 2.5 times more permeable than drug solution. The higher amount of drug was observed in brain in case of duloxetine-NLCs. The study suggests that intranasal infusion of NLCs were found to be the potential route for the delivery of drugs to the brain for the treatment of depression.
Jain et al. [Citation52] designed artemether-loaded nanostructured carriers for the treatment cerebral malaria. Artemether-NLCs were developed by microemulsion method and optimized by central-composite design. The particle size and zeta potential was found to be 123.4 nm and zeta potential −34.4 mV. In vitro cytotoxicity on the SVGp12 cell line and nasal ciliotoxicity studies showed that the formulations were safe and non-toxic for intranasal administration. The pharmacokinetic and brain uptake studies demonstrated that higher concentration of drug was found in brain in case of intranasal administration of artemether-loaded NLCs. Brain-to-blood ratio for different routes were determined 2.619 (artemether-NLCs intranasal route), 1.642 for artemether–solution intranasal route and artemether-solution by intravenous route at 0.5 h. The higher drug targeting efficiency and drug transport percentage to the brain via intranasal route was observed in artemether-NLCs compared to artemether-solution.
Gartziandia et al. [Citation23] designed and optimized a chitosan coated NLCs for brain delivery of proteins by intranasal route. The optimized formulation displayed a particle size, zeta potential and entrapment efficiency of 114 nm, +28 mV and 90.28 ± 0.4%. The in vitro studies cell uptake and toxicity studies demonstrated that no signs of toxicity. In vivo biodistribution study by fluorescence imaging demonstrated the efficient brain delivery through nose to brain.
Khan et al. [Citation53] investigated the efficacy of temozolomide nanostructured lipid carriers (TMZ-NLCs) to enhance brain targeting via nasal route administration. The formulation was optimized by using a four-factor, three-level Box–Behnken design. The optimized TMZ-NLCs were evaluated for their surface morphology as well as ex vivo permeation and in vivo studies. All TMZ-NLCs formulations showed sizes in the nanometer range, with high drug loading and prolonged drug release. The optimized formulation (TMZ-NLCs) displayed an entrapment efficiency of 81.64 ± 3.71%, zeta potential of 15.21 ± 3.11 mV, and polydispersity index of less than 0.2. The enhancement ratio was found to be 2.32-fold that of the control formulation (TMZ-disp). In vivo studies in mice demonstrated that the brain/blood ratio of TMZ-NLCs were found to be significantly higher compared to that of TMZ-disp (intranasal, intravenous). Scintigraphy images of mouse brain displayed the presence of a high concentration of TMZ-NLCs. The findings of this study substantiate the existence of a direct nose-to-brain delivery route for NLCs.
Madane and Mahajan [Citation54] developed a curcumin-loaded nanostructured lipid carriers (NLCs) for the treatment of brain tumour. To improve blood–brain barrier (BBB) permeability and to reach the brain, NLCs system as a carrier for curcumin was developed. The curcumin-loaded NLCs were prepared by hot high-pressure homogenization. Curcumin-loaded NLCs were subjected to the evaluation studies such as particle size, zeta potential, poly-dispersibility index, entrapment efficiency, cytotoxicity and in vivo biodistribution studies. The study results showed that particle size of 146.8 nm, zeta potential of −21.4 mV, poly dispersibility 0.18 and entrapment efficiency 90.86%. Increased cytotoxicity of curcumin-NLCs were observed in astrocytoma-glioblastoma cancer cell line (U373MG) as compared to the curcumin. In vivo biodistribution studies revealed higher concentration of drug in brain after intranasal administration of curcumin-NLCSs compared to drug solution. The study suggests that NLCs offers a promising drug delivery system for the treatment of brain cancer.
Singh et al. [Citation55] developed asenapine-loaded NLCs for the treatment of schizophrenia via intranasal route. The asenapine-NLCs particle size, zeta potential and entrapment efficiency was found to be 167.30 ± 7.52 nm, −4.33 ± 1.27 mV and 83.50 ± 2.48%, respectively. The surface morphology showed smooth surface and spherical shape. The biodistribution study revealed that higher peak drug concentration of asenapine-loaded NLCs Cmax of 74.13 ± 6.73 ng/mL, AUC0–24 h of 560.93 ± 27.85 ng/mL and the mean residence time (MRT) of 7.1 ± 0.13 h as compared to the asenapine via intranasal route. The behavioural study results demonstrated that the decrease in extra-pyramidal side effects with increasing anti-psychotic effect was observed after the treatment of 1–2 weeks. The findings suggest that NLCs could be a better drug delivery system for the delivery of asenapine to the brain via intranasal route for the treatment of schizophrenia.
Sharma et al. [Citation56] developed embedin-loaded NLCs to determine the potential of NLCs in brain targeting for the management of epilepsy. Embedin-NLCs were optimized and the optimized batch shows the average particle size and polydispersity index (PDI) were found to be 152 ± 19.7 nm and 0.143 ± 0.023. NLCs were also significantly attenuated pentylenetetrazole (PTZ)-induced biochemical parameters in comparison to pure embedin that results in an increase in the level of malondialdehyde (MDA), nitrite and reduction in the level of glutathione. From the results, it was concluded that embedin-NLCs as a beneficial carrier to achieve sustained release and brain targeting through nasal route. Recent developments of NLCs in brain targeting were summarized in .
Table 2. Recent development of NLC for brain targeting.
Conclusions
Considering the NLCs via intranasal route favours’ the treatment of several CNS disorders, it is expected that several products will enter into the market. Targeting of active moiety directly into the brain bypassing BBB with better therapeutic efficacy will lead to the successful nanobased intranasal products. The wide spread interest of nasal drug delivery to the brain using NLCs may helpful in the reducing the dose, and side effects related to the conventional therapies. NLCs improve the nasal absorption by increasing nasal permeation, retention time, and avoid enzymatic degradation thereby achieving high therapeutic efficacy.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11:1–18.
- Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. Neurorx. 2005;2:541–553.
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci. 2010;31:246–254.
- Thorne RG, Emory CR, Ala TA, et al. Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692:278–282.
- Mathison S, Nagilla R, Kompella UB. Nasal route for direct delivery of solutes to the central nervous system: fact or fiction? J Drug Target. 1998;5:415–441.
- Chow HS, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci. 1999;88:754–758.
- Chow HH, Anavy N, Villalobos A. Direct nose-brain transport of benzoylecgonine following intranasal administration in rats. J Pharm Sci. 2001;90:1729–1735.
- Colombo G, Lorenzini L, Zironi E, et al. Brain distribution of ribavirin after intranasal administration. Antiviral Res. 2011;92:408–414.
- Illum L. Nasal drug delivery-possibilities, problems and solutions. J Control Release. 2003;87:187–198.
- Ali J, Ali M, Baboota S. Potential of nanoparticulate drug delivery systems by intranasal administration. Curr Pharm Res. 2010;10:1644–1653.
- Medina C, Santos-Martinez MJ, Radomski A, et al. Nanoparticles: pharmacological and toxicological significance. Br. J. Pharmacol. 2009;150:552–558.
- Sharma HS, Sharma A. Nanoparticles aggravate heat stress induced cognitive deficits, blood–brain barrier disruption, edema formation and brain pathology. Prog Brain Res. 2007;162:245–273.
- Schipper NG, Verhoef JC, Merkus FW. The nasal mucociliary clearance: relevance to nasal drug delivery. Pharm Res. 1991;8:807–814.
- Altner H, Altner-Kolnberger I. Freeze-fracture and tracer experiments on the permeability of the Zonulae occludentes in the olfactory mucosa of vertebrates. Cell Tissue Res. 1974;154:51–59.
- Dhuria SV, Hanson LR, Frey WH. Novel vasoconstrictor formulation to enhance intranasal targeting of neuropeptide therapeutics to the central nervous system. J Pharmacol Exp Ther. 2009;328:312–320.
- Frey WH, Liu J, Thorne RG, Rahman YE. Intranasal delivery of 125I-labeled nerve growth factor to the brain via the olfactory route. In: Iqbal K, Mortimer JA, Winblad B, Wisniewski HM, editors. Research advances in Alzheimer’s disease and related disorders. New York (NY): John Wiley and Sons Ltd.; 1995. p. 329–335.
- Frey WH, Liu J, Chen X, et al. Delivery of 125I-NGF to the brain via the olfactory route. Drug Delivery.1997;4:87–92.
- Chen XQ, Fawcett JR, Rahman YE, et al. Delivery of nerve growth factor to the brain via the olfactory pathway. J Alzheimers Dis. 1998;1:35–44.
- Dhuria SV, Hanson LR, Frey WH II. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharmaceut Sci. 2010;99:1654–1673.
- Liu XF, Fawcett JR, Hanson LR, Frey WH. 2nd. The window of opportunity for treatment of focal cerebral ischemic damage with noninvasive intranasal insulin-like growth factor-I in rats. J Stroke Cerebrovasc Dis. 2004;13:16.
- Thorne RG, Pronk GJ, Padmanabhan V, et al. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496.
- Arora P, Sharma S, Garg S. Permeability issues in nasal drug delivery. Drug Deliv Tech. 2002;7:67–97.
- Gartziandia O, Herran E, Pedraz JL, et al. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf B Biointerfaces. 2015;134:304–313.
- Kimura R, Miwa M, Kato Y, et al. Nasal absorption of tetraethylammonium in rats. Arch Int Pharmacodyn Ther. 1989;302:7–17.
- Schaefer ML, Bottger B, Silver WL, et al. Trigeminal collaterals in the nasal epithelium and olfactory bulb: a potential route for direct modulation of olfactory information by trigeminal stimuli. J Comp Neurol. 2002;444:221–226.
- Migranal (dihydroergotamine mesylate) for the treatment of migraine headaches: an introduction. 2017 [cited 2017]. Available from: https://migraine.com/migraine-causes-and-triggers/
- Migranal nasal spray. 2017 [cited 2017]. Available from: http://www.migraines.org/treatment/promgrns.htm
- Stimate. 2017 [cited 2017]. Available from: https://www.drugs.com/pro/stimate.html
- Stimate®. 2010 [cited 2017]; 1--15. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020355s013lbl.pdf
- Nafarelin (Nasal route). 2016 [cited 2017]. Available from: https://www.drugs.com/cons/nafarelin-nasal.html
- Nafarelin acetate spray-Nasal Synarel. 2016. Available from: http://www.medicinenet.com/nafarelin_acetate-nasal_spray/article.htm [cited 2017]
- Butorphanol tartarate. 2016 [cited 2017]. Available from: https://medlineplus.gov/druginfo/meds/a601204.html
- Nasal Spray. 2017 [cited 2017]. Available from: https://migraine.com/migraine-treatment/nasal-spray/
- Zoming. 2017 [cited 2017]. Available from: https://migraine.com/migraine-treatment/zomig/
- DDAVP Nasal Spray. 2016 [cited 2017]. Available from: https://www.drugs.com/pro/ddavp-nasal-spray.html
- DDAVP Nasal spray. 2007 [cited 2017]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/017922s038,018938s027,019955s013lbl.pdf
- Md S, Khan RA, Mustafa G, et al. Bromocriptine loaded chitosan nanoparticles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci. 2013;48:393–405.
- Haque S, Md S, Fazil M, et al. Venlafaxine loaded chitosan NPs for brain targeting: pharmacokinetic and pharmacodynamic evaluation. Carbohydr Polym. 2012;89:72–79.
- Alam S, Khan ZI, Mustafa G, et al. Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. Int J Nanomed. 2012;7:5705–5718.
- Chen Y, Dalwadi G, Benson HAE. Drug delivery across the blood-brain barrier. Curr Drug Deliv. 2004;1:361–376.
- Kao YY, Cheng TJ, Yang DM, et al. Demonstration of an olfactory bulb-brain translocation pathway for ZnO nanoparticles in rodent cells in vitro and in vivo. J Mol Neurosci. 2012;48:464–471.
- Raghnaill MN, Bramini M, Ye D, et al. Paracrine signalling of inflammatory cytokines from an in vitro blood brain barrier model upon exposure to polymeric nanoparticles. Analyst. 2014;139:923–930.
- Wu J, Ding TT, Sun J. Neurotoxic potential of iron oxide nanoparticles in the rat brain striatum and hippocampus. Neurotoxicology. 2013;34:243–253.
- Han DD, Tian YT, Zhang T, et al. Nano-zinc oxide damages spatial cognition capability via over-enhanced long-term potentiation in hippocampus of Wistar rats. Int J Nanomedicine. 2011;6:1453–1461.
- Chen JY, Dong X, Xin YY, et al. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat Toxicol. 2011;101:493–499.
- Alam MI, Baboota S, Ahuja A, et al. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int J Pharm. 2014;470:99–106.
- Alam MI, Baboota S, Ahuja A, et al. Intranasal administration of nanostructured lipid carriers containing CNS acting drug: pharmacodynamics studies and estimation in blood and brain. J Psychiatr Res. 2012;46:1133–1138.
- Mittal D, Ali A, Md S, et al. Insights into direct nose to brain delivery: current status and future perspective. Drug Deliv. 2014;21: 75–86.
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628.
- Eskandari S, Varshosaz J, Minaiyan M, et al. Brain delivery of valproic acid via intranasal administration of nanostructured lipid carriers: in vivo pharmacodynamic studies using rat electroshock model. Int J Nanomedicine. 2011;6:363–371.
- Alam MI,S, Baboota A, Ahuja M, et al. Intranasal infusion of nanostructured lipid carriers (NLCS) containing CNS acting drug and estimation in brain and blood. Drug Deliv. 2013;20:247–251.
- Jain K, Sood S, Gowthamarajan K. Optimization of artemether-loaded NLCS for intranasal delivery using central composite design. Drug Deliv. 2015;22:940–954.
- Khan A, Imam SS, Aqil M, et al. Brain targeting of temozolomide via the intranasal route using lipid-based nanoparticles: brain pharmacokinetic and scintigraphic analyses. Mol Pharmaceutics. 2016;13:3773–3782.
- Madane RG, Mahajan HS. Curcumin-loaded nanostructured lipid carriers (NLCSs) for nasal administration: design, characterization, and in vivo study. Drug Deliv. 2016;23:1326–1334.
- Singh SK, Dadhania P, Vuddanda PR, et al. Intranasal delivery of asenapine loaded nanostructured lipid carriers: formulation, characterization, pharmacokinetic and behavioural assessment. RSC Adv. 2016;6:2032–2045.
- Sharma N, Bhandari S, Deshmukh R, et al. Development and characterization of embelin-loaded nanolipid carriers for brain targeting. Artif Cells, NanomedBiotechnol. 2017;45:409–413.