Abstract
The interest in developing new drug carriers for delivery to the liver using natural polysaccharides with a high galactose content has necessitated the study of the pharmacokinetics and tissue distribution of these polysaccharides. In this paper, a new method was established for the microanalysis of Angelica sinensis polysaccharide (ASP) in biosamples. Fluorescein-labelled ASP (FA) was rapidly eliminated from the bloodstream and distributed to the liver with high specificity following intravenous injection. The analysis of the hepatocellular localization demonstrated that FA was predominantly endocytosed by the parenchymal cells, an observation consistent with the results obtained from microscopy studies. Additionally, FA showed a high affinity for asialoglycoprotein receptor-rich cells, while minimal binding of FA to asialoglycoprotein receptor-poor cells was observed. Moreover, the absorption of FA was markedly inhibited by the co-administration of neogalactosylalbumin (NGA) both in vivo and in vitro. To allow for the visualization of the systemic circulation of ASP, 99mTc-DTPA-ASP was synthesized and in vivo imaging was performed with single photon emission computed tomography (SPECT). It also showed a high aggregation of 99mTc-DTPA-ASP in liver. These results suggest that the distribution of ASP to the liver occurs via asialoglycoprotein receptor (ASGPR) mediated endocytosis and ASP could potentially be applied as a new carrier for delivering drugs to the liver.
Introduction
Liver diseases, currently the fifth leading cause of death can impair liver function and cause organ damage. Though drugs with molecular weights greater than 300 Da can accumulate in the liver, their rapid elimination and non-specific target cell accumulation often result in poor success rates [Citation1]. Therefore, there is a strong interest in studying more effective therapeutic methods for treating liver disease [Citation2,Citation3]. Developments in the study of the pathogenesis of liver diseases have revealed that different diseases affect different types of liver cells. In light of this specificity, the delivery of drugs to specific types of liver cells, usually achieved by receptor-mediated endocytosis, can increase the efficacy of these drugs and reduce the risk of side effects in other organs.
Although there is great diversity in the receptors located on hepatocytes’ surface, such as glycyrrhetinic acid receptor, integrin receptor and others [Citation4–6], that could be used as drug targets for hepatocytes, the asialoglycoprotein receptor (ASGPR), which is present in abundance on hepatocytes and minimally expressed on extrahepatic cells, is an ideal target for hepatocyte-specific delivery. ASGPR internalizes large molecules through clathrin-mediated endocytosis and show high specific affinities for galactose, N-acetylgalactosamine and glucose. A variety of ligand-anchored nanocarriers have been designed in recent years to make use of the favourable properties of ASGPR [Citation7,Citation8]. However, due to the undesirable properties of chemosynthetic vectors bearing specific ligands, such as poor water solubility and highly toxic side effects, research on using natural polysaccharides as drug delivery carriers instead of synthetic vectors are underway [Citation9–11]. It has been demonstrated that both arabinogalactan and pullulan, two widely used natural polysaccharides, have a high affinity for ASGPR [Citation12,Citation13]. Arabinogalactan, a typical galactose-based polymer extracted from the plant Larix occidentalis, is a highly branched natural polysaccharide containing arabinose and galactose in a ratio of 1:6. It was observed to be rapidly eliminate from the bloodstream and pass to the liver following intravenous injection, making it a viable liver-targeting carrier. Dox liposome modified with cholesterol arabinogalactan exhibited an effect on hepatocellular carcinoma (HCC) [Citation14]. Moreover, the enhancement of immune properties of arabinogalactan could enhance pharmacological activity [Citation15]. Pullulan is a linear polysaccharide containing only glucose. Pullulan-functionalized doxorubicin nanoparticles for ASGPR mediated uptake in HepG2 cells have been evaluated [Citation16]. Furthermore, the prolonged in vivo blood circulation time of pullulan provides an added advantage [Citation17]. Owing to their high water solubility, multiple hydroxyl groups that can readily be modified chemically, non-toxicity and lack of immunogenicity, natural polysaccharides are considered the most promising candidates for nanometric carriers.
Angelica polysaccharides, extracted from the roots of Angelica sinensis (Oliv.) Diels, have drawn much attention for their outstanding pharmacological properties, such as immunomodulatory activity, antitumour activity, etc. [Citation18–20]. It has also been applied to drug delivery systems. The Angelica polysaccharide, containing mainly glucose, is a promising colon-specific drug carrier to deliver dexamethasone to the colon [Citation21]. A novel non-viral carrier based on Angelica sinensis polysaccharide (ASP) nanoparticles was successfully used for mesenchymal stem cell gene delivery [Citation22]. In our previous studies, a novel ASP containing high amount of galactose was obtained and a possible primary structure of ASP was proposed [Citation23]. Related experiments demonstrated that ASP plays a role primarily in the function of the liver [Citation24,Citation25]. However, the effect of the potential mechanism influenced by the in vivo behaviour of ASP is still unclear, necessitating the study of its pharmacokinetics and tissue distribution. A polysaccharide iron complex, using ASP as a carrier conjugated with FeOOH, was also synthesized for the treatment of iron deficiency anaemia [Citation26]. It was observed that the complex was distributed to the liver to a large degree, indicating that ASP could serve as a potential carrier for delivering drugs to the liver.
It has been emphasized that the pharmacokinetics of polymeric drugs are governed to a large extent by the carrier moiety. Therefore, to lay the foundation for using ASP as a drug delivery carrier, investigations of the pharmacokinetics and biodistribution of ASP both in vitro and in vivo, focusing especially on receptor-mediated hepatic uptake, are imperative. In this paper, we first established a specific high performance size exclusion chromatography-fluorescence detection (HPSEC-FD) method to quantitatively determinate the concentration of ASP in biosamples and evaluated the pharmacokinetics and tissue distribution of ASP in rats accordingly. Moreover, we also explored the receptor-mediated uptake of ASP in vivo and in vitro. Additionally, to visibly track the systemic circulation of ASP after intravenous administration, ASP was radiolabelled with 99mTc and then imaged using single photon emission computed tomography (SPECT).
Material and methods
Materials
The dry roots of Angelica sinensis (Oliv.) Diels were collected from Minxian County in Gansu Province in China. Dextran, pullulan and fluorescein isothiocyanate isomer I (FITC) were purchased from Sigma-Aldrich (St. Louis, MO). The HepG2, Bel-7402 and HeLa cell lines were purchased from the China Centre for Type Culture Collection. William’ E medium, RPMI-1640 and foetal bovine serum were purchased from Gibco (Grand Island, NY). 99mTc was obtained in the form of its sodium salt, Na99mTcO4, eluted from a 99mTc/99Mo generator (Beijing Atom High Tech Co., Ltd., Beijing, China). Instant thin-layer chromatography-silica gel (ITLC-SG) chromatographic strips were purchased from Pall Life Sciences. All other reagents and chemicals were of analytical grade.
Animals
All animal studies were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology, and were carried out in accordance with the approved guidelines. SD rats, Kunming mice and Japanese White rabbits were obtained from the Centre for Experimental Animal Research (Wuhan, China) and maintained in polypropylene cages at 23 ± 2 °C, a relative humidity of 50 ± 20% and 12 h light/dark cycles. All the animals had ad libitum access to food and water.
Preparation of ASP
ASP was prepared according to our previous study [Citation23]. Briefly, the fragment roots of Angelica sinensis (Oliv.) Diels were first treated with 95% ethanol and then extracted twice with hot water. After adding Ca(OH)2 to the aqueous extracts, the supernatant were collected and the pH of the supernatant was adjusted with dilute sulphuric acid. The resulting solution was precipitated with an equal volume of ethanol and centrifuged to obtain the crude polysaccharide. Then, a repeated freeze-thaw method was adopted to remove the protein among the crude polysaccharide. For further purification, the crude polysaccharide was applied to a Sephadex G-50 chromatography column and eluted with distilled water. The eluent was collected and assessed using the phenol-sulphuric acid method [Citation27]. As a result, one purified fraction was collected and lyophilized, affording ASP.
Preparation of fluorescein-labelled ASP (FA)
FA was prepared according to previous literature [Citation28]. Briefly, ASP (1000 mg) was dissolved in dimethyl sulphoxide (10 ml) containing three drops of pyridine. FITC (100 mg) was added, followed by the addition of dibutyltin dilaurate (20 mg). The mixture was then heated for 2 h at 95 °C. After several precipitations in ethanol to fully remove free FITC and other excess reagents, the FA fraction was dialyzed against distilled water and then freeze-dried. The fluorescein content of FA was calculated using a calibration curve of FITC. Fluorescein-labelled dextran (FD) and fluorescein-labelled pullulan (FP) were synthesized the same way.
Plasma level and tissue distribution studies of FA
Analytical conditions
Treatment of the biological samples before detection was performed according to procedures described in the literature [Citation29]. A Prominence HPLC system (Agilent) coupled with a variable-wavelength fluorescent detector (Jasco, Japan) was used to determine the concentrations of FA in biological samples. Chromatographic separation was performed on a PL aquagel-OH MIXED column (300 mm × 7.5 mm, 8 μm) at 25 °C, while the excitation and emission wavelengths of the fluorescent detector were set at 494 and 520 nm, respectively. The eluent was 0.2 M NaCl in 0.05 M phosphate buffer (pH 7.0), and the flow rate was set at 0.5 mL/min.
Preparation of standard and quality control samples
To prepare the standard calibration samples, a series of standard FA solutions were prepared with phosphate buffer (pH =7.4) and then mixed with blank plasma or tissue homogenates. After the above operations, standard calibration samples ranging from 2.5 to 400 μg/mL were obtained. The quality samples were prepared in the same way.
Plasma level and tissue distribution
For pharmacokinetics studies, a cannula was inserted into the femoral artery of rats under anaesthesia prior to an injection with a single dose of FA (12 mg/kg) through the tail vein. Blood samples were obtained periodically (1, 5, 10, 15, 30, 60, 90 and 120 min post-dose) from the femoral artery cannula and then centrifuged for 10 min to collect the supernatant. To study tissue distribution, rats were administered the FA solution via tail vein injection at a single dose of 12 mg/kg and euthanized at a pre-defined time (30, 60 and 120 min post-dose). The liver, spleen, heart, lungs, brain, stomach, kidney and intestine were promptly excised, washed and weighed. These samples were frozen at −20 °C until analysis.
Distribution of FA in liver parenchymal and non-parenchymal cells
The liver of the rats was isolated immediately at 2 h after intravenous injection with a dose of 12 mg/kg FA or FD into rats. Parenchymal cells and non-parenchymal cells were separated by Percoll centrifugation, and the distributions of FA and FD were examined. Another group of rats that were injected with FA or FP, were sacrificed and the liver tissues were also excised. The tissue specimens were examined with a transmitted light fluorescence microscope (Hitachi, Japan).
Absorption of FA by liver parenchymal cells
Hepatic parenchymal cells were isolated by collagenase perfusion and verified by the Trypan blue exclusion test. Then the cells were seeded in 12-well rat tail collagen-pre-coated plates at a density of 2 × 106 cells/well and cultured for 24 h. After attaching to the collagen, the monolayers of hepatocytes were washed with PBS and treated with FA at different concentrations (0, 50, 100 and 200 μg/mL) in the dark for 120 min. Cells were harvested and resuspended in William’s E medium, followed by flow cytometer analysis. The fluorescence intensity and uptake rates, which were calculated as the number of cells occupied by FA divided by the total cell count, were determined.
Binding of FA to HepG2, Bel-7402 and HeLa cells
HepG2, Bel-7402 and HeLa cells were plated in 12-well plates at a density of 2 × 106 cells/well and incubated in a 3 mL/well culture medium for 48 h. The medium was removed, and the cells were flushed with RPMI-1640 containing 10 mM Hepes and 50 μM CaCl2 (medium A). Then, 3 mL/well medium A was added, and the cells were incubated for 1 h at 37 °C; following this, the cells were treated with various concentrations of FA (0, 25, 50, 75, 100, 150, 200, 300, 400, 600 and 800 μg/mL) in the dark for 30 min. Afterwards, the cells were washed, harvested and analysed with a flow cytometer. For blocking experiments, cells were incubated with NGA (2.4 mg/mL) for 30 min prior to incubation with FA (0.6 mg/mL).
Uptake of FA in mice liver in vivo
The mice were intravenously injected with a single dose of FA (6 mg/kg) or co-administered with NGA (120 mg/kg). Livers of mice were excised after cervical dislocation at 15, 30, 60, 90 and 120 min after administration in each group. For positive control experiments, 120 mg/kg of dextran combined with 6 mg/kg of FA were intravenously injected.
Preparation of 99mTc-DTPA-ASP
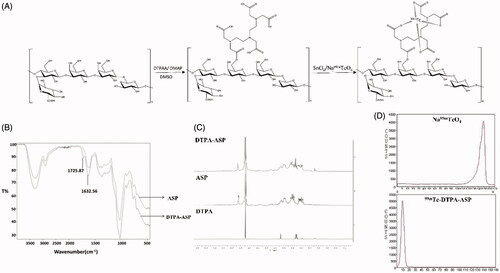
DTPA-ASP was first synthesized by reacting DTPA anhydride (300 mg) with ASP (150 mg) in anhydrous DMSO (80 mL) using DMAP (100 mg) as a catalytic agent. After stirring at 40 °C for 24 h, the mixture was further purified using a Sephadex G-50 column. The final product was lyophilized and characterized by 1H NMR and FT-IR. DTPA-ASP (5 mg) was then radiolabelled with generator-eluted 99mTc-pertechnetate (20 mCi) using stannous chloride as a reducing agent. The radiolabelled complex was then purified using a PD-10 column to yield 99mTc-DTPA-ASP. The radiochemical purity was evaluated using ITLC-SG strips with saline as the mobile phase. The 99mTc-DTPA–ASP was used without further purification for subsequent biodistribution and imaging studies.
In vivo SPECT imaging of 99mTc-DTPA-ASP
A SPECT imaging assay was performed in male rabbit. After the rabbit was anaesthetized, approximately 55.5 MBq of 99mTc-DTPA-ASP (1 × 10−9 mol/kg of body weight) was injected through the marginal ear vein. Static SPECT images were acquired at 15, 30, 60 and 120 min after the tracer injection. The scan time was 2 min. Meanwhile, a blocking study was carried out via the pre-administration of NGA (1 × 10−7 mol/kg of body weight) as a blocking agent 5 min before the intravenous injection of the radiotracer.
Results and discussion
Characterization of fluorescein-labelled conjugates
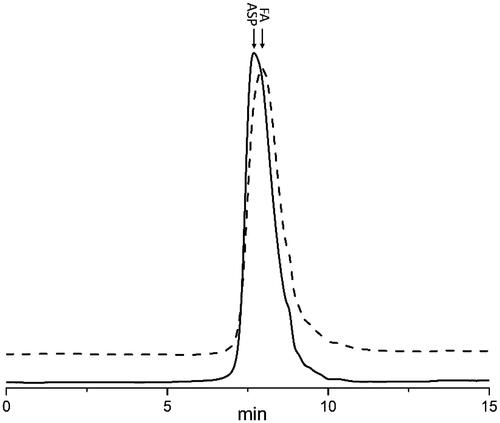
To develop a sensitive and specific procedure to quantify ASP in biological media, the pre-labelling method chosen is crucial. In this study, FA was successfully synthesized and characterized by high performance gel permeation chromatography (HPGPC). As shown in , both ASP and FA were eluted as a single peak, and no notable differences in the elution position and peak shape between them were found. Furthermore, no degradation was observed during the reaction. The MWs of ASP and FA were estimated to be 87.80 and 88.70 kDa, respectively. These results were consistent with the FITC substitution degree (%) of ASP, which was calculated to be 1.18% using the FITC concentration related to absorbance as the linear regression equation. Because of the very low degree of substitution of FITC, the pharmacokinetics parameters for FA following intravenous administration were similar to that of ASP [Citation30].
Method validation
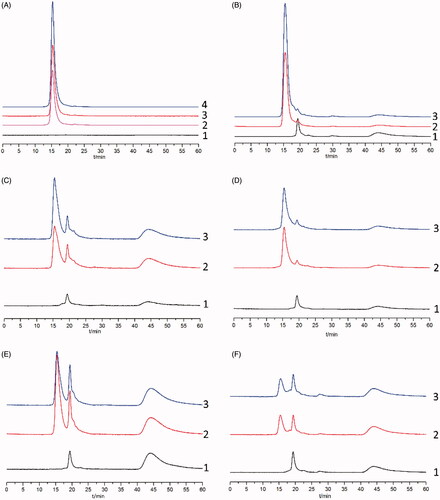
The specificity of the method was evaluated by comparing the chromatograms of processed blank biological samples with those of blank biosamples mingled with FA and biosamples collected at 30 min after the intravenous injection of FA. Chromatograms of FA in plasma and tissue samples are shown in . The typical retention time for FA was approximately 15.6 min and good separation was observed, indicating minimal interference from endogenous substances. A similar study focused on determining the concentrations of Radix ophiopogonis polysaccharide (ROP) in biosamples showed that fluorescein-labelled ROP can also be separated well from endogenous substances [Citation31]. However, the fluorescein pre-labelling method can only be used for the microanalysis of polysaccharide-protein complexes (LPPF) in mouse plasma, spleens and lungs [Citation32]. Related peaks of blank biosamples, such as heart, liver and kidney, were partially overlapped with those of LPPF, which was primarily caused by the insufficient purity of LPPF.
Figure 2. Representative chromatograms for the determination of FA in plasma (A) and various tissues (B: liver; C: stomach; D: spleen; E: heart; F: intestines) by HPGPC. (1) Blank plasma or tissue; (2) Blank plasma or tissue spiked with standard FA solution; (3) Plasma or tissue samples collected after intravenous injection of FA; (4) FA dissolved in phosphate buffer (pH =7.4).
The standard curves of peak height (Y) to FA concentration (C) were constructed using the 1/C weighted linear least squares regression model. The standard curves, correlation coefficients and linear ranges of the quantitative determination of FA in plasma and various tissues are listed in . Commendable linearity was obtained in the concentration range of 1–250 μg/mL in all studied biosamples, and the correlation coefficients were larger than 0.995. The detection limit (DL) and quantitation limit (QL) of the method calculated from the signal to noise ratio (DL and QL correspond to 3- and 10-fold of the noise level, respectively) were 0.06 and 0.2 μg/mL, respectively. The accuracy and precision of the proposed method were evaluated by adding different concentrations of FA to blank plasma and tissues. These results are summarized in Supplementary Table S1. For all tissues, the intra-day precision measured was lower than 10%, and the accuracy evaluated by a recovery assessment ranged from 90% to 115%, which were in compliance with the testing requirements of the following experiment.
Table 1. Calibration curves for FA in rat plasma and tissues (n = 5).
Table 2. Distribution of FA in tissues (n = 5)..
Plasma level and tissue distribution of FA
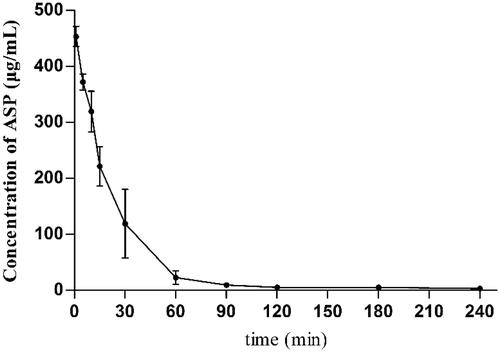
The plasma concentration-time profile of FA following an intravenous injection at a single dose of 12 mg/kg, shown in , was consistent with a two-compartment model. It has been reported that the half-life period of polysaccharides is related to different molecular weight and dosages [Citation33]. For instance, a typical two-compartment model of dextran in mouse plasma after intravenous administration was authenticated in previous studies [Citation34], and the results demonstrated that the half-life period of dextran in blood increased along with the increase in its molecular weight. However, compared to dextran, the results of a pharmacokinetic study of FA did not confirm this conclusion. With a higher molecular weight and higher dosages (80 kDa, 12 mg/kg) relative to dextran (70 kDa, 5 mg/kg), the half-life period of FA in blood, determined to be 13.45 min, was significantly shorter than the expected value. Since ASP mainly contains galactose and dextran contains only glucose, differences in monosaccharide composition between ASP and dextran may be responsible for this phenomenon. Additionally, the large extent of organ accumulation also usually results in a rapid elimination from the bloodstream after the i.v. injection of water-soluble macromolecules. Therefore, we assumed that ASP could be primarily absorbed by the liver via the ASGPR. The hypothesis was then tested using a tissue distribution study of FA. The concentrations of FA in tissues at 30, 60 and 120 min are listed in . In all tissues studied, the highest levels of FA were observed at 60 min post-dose, and FA became undetectable in the brain beyond 120 min. It was found that the accumulation of FA in the liver was appreciably higher than in other tissues at all the time points, indicating that FA has a high affinity for the liver.
Receptor-mediated endocytosis of FA in liver
To further elucidate the specific mechanism of the receptor-mediated uptake of ASP, observations from both in vitro and in vivo experiments were combined. Rat livers contain two main cell types, parenchymal cells and non-parenchymal cells, which constitute 80 and 20% of liver cells, respectively. The hepatic cellular localization of FA was examined and the results were compared with those of FD. As shown in , FA was predominantly distributed into parenchymal cells, and the concentration was approximately eight times higher than that of the non-parenchymal cells. This finding was also confirmed by fluorescence microscopy (Figure 4(B-1)). However, FD was taken up by both types of cells equally. Dextran is a polysaccharide containing glucose molecules, and it is a potential ligand for the mannose receptor, which is expressed on Kupffer cells. The interaction of dextran with the ASGPR exists but was weak [Citation35]. Fluorescence microscopy was also carried out to determine the hepatocellular localization of pullulan (Figure 4(B-2)). As shown in Supplementary Figure S2, the absorption of both FA and FP in parenchymal cells are much higher than that in biliary epithelial cells, which located in portal area. This is mainly because the absence of ASGPR on biliary epithelial cell membrane. Fascinatingly, more fluorescent green granules were observed in the ASP group than in the pullulan group, indicating that ASP might have a higher affinity for hepatocytes than pullulan. As is well recognized, pullulan can bind to the ASGPR with a high affinity. However, arabinogalactan, a branched polysaccharide with a high density of the galactose moiety, showed an even higher affinity to the ASGPR than pullulan [Citation36]. The galactose density of ASP is higher than that of pullulan. Hence, the reasonable explanation for the higher affinity of ASP to the hepatocytes compared to pullulan is that ASP has a high density of galactose combined with the branched structure.
Figure 4. Hepatocytes uptake of FA. (A) Distributions of FA and FD in liver parenchymal and non-parenchymal cells 2 h after intravenous injection at a dose of 6 mg/kg. Values are given as mean ± SD (n = 3). (B) Fluorescence microscopic examination of paraffin sections of mice liver 2 h after intravenous injection of FA (B1) and FP (B2). The bar represents a length of 50 μm. (C) Fluorescence intensity of FA in liver parenchymal cells. (D) The uptake rate of FA in liver parenchymal cells. Values are given as mean ± SD (n = 3).
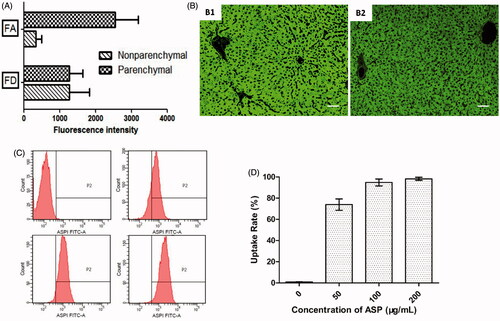
The parenchymal cells were isolated and cultured with media containing various concentrations of FA. The percentages of cells occupied by FA and their fluorescence intensity were analysed. As shown in , a marked concentration-dependency was found for the fluorescence intensity with the stepwise elevation of ASP levels. Additionally, because of the saturation of the hepatic uptake of FA, shows that nearly 100% of the hepatocytes were occupied by ASP when the concentration was at or above 100 μg/mL. Receptor-mediated endocytosis is a natural process involving the binding of a ligand to specific cell surface receptors first, followed by the internalization of receptor–ligand complexes through coated vesicles into endosomal compartments. It has been investigated that the cell dispositions of arabinogalactan and asialofetuin were achieved at a steady state during both the binding process and internalization process, which agrees well with our results [Citation37]. Moreover, the slight absorption of FA was found in separated Kupffer cells (Supplementary Figure S3) after treated with 200 μg/mL of FA, indicating that receptors expressed on Kupffer cell membrane surface, such as Kupffer cell receptor, mannose receptor and complement receptor, displayed little affinity to ASP [Citation38].
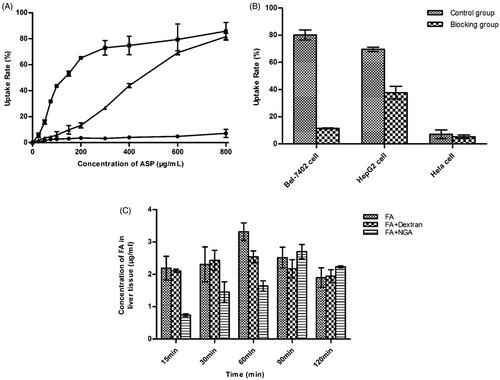
It has been reported that ASGPR are overexpressed in HCC cell lines, including HepG2 and Bel7402 cells, but under expressed in HeLa cells [Citation39]. In in vitro experiments, ASGPR-rich HepG2 cells and Bel-7402 cells were chosen as targets while ASGPR-poor HeLa cells were employed as negative control cells. represents the uptake rate of various concentrations of FA into HepG2 cells, Bel-7402 cells and HeLa cells. A marked concentration dependency was found for the uptake of FA in HepG2 cells. Similar results were obtained with Bel-7402 cells. However, FA was only endocytosed in low quantities by HeLa cells regardless of the concentration of FA. The difference in the cellular uptake of FA among HepG2, Bel-7402 and HeLa cells might be largely dependent on the differences in ASGPR expression on the cell membranes of these cell types. The cellular uptake results also suggested that more ASP was endocytosed into HepG2 cells than into Bel-7402 cells before reaching a steady state, given equal concentrations of ASP. The positive ASGPR expression of the HCC cell lines was demonstrated to be different. In the two different cell lines, the ASGPR expression in Bel-7402 cells was found to be higher than in HepG2 cells [Citation40]. The cellular uptake results showing that more ASP was endocytosed into HepG2 cells than into Bel-7402 cells were not consistent with the ASGPR expression in those two HCC cells. A possible explanation for this inconsistency is that in addition to endocytosis through the ASGPR, ASP might exploit other mechanisms to enter HepG2 cells. It has also been verified in an antagonistic experiment. After being blocked with NGA, the activity of absorption of FA was reduced in target cells, as shown in . The uptake ratio between the unblocked and the antagonistic group was 2.3 and 4.2 in HepG2 and Bel-7402 cells, respectively. The absorption of FA in HepG2 cells was not blocked as completely as in Bel-7402 cells by NGA. However, in negative control cells, the difference between the blocked and unblocked groups was not significant. Similar results were also observed during the study of the accumulation of FA in mouse liver. As shown in , the time point corresponding to the maximum accumulation of FA in hepatic cells was retarded by the co-administration of NGA, whereas dextran had a comparatively faint inhibition to the hepatic uptake of FA.
Figure 5. (A) Uptake rate of cells treated with different concentrations of FA (n = 3). ▪, HepG2 cells, •, Bel-7402 cells, ▲, Hela cells; (B) Effect of NGA on the uptake of FA to cells (n = 3); (C) Effect of NGA or dextran on the uptake of FA to mice liver (n = 3).
Based on the above results, we can deduce that the large amount of ASP accumulated in the liver in biodistribution studies was endocytosed via ASGPR. To further confirm this conclusion and track the systemic circulation of ASP visibly, the radiolabelled ASP was synthesized in a subsequent experiment.
Preparation of 99mTc-DTPA-ASP
The reaction procedure is described in . DTPA, a bifunctional chelator known to form stable complexes with metal ions, can easily and efficiently be labelled with 99mTc. It can also be conjugated with acidic polysaccharides to give an exclusive product through two possible ways [Citation41]. The IR spectrum and 1H NMR spectrum were utilized to confirm the identity of DTPA-ASP. As shown in , the absorption peak at 1725 cm−1 corresponding to νC=O stretching was enhanced significantly in the IR spectrum. The signals of DTPA were obscured because they are in the same region as ASP (3.2–4.0 ppm) in the 1H NMR spectrum. However, two characteristic peaks consistent with the presence of DTPA were observed at 3.5 ppm and 3.08 ppm (). The radiochemical purity of 99mTc-DTPA-ASP was greater than 96% according to the results obtained by chromatographic analysis (). While 99mTc-DTPA-ASP remained at the point of spotting, hydrolysed 99mTc impurities and other radioactive impurities moved with the solvent front [Citation42]. The results of measuring the in vitro stability of 99mTc-DTPA-ASP at different time points are shown in Supplementary Figure S1. The radiochemical purity was still over 90% in PBS (pH 7.4) and over 80% in mouse serum after 24 h at room temperature, which met the requirements of the following experiments.
In vivo SPECT imaging
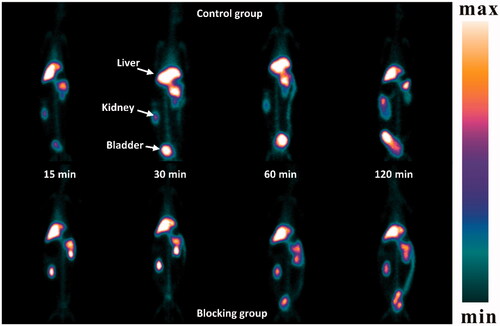
A SPECT study of 99mTc-DTPA-ASP in Japanese White rabbits was conducted (). In the control group, 99mTc-DTPA-ASP had high liver accumulation. No free 99mTc was detected in the thyroid, indicating the complexation was stable even 2 h after administration in vivo. The outline of the liver was clear at all time points even though the radioactivity was eliminated from the liver after 60 min. Unlike 99mTc galactosylated chitosan, an appreciable low level of 99mTc-DTPA-ASP was found in the kidney at all time points, suggesting the radioactivity was eliminated from the liver by hepatobiliary excretion rather than excretion via the kidneys [Citation43]. Conversely, the radioactivity level in other organs was relatively low. After inhibition, the outline of the livers was observed to shrink. Additionally, the accumulation of radioactivity in the kidneys caused them to become visible in the images. In vivo SPECT imaging provided a visual confirmation that the results observed were in accordance with the biodistribution data, which was performed in mice. The related results are listed in Supplementary Table S2. Because liver disease usually leads to a change in the amount of ASGPR [Citation44], these findings above suggest that 99mTc-DTPA-ASP is an underlying contrast agent for the clinical molecular imaging of liver disease.
Conclusions
Taken together, our results showed that the method of a combination of fluorescein pre-labelling and HPSEC-FD could be used for the quantitative determination of ASP in biosamples. Using this method, a typical two-compartment model of ASP in rat plasma and a high affinity of ASP for the liver after intravenous injection was identified. Additionally, in vivo and in vitro studies on the receptor-mediated uptake of FA demonstrated that ASP was endocytosed by liver parenchymal cells via the ASGPR and displayed a higher affinity for the ASGPR than traditional polysaccharides such as pullulan, indicating that ASP could serve as a new carrier for delivering drugs to the liver. In addition, SPECT imaging assays revealed that ASP also has the potential to be used as a contrast agent. Relevant aspects of this work need to be further studied.
Supplementary_material.docx
Download MS Word (3.3 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- D'Souza AA, Devarajan PV. Asialoglycoprotein receptor mediated hepatocyte targeting – strategies and applications. J Control Release. 2015;203:126–139.
- Guo DL, Wang ZG, Xiong LK, et al. Hepatogenic differentiation from human adipose-derived stem cells and application for mouse acute liver injury. Artif Cell Nanomed B. 2017;45:224–232.
- Usmani A, Mishra A, Ahmad M. Nanomedicines: a theranostic approach for hepatocellular carcinoma. Artif Cells Nanomedicine Biotechnol. 2017 [Sep 8]; [11 p.]. DOI: https://doi.org/10.1080/21691401.2017.1374282
- Hodgins NO, Al-Jamal WT, Wang JT, et al. Investigating in vitro and in vivo alphavbeta6 integrin receptor-targeting liposomal alendronate for combinatory gammadelta T cell immunotherapy. J Control Release. 2017;256:141–152.
- Zhang L, Yao J, Zhou JP, et al. Glycyrrhetinic acid-graft-hyaluronic acid conjugate as a carrier for synergistic targeted delivery of antitumor drugs. Int J Pharmaceut. 2013;441:654–664.
- Chen DQ, Sun JF, Sun KX, et al. In vivo evaluation of novel ketal-based oligosaccharides of hyaluronan micelles as multifunctional CD44 receptor-targeting and tumor pH-responsive carriers. Artif Cell Nanomed B. 2016;44:898–902.
- Luo LH, Zheng PJ, Nie H, et al. Pharmacokinetics and tissue distribution of docetaxel liposome mediated by a novel galactosylated cholesterol derivatives synthesized by lipase-catalyzed esterification in non-aqueous phase. Drug Deliv. 2016;23:1282–1290.
- Liang MH, Zheng XL, Tu LL, et al. The liver-targeting study of the N-galactosylated chitosan in vivo and in vitro. Artif Cells Nanomed Biotechnol. 2014;42:423–428.
- Wang W, He S, Hong T, et al. Synthesis, self-assembly, and in vitro toxicity of fatty acids-modified Bletilla striata polysaccharide. Artif Cells Nanomed Biotechnol. 2017;45:69–75.
- Sharma M, Sahu K, Singh SP, et al. Wound healing activity of curcumin conjugated to hyaluronic acid: in vitro and in vivo evaluation. Artif Cells Nanomedicine Biotechnol. 2017 [Jul 28]; [9 p.]. DOI: https://doi.org/10.1080/21691401.2017.1358731
- Safdar MH, Hussain Z, Abourehab MAS, et al. New developments and clinical transition of hyaluronic acid-based nanotherapeutics for treatment of cancer: reversing multidrug resistance, tumour-specific targetability and improved anticancer efficacy. Artif Cells Nanomedicine Biotechnol. 2017 [Oct 30]; [14 p.]. DOI: https://doi.org/10.1080/21691401.2017.1397001
- Kaneo Y, Ueno T, Tanaka T, et al. Pharmacokinetics and biodisposition of fluorescein-labeled arabinogalactan in rats. Int J Pharm. 2000;201:59–69.
- Kaneo Y, Tanaka T, Nakano T, et al. Evidence for receptor-mediated hepatic uptake of pullulan in rats. J Control Release. 2001;70:365–373.
- Pathak P, Dhawan V, Magarkar A, et al. Design of cholesterol arabinogalactan anchored liposomes for asialoglycoprotein receptor mediated targeting to hepatocellular carcinoma: in silico modeling, in vitro and in vivo evaluation. Int J Pharmaceut. 2016;509:149–158.
- Kim LS, Waters RF, Burkholder PM. Immunological activity of larch arabinogalactan and Echinacea: a preliminary, randomized, double-blind, placebo-controlled trial. Altern Med Rev:. 2002;7:138–149.
- Guhagarkar SA, Majee SB, Samad A, et al. Evaluation of pullulan-functionalized doxorubicin nanoparticles for asialoglycoprotein receptor-mediated uptake in Hep G2 cell line. Cancer Nano. 2011;2:49–55.
- Pranatharthiharan S, Patel MD, Malshe VC, et al. Asialoglycoprotein receptor targeted delivery of doxorubicin nanoparticles for hepatocellular carcinoma. Drug Deliv. 2017;24:20–29.
- Liu PJ, Hsieh WT, Huang SH, et al. Hematopoietic effect of water-soluble polysaccharides from Angelica sinensis on mice with acute blood loss. Exp Hematol. 2010;38:437–445.
- Wang QK, Chen CX, Guo YJ, et al. Dietary polysaccharide from Angelica sinensis enhanced cellular defence responses and disease resistance of grouper Epinephelus malabaricus. Aquacult Int. 2011;19:945–956.
- Cao W, Li XQ, Wang X, et al. Characterizations and anti-tumor activities of three acidic polysaccharides from Angelica sinensis (Oliv.) Diels. Int J Biol Macromol. 2010;46:115–122.
- Zhou S, Zhang B, Liu X, et al. A new natural angelica polysaccharide based colon-specific drug delivery system. J Pharm Sci. 2009;98:4756–4768.
- Deng W, Fu M, Cao Y, et al. Angelica sinensis polysaccharide nanoparticles as novel non-viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine Nanotechnol Biol Med. 2013;9:1181–1191.
- Zhang Y, Zhou T, Wang H, et al. Structural characterization and in vitro antitumor activity of an acidic polysaccharide from Angelica sinensis (Oliv.) Diels. Carbohydr Polym. 2016;147:401–408.
- Zhang Y, Cheng Y, Wang N, et al. The action of JAK, SMAD and ERK signal pathways on hepcidin suppression by polysaccharides from Angelica sinensis in rats with iron deficiency anemia. Food Funct. 2014;5:1381–1388.
- Wang K, Song Z, Wang H, et al. Angelica sinensis polysaccharide attenuates concanavalin A-induced liver injury in mice. Int Immunopharmacol. 2016;31:140–148.
- Wang KP, Chen ZX, Zhang Y, et al. Molecular weight and proposed structure of the Angelica sinensis polysaccharide-iron complex. Chin J Chem. 2008;26:1068–1074.
- Yuan Q, Zhao L, Cha Q, et al. Structural characterization and immunostimulatory activity of a homogeneous polysaccharide from Sinonovacula constricta. J Agric Food Chem. 2015;63:7986–7994.
- De Belder A, Granath K. Preparation and properties of fluorescein-labelled dextrans. Carbohydr Res. 1973;30:375–378.
- Lin X, Wang Z-J, Wang S, et al. Comparison of tissue distribution of a PEGylated Radix Ophiopogonis polysaccharide in mice with normal and ischemic myocardium. Eur J Pharm Biopharm. 2011;79:621–626.
- Bachelet L, Bertholon I, Lavigne D, et al. Affinity of low molecular weight fucoidan for P-selectin triggers its binding to activated human platelets. Biochim Biophys Acta Gen Subjects. 2009;1790:141–146.
- Lin XA, Wang ZJ, Sun GL, et al. A sensitive and specific HPGPC-FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomed Chromatogr. 2010;24:820–825.
- Min T, Sun J, Yi Y, et al. Microanalysis, pharmacokinetics and tissue distribution of polysaccharide-Protein complexes from longan pulp in mice. Int J Mol Sci. 2015;16:24403–24416.
- Yi Y, Wang HX, He JR. Research progresses of pharmacokinetics of polysaccharides. Acta Pharm Sin. 2014;49:443–449.
- Yamaoka T, Tabata Y, Ikada Y. Body distribution profile of polysaccharides after intravenous administration. Drug Deliv. 1993;1:75–82.
- Nishikawa M, Yamashita F, Takakura Y, et al. Demonstration of the receptor-mediated hepatic uptake of dextran in mice. J Pharm Pharmacol. 1992;44:396–401.
- Tanaka T, Fujishima Y, Hanano S, et al. Intracellular disposition of polysaccharides in rat liver parenchymal and nonparenchymal cells. Int J Pharm. 2004;286:9–17.
- Tanaka T, Abo Y, Hamano S, et al. Intracellular disposition of arabinogalactan and asialofetuin in HepG2 cells. J Drug Deliv Sci Technol. 2013;23:435–438.
- Kang JH, Toita R, Murata M. Liver cell-targeted delivery of therapeutic molecules. Crit Rev Biotechnol. 2016;36:132–143.
- Wu XM, Tan YJ, Toh HT, et al. Stimuli-responsive multifunctional glyconanoparticle platforms for targeted drug delivery and cancer cell imaging. Chem Sci. 2017;8:3980–3988.
- Mu H, Lin KX, Zhao H, et al. Identification of biomarkers for hepatocellular carcinoma by semiquantitative immunocytochemistry. World J Gsatroenterol. 2014;20:5826–5838.
- Buffa R, Běťák J, Kettou S, et al. A novel DTPA cross-linking of hyaluronic acid metal complexation thereof. Carbohydr Res. 2011;346:1909–1915.
- Balogh L, Polyak A, Mathe D, et al. Absorption, uptake and tissue affinity of high-molecular-weight Hyaluronan after oral administration in rats and dogs. J Agric Food Chem. 2008;56:10582–10593.
- Kim E-M, Jeong H-J, Kim S-L, et al. Asialoglycoprotein-receptor-targeted hepatocyte imaging using 99m Tc galactosylated chitosan. Nucl Med Biol. 2006;33:529–534.
- Ke Z, Li J, Zhe J, et al. Flow cytometric analysis of Asialoglycoprotein receptor expression predicts hepatic functional reserve after hepatectomy. J Coll Physicians Surg Pak. 2014;24:820–824.