?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cartilage defect is common in clinical but notoriously difficult to treat for low regenerative and migratory capacity of chondrocytes. Biodegradable tissue engineering nano-scaffold with a lot of advantages has been the direction of material to repair cartilage defect in recent years. The objective of our study is to establish a biodegradable drug-loading synthetic polymer (PLA) and biopolymer (Gelatine) composite 3D nano-scaffold to support the treatment of cartilage defect. We designed a microfluidic chip-based drug-screening device to select the optimum concentration of resveratrol, which has strong protective capability for chondrocyte. Then biodegradable resveratrol-loading PLA/Gelatine 3D nano-scaffolds were fabricated and used to repair the cartilage defects. As a result, we successfully cultured primary chondrocytes and screened the appropriate concentrations of resveratrol by the microfluidic device. We also smoothly obtained superior biodegradable resveratrol-loading PLA/Gelatine 3D nano-scaffolds and compared the properties and therapeutic effects of cartilage defect in rats. In summary, our microfluidic device is a simple but efficient platform for drug screening and resveratrol-loading PLA/Gelatine 3D nano-scaffolds could greatly promote the cartilage formation. It would be possible for materials and medical researchers to explore individualized pharmacotherapy and drug-loading synthetic polymer and biopolymer composite tissue engineering scaffolds for the repair of cartilage defect in future.
Introduction
Articular cartilage defect is one of the most common diseases resulted from trauma, degeneration, inflammation, and neoplasm [Citation1–3]. Due to the lack of blood supply and single cell component, it is difficult to cure after cartilage injury. In order to improve the therapeutic activity of cartilage defect, a variety of methods including autologous or allogeneic cartilage transplantation, chondrocyte transplantation, and periosteum transplantation were used in research and clinical [Citation4–6]. However, autologous transplantation is confronted with the problem of limited sources and secondary pathology in donor area; and allogeneic transplantation would inevitably cause immunologic rejections and infections [Citation7,Citation8]. Furthermore, the original structure of cartilage often could not be recovered completely by biological treatment methods, especially for large defects (>4 mm), then the function of cartilage would also be affected correspondingly [Citation9,Citation10]. Therefore, it is vital to look for a reliable therapy to repair cartilage defect well both in structure and function, and biodegradable tissue engineering scaffold with a lot of advantages was paid more attention in recent years.
Scaffold plays a key role in tissue engineering, which works as a substrate for cell junctions and growing. In recent years, hydrogel [Citation11], freeze-dried composite scaffold [Citation12], and 3D printing scaffold [Citation13] had been usually used for cartilage repair. However, hydrogel scaffold lacks integral macropores for cell growth and tissue formation, freeze-dried composite scaffold lacks the fibrous structure for cell proliferation, and 3D printing scaffold is limited by high requirement for printing materials. Electrospun nanofibre scaffolds have the unique advantages and can be intensively utilized in tissue engineering, owing to their fibrous structure mimicking the physical dimensions of natural fibrous extracellular matrix. The major limitation of conventional 2D electrospun nanofibre mat is the low porosity for cell attach and nutrient transport. Recently, novel pathways of fabricating 3D nanofibrous scaffolds have been reported in literatures [Citation14,Citation15]. By combining electrospinning, homogeneous dispersion and freeze-drying technique, electrospun nanofibre-assembled cellular 3D scaffold could be obtained.
Biodegradable synthetic polymer like poly lactic acid (PLA) is widely used in construction of tissue engineering scaffold for tissue repair, using electrostatic spinning or other techniques [Citation16]. However, poor hydrophilicity and low affinity with cells restrict the application of synthetic polymer in clinical and research [Citation17]. To solve the problem above, some researchers tried to combine high hydrophilic materials with synthetic polymer to improve the characters [Citation18]. Between all the materials with high hydrophilicity and good affinity with cells, biopolymers are undoubtedly the optimal choice. On the one hand, biopolymers could improve the hydrophilicity and affinity with cells of synthetic polymer; on the other hand, the synthetic polymer could bring about high mechanical stability of biopolymers [Citation19]. In this study, we tried to establish a ‘synthetic polymer–biopolymer’ composite scaffold, using the material of PLA and Gelatine, to repair the cartilage defect of rats. And for optimal effects, the ‘synthetic polymer–biopolymer’ composite scaffold was fabricated into 3D nanofibre sponges structure by specific process and a chondyocyte protective drug (resveratrol) was also added in it.
Biological factor is of great significance to promote the repair of cartilage defect [Citation20]. Here, we select a kind of biological strong natural polyphenols (resveratrol) with the characteristics of anti-senescence, anti-inflammation, and reducing the damage of extracellular matrix, which could play a similar role in cartilage repair [Citation21–23]. In order to seek out the appropriate concentrations of biological factor, we designed and fabricated a new platform named microfluidic device to screen the proper concentrations of resveratrol for the regeneration of chondrocytes. Microfluidic technology, also named lab on a chip, is widely used in the biological and clinical field, which miniaturized the laboratory operations, including cell culture, biochemical reaction, drug affect, result analyze, on a device with a few square centimetres [Citation24]. Compare with conventional static 96-well plate for cell culture, microfluidic device could provide cells with fresh nutrition and oxygen by a controlled fluid culture medium. Furthermore, multiple concentration gradients of biological factor, which designed in our device, could greatly promote the screening efficiency of resveratrol.
In the present study, we established a high efficient microfluidic drug-screening device on which the optimum concentration of resveratrol for chondrocytes could be obtained. Then, biodegradable resveratrol-loading synthetic polymer (PLA) and biopolymer (gelatine) composite 3D nano-scaffolds with high porosity, interconnected pore structure, and good mechanical biostability were fabricated, and the relevant characters were test. At last, the biodegradable resveratrol-loading PLA/Gelatine 3D nano-scaffolds were used to repair cartilage defects. With this method, we could get the appropriate concentration of resveratrol and obtained superior biodegradable resveratrol-loading PLA/Gelatine 3D nano-scaffolds. Furthermore, the properties and therapeutic effects for cartilage defect by different nano-scaffolds were compared and investigated effectively as well.
Materials and methods
Isolation and cell culture of chondrocytes
The femoral inferior segments of SD rats (1-week old) were isolated and cut into pieces (1 mm3) under a dissecting microscope. Then the cartilages were digested by a type II collagenase (0.2%, Sigma, St. Louis, MO) for 4 h in a laboratory shaker with 37 °C. After that, the collagenase was neutralized by complete DMEM medium (Gibco, Carlsbad, CA) with the same volume. The medium with chondrocytes was centrifugalized 1000g, 5 min by a concentrator, throwed away the supernate liquid, and resuspended the chondrocytes by complete DMEM medium. The chondrocytes would be cultured in an incubator with 37 °C, 5% CO2 during the culture period and identified by toluidine blue staining.
Establishment of microfluidic device
Design and fabrication
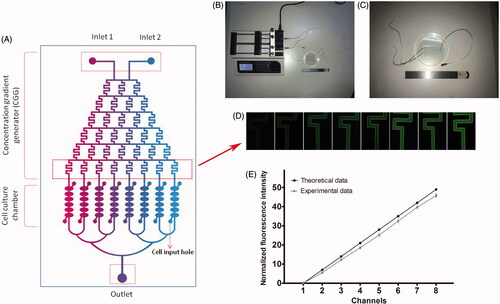
The microfluidic device was designed to screen the effect concentrations of resveratrol for chondrocytes, which mainly consisted of an upstream concentration gradient generator (CGG) and downstream parallel cell culture chambers (). The upstream microfluidic device was made of two solution inlets (1.4-mm diameter) and a CGG including six parallel solution microchannels (200 μm in width and 100 μm height). The downstream microfluidic device was made of 8 parallel cell culture chambers with 6 rooms (1.6 mm ×800 μm × 100 μm), 2 cell inlet holes (0.8-mm diameter) in each chamber unit and a waste liquid outlet (1.8-mm diameter) (). In our study, chondrocytes were cultured in the downstream parallel cell culture chambers and fresh culture medium with resveratrol was made to flow through the cell chambers using a syringe pump (). The microfluidic device was mainly fabricated with a polydimethylsiloxane (PDMS, Sylgard 184, Dow Corning, Midland, MI) replica shaped on a mould that was prepared by soft lithography technology and a glass substrate. The mould was designed at first by AutoCAD software (Autodesk, Inc, San Rafael, CA) and then duplicate into a silicon wafer by SU8-2035 negative photoresist (Microchem Corp, Newton, MA) according to 1:1 ration. The mixed PDMS (10:1) was poured on the silicon wafer, vacuumized, and cured on 80 °C for 1 h. After that the cooling PDMS layer was cut, punched, and bonded together with a clean glass substrate by oxygen plasma for 1 min. At last, the microfluidic device should be sterilized by ultraviolet rays for 1 h before use.
Figure 1. Design and validation of microfluidic device. (A) Microfluidic device is made up of an upstream CGG and downstream parallel cell culture chambers. Coloured liquids were injected into the device from inlet 1 and inlet 2, then concentration gradient was generated in CGG. (B and C) Illustration of microfluidic device, pump, syringe, and pipeline. (D and E) Fluorescent images of AO in the final unit of CGG and normal fluorescence intensity of theoretical data and experimental data. Values are presented as mean ± SD.
CGG performance validation
In our microfluidic device, mediums from inlets 1 and 2 flowed through the CGG to the downstream parallel cell culture chambers, via a syringe pump (LSP04-1A, Baoding Longer Precision Pump Co., Ltd., Baoding, China) and pipes. With the help of CGG, solutions 1 and 2 were mixed and different concentration gradients were generated. According to Reynolds effect and the previous work and equation described by Jeon [Citation25], when the liquid flowed through the micro-channels with a lower speed, it would exhibit laminar flow. In our microfluidic device, with the fluid speed of 0.1 μl/min, different laminar flow concentration gradients would be generated by means of campulitropal micro-channel design (). When the solution 1 with concentration 0 and solution 2 with concentration R were injected into CGG, the specific concentrations in this microfluidic device were as follows: 0, 1/7R, 2/7R, 3/7R, 4/7R, 5/7R, 6/7R, and 1R. In our study, to verify the performance of CGG, we used solution PBS injected into inlet 1 and solution Acridine orange (AO, 5 μg/ml, Sigma-Aldrich, St. Louis, MO) injected into inlet 2, respectively, and different eight outlets of CGG were imaged with a fluorescence microscope (DM4, Leica, Wetzler, Germany). The fluorescence intensity was quantified by Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD) and compared with the theoretical values (). All experiments were repeated three times.
Resveratrol screening
We used complete DMEM medium inject into inlet 1 and complete DMEM medium with Resveratrol (200 μmol/L) inject into inlet 2 in our microfluidic device. The theoretical concentrations of Resveratrol should be 0, 28.57, 57.14, 85.71, 114.28, 142.86, 171.43, and 200 μmol/L in the eight downstream parallel cell culture chambers. Chondrocytes were adjusted to 1 × 104 cells/ml and 0.8 µl cells were seeded in 8 parallel cell culture chambers in our microfluidic device. After 1 h, pure complete cell culture medium was injected into inlet 1 and complete medium with Resveratrol (200 μmol/L) was injected into inlet 2. With the fluid speed of 0.1 μl/min, the concentration gradient would be established in 30 min and the outlet would be connected with a conduit. The microfluidic device would be stored in an incubator with 37 °C, 5% CO2 during the culture period.
Fabrication and character evaluation of biodegradable resveratrol-loading synthetic polymer (PLA) and biopolymer (Gelatine) composite 3D nano-scaffolds
Fabrication of resveratrol-loading PLA/Gelatine 3D nanofibre Membranes and sponges
PLA/Gelatine nanofibres (with or without resveratrol) were prepared by electrospinning technology, which consisted of a high voltage power supply, a digitally controlled syringe pump, and a laboratory-produced plat (30 cm × 30 cm) covered with conductive non-wovens to collect electrospun nanofibrous mats. Four PLA/Gelatine solutions (w/v (%) = 10) were prepared by dissolving mixed 0.1 g PLA and 0.75 g Gelatine (w/w = 1:5) in 9 ml HFIP and stirring at room temperature for 5 h. Then various amounts of resveratrol were accurately weighed, dissolved in 0.1 ml ethanol, and mixed with the four prepared PLA/Gelatine solutions, respectively, stirred until homogeneous. The concentrations of resveratrol in the final solutions should be 0 μmol/L, 57.14 μmol/L, 114.28 μmol/L, and 200 μmol/L according to the screening results by microfluidic device. The four solutions were, respectively, electro-spun with a stainless-steel needle under same parameters, flow rate, and applied voltage (3 ml/h and 15 kV), the distance between needle and collector was 10 cm. All of the collected PLA/Gelatine nanofibre membranes were placed in vacuum overnight to remove the residual solvent (). Resveratrol-loading PLA/Gelatine 3 D nanofibre sponges were fabricated with the method ever reported by Chen [Citation26] and shown briefly in . The nanofibre membranes were cut into small pieces (0.5 cm × 0.5 cm), and 1 g membrane pieces were weighed and added into 100 ml tert-butanol to disperse with a homogenizer (Wiggens D-500) at speed of 10000 rpm for 20 min. Then, the dispersed nanofibres were poured into a 24-well cell culture plate and freeze-dried for 24 h. After that, the freeze-dried 3 D nanofibre sponges were heated at 190 °C for 2 h in air for crosslink to obtain the final scaffold ().
Figure 2. Schematic illustration for the fabrication of resveratrol-loading PLA/Gelatine 3D scaffold and animal testing.
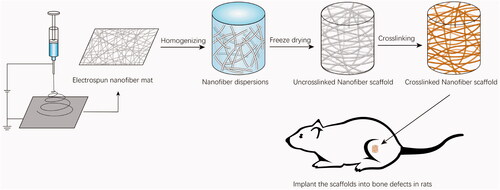
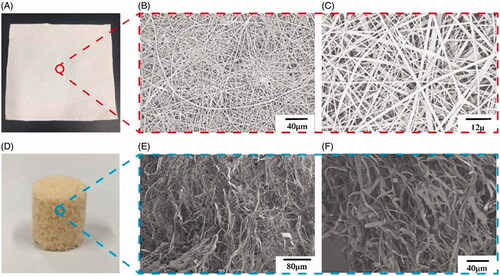
Figure 3. Digital photographs of resveratrol-loading PLA/Gelatine 3D nanofibre membrane and sponge. (A) General observation of resveratrol-loading PLA/Gelatine 3D nanofibre membrane; (B and C) SEM images of resveratrol-loading PLA/Gelatine nanofibre membrane with low and high magnifications. (D) General observation of resveratrol-loading PLA/Gelatine 3D nanofibre sponge; (E and F) SEM images of resveratrol-loading PLA/Gelatine 3D nanofibre sponge with low and high magnifications. SEM: scanning electronic microscope.
Characters evaluation of biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds
Morphology
The morphology of resveratrol-loading PLA/Gelatine nanofibre membranes and sponges were examined using a digital camera (Canon EOS 1300D, Tokyo, Japan) and scanning electron microscope (SEM, HITACHI-8010, Tokyo, Japan). Prior to evaluation, the fibres were coated with gold.
Densities
The densities (ρ) of resveratrol-loading PLA/Gelatine 3 D nanofibre sponge were calculated by the following equation [Citation17]:
(1)
(1)
Here, m, v, d, and h stand for mass, volume, diameter, and height of the scaffold, respectively.
Compressive mechanical properties
Compressive mechanical properties of the resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds were carried out by compression testing machine (MARK-10, ESM303, TA Instruments, New Castle, DE). The samples with diameters of 12 mm and height of 15 mm were used for mechanical property study. The compression strain − stress curves with compression strain (ε = 60%) were measured at a strain rate of 300 mm/min.
Resveratrol standard curve
The standard curve was created by a high-performance liquid chromatography (HPLC) assay. To create a standard curve of resveratrol concentration, different amounts of resveratrol were dissolved into PBS to prepare 0 mg/L, 2.5 mg/L, 5 mg/L, 10 mg/L, 20 mg/L, 30 mg/L, and 40 mg/L resveratrol solutions. The absorbency was monitored by a ultra-violet light detector (UV230II) at a wavelength of 306 nm with the flow rate set at 1.0 ml/min. UV absorbance at 306 nm (A306) over the range of resveratrol concentrations were read. Then, A306 values were plotted against concentrations to obtain a standard curve. All experiments were repeated three times using independent resveratrol stock solutions.
Resveratrol release characters
PLA/Gelatine 3D nanofibre scaffolds loading with 57.14 μmol/L, 114.28 μmol/L, and 200 μmol/L resveratrol were selected to evaluate the drug release character. About 500 mg scaffolds were weighed and submerged in 5 ml PBS at 37 °C. About 100 μl PBS was took out and assessed by HPLC assay at 306 nm (A306) each time. The remaining PBS was removed and replaced with 5 ml fresh PBS. A306 values were converted to resveratrol concentrations using previously obtained standard curve. All samples were assayed three times.
Repair of cartilage defect by biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds and evaluation
Experimental animals
Thirty male 6 week Sprague–Dawley (SD) rats were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. The body weight of rats was 140–180 g and the genetic background was clear. The experimental animals were housed in rat cages in a specific pathogen-free (SPF) barrier region in Experimental Animal Centre of Peking University People’s Hospital at a room temperature of 24 ± 2 °C and relative humidity of 50–55%. The rats were free access to food and sterile water. The centre was kept 12 h light/dark cycle every day. All rat experimental procedures and treatments were performed according to the experimental animal management regulations.
Treatment of animals
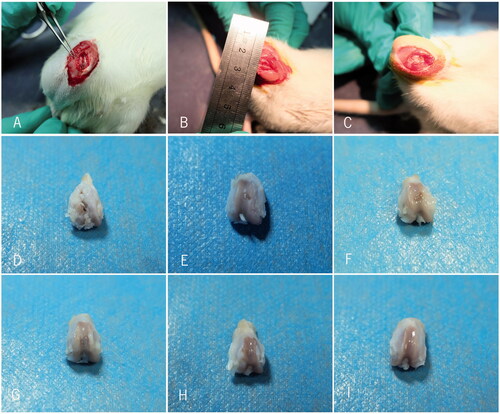
SD rats was anaesthetized by small animal anaesthesia machine and disinfected by iodophor. The right knee joint incision was performed under aseptic condition, articular surface of medial and lateral condyle in femoral was fully exposed. A defect hole was drilled by Kirschner wire with a diameter of 2 mm in the middle of the knee joint femoral condyle non-weight-bearing area and the depth should reach the full thickness of subchondral bone. SD rats were randomly divided into five groups: group A (control group, n = 6): no scaffold was inserted in the bore hole; group B (n = 6): blank PLA/Gelatine scaffold was inserted in the bore hole; group C (n = 6): 57.14 μmol/L resveratrol-loading PLA/Gelatine 3D nanofibre scaffold was inserted in the bore hole; group D (n = 6): 114.28 μmol/L resveratrol-loading PLA/Gelatine 3D nanofibre scaffold was inserted in the bore hole; group E (n = 6): 200 μmol/L resveratrol-loading PLA/Gelatine 3D nanofibre scaffold was inserted in the bore hole. Sham group: all the left knee joint femoral condyles of rats were regarded as sham operation, only cut articular capsule open and exposed the middle femoral non-weight-bearing area of knee joint. The wound was sutured routinely and well pressed and fixed ().
General observation
After 3 months, SD rats were sacrificed with cervical dislocation method, the bilateral knee joint capsule was opened, and the distal femur areas were observed to find out whether there were joint effusion, synovial swelling or other features of synovitis.
3D reconstruction of microCT
The articular cartilage specimens of rats were scanned under microCT; the CT values were set as SPOV 32 cm, 120 KV, 100 mA, TI0.5; and the surface and internal structures were observed.
Evaluation of immunohistochemistry
HE staining: tissue specimens were fixed in 4% paraformaldehyde for 24 h, decalcified, and embedded in paraffin, serial 4-μm sections were obtained and HE-stained. The tissue sections were treated with haematoxylin for 5 min, differentiated with liquid differentiation for 2 s, and immersed in ammonia for 5 s. Next, the sections were treated with eosin for 1 min. Safranin O- fast green staining: tissue specimens were fixed in 4% paraformaldehyde for 24 h, decalcified, and embedded in paraffin. Serial 4-μm sections were obtained and safranin O- fast green-stained. The tissue sections were treated with Weigert for 5 min differentiated with acidic differentiation solution for 15 s, treated with solid green for 5 min, and treated with weak acid solution for 15 s. Next, the sections were treated with safranin O for 5 min. Toluidine blue staining:tissue specimens were fixed in 4% paraformaldehyde for 24 h, decalcified, and embedded in paraffin. Serial 4-μm sections were obtained and toluidine blue-stained. The tissue sections were treated with 1% toluidine blue for 60 min. Alcian blue staining: tissue specimens were fixed in 4% paraformaldehyde for 24 h, decalcified, and embedded in paraffin. Serial 4-μm sections were obtained and alcian blue-stained. The tissue sections were treated with Alcian blue acidizing fluid for 3 min, treated with alcian blue for 30 min.
Results
Isolation and cell culture of chondrocytes
Chondrocytes were successfully isolated and cultured in dishes by double enzymes digestion. Subcultured chondrocytes had the normal morphology of polygons. With the staining of toluidine blue, we found the purity of third-generation chondrocyte was more than 90%, which could satisfy the experimental demand of subsequent research ().
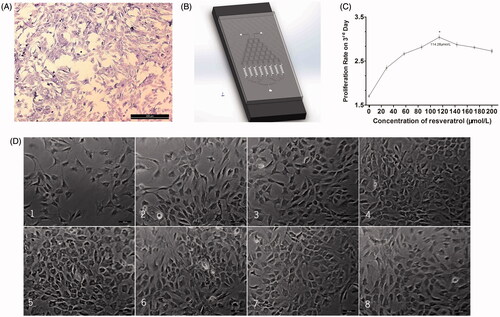
Figure 5. Resveratrol screening on microfluidic device. (A) Toluidine blue staining of chondrocytes. (B) Microfluidic device. (C) Chondrocyte proliferation curve on third day with different concentrations of resveratrol on microfluidic device. (D) The growth conditions of chondrocyte in parallel cell culture chambers. Scale bar =100 μm. Values are presented as mean ± SD. * p < .05.
CGG performance validation of microfluidic device
We used solution PBS (colourless) inject into inlet 1 and solution AO (green colour under fluorescence microscope) inject into inlet 2 in our microfluidic device. The theoretical concentrations should be 0, 1/7R, 2/7R, 3/7R, 4/7R, 5/7R, 6/7R, and 1R in the eight downstream parallel cell culture chambers, respectively. In our study, the experimental data was observed and quantified. There was a good association between experimental data and theoretical data, which proved that our microfluidic device could satisfy the experimental demand of subsequent research ()
Resveratrol screening on microfluidic device
After the chondrocytes were seeded in our microfluidic device, we used complete DMEM medium inject into inlet 1 and complete DMEM medium with Resveratrol (200 μmol/L) inject into inlet 2 in our microfluidic device, eight different concentrations of Resveratrol would be generated. After 72 h, we randomly selected different five visual fields under an inverted microscope and counted the number of chondrocytes directly. The results showed that the proliferation rate of chondrocytes under the concentrations of 114.28 μmol/L Resveratrol was best (3.04 ± 0.05), and there was significant differences compared with other groups ().
Characters evaluation of biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds
Morphology characterization
The PLA/Gelatine nanofibre membranes and 3D nanofibre sponges with various amounts of resveratrol have the similar morphological structures, as the amount of resveratrol is very small compared with Gelatine and PLA so it almost has no effect on the electrospinning process. The digital photographs of resveratrol-loading PLA/Gelatine nanofibre membrane and 3D nanofibre sponge are shown in . We could clearly see that the fibrous membrane was densely packed in the structure while the sponge was loose and porous. shows the SEM images of resveratrol-loading PLA/Gelatine nanofibre membrane. The nanofibrous mats were consisted of almost randomly overlaid nanofibres with diameters ranging from 200 nm to 2200 nm. The morphologies of nanofibres were relatively uniform and basically strip shape. shows the SEM images of resveratrol-loading PLA/Gelatine 3D nanofibre sponge. We could see that the morphologies of resveratrol-loading PLA/Gelatine nanofibres were well retained in 3D scaffolds constituted of electrospun nanofibres. The fibrous structure of scaffolds was similar to natural ECM. The pores on the scaffold and gap spaces between the nanofibres were interconnected with each other, which would be beneficial for nutrition supply and cell migration.
Density and compressive mechanical property
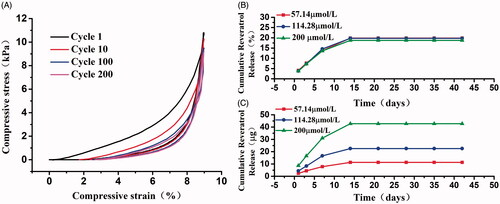
The density of resveratrol-loading PLA/Gelatine 3D nanofibre sponge was 92.68 mg/cm3, which was calculated by formula (1). The nanofibre sponges had good elasticity and mechanical strength and could play a certain role in supporting the repair of cartilage defect. shows a cyclic compression test of 200 loading–unloading fatigue cycles with 60% strain. The nanofibre sponges had a good compression recovery capability. After 200 cyclic compressions, the scaffolds exhibited small plastic deformation (15.73% at 10th, 19.06% at 100th, 19.73% at 200th) and no significant decrease in the strength was observed.
Figure 6. Characters evaluation of biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds. (A) Compressive stress − strain curves of 3D nanofibre scaffold with different cycles; (B) released amount of resveratrol; (C) released percentage of the total resveratrol loading. Values are presented as mean ± SD.
The release characteristics of resveratrol
The accumulated release rates of resveratrol in 57.14 μmol/L, 114.28 μmol/L, and 200 μmol/L drug-loading PLA/Gelatine scaffolds are shown in . Curves of these three kinds of scaffold practically have the same trend. Resveratrol was released in a burst way in the first 7 d, then the release rate slowed down over the 7–14 d, and the release of resveratrol basically ceased after 14 d. The final released percentage of the total loading resveratrol were 19.85%, 19.75%, and 18.72%, which had a slight decline with the increase of resveratrol concentration.
Repair of cartilage defect by biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds and evaluation
General observation
After 3 months, we found that compared with group A, the defects of knee joint were more complete, superficial, and smooth in groups B, C, D, E. The luster and colour were more close to the sham group. It proved that all PLA/Gelatine scaffolds were beneficial to promote the repair of cartilage defects. Furthermore, the repair effects of groups C, D, and E were better than group B, which proved that resveratrol played an important role in the recovery process. Group D (with 114.28 μmol/L resveratrol) had the optimal luster and colour after repair.
Evaluation of microCT reconstruction
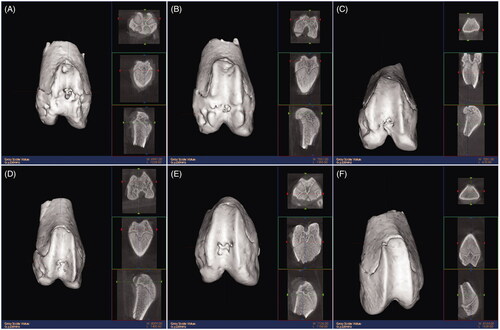
After 3 months, the cartilage tissue of SD rats was scanned by microCT and reconstructed after scanning. Both the scanning plain and reconstructed three-dimensional images showed that cartilage collapse defects in group A were obvious, bone trabecula structure was disorder. In the sham group, the cartilage surface was smooth and the trabecular structure was neat. In group B, the cartilage defect still collapsed but the cartilage surface and scaffold started to integration. The trabecular bone structure remained disordered. In group C, the defect collapse became shallow. The cartilage surface and scaffold integrated more. The trabecular bone structure gradually recovered. In groups D and E, the defect collapses were the shallowest. In group D, the cartilage surface and scaffold were well integrated, a large number of bone trabeculars were generated, and the defect was almost flat and completely fused compared with surrounding cartilage. The structure between the trabecular was intact with good porosity. Group D (with 114.28 μmol/L resveratrol) had the best repair effect for the cartilage defects ().
Immunohistochemistry
HE staining, safranin O-fast green staining, alcian blue staining, and toluene staining were used to dyeing the cartilage to compare the difference between groups. Through these staining methods, we found that in the sham group, a smooth cartilage surface and a clear structure were observed, the cartilage tissue was thick. Surface layer, transitional layer, radiation layer, and calcified layer could be distinguished clearly. A large number of chondrocytes had a moderate size and a regular arrangement. The tidal line was complete and the subchondral bone was regular. All kinds of dyeing were uniform ().
Figure 8. Immunohistochemistry of the cartilage. (A–F) HE staining of the cartilage in repaired groups A–E and sham group, respectively; (G–L) Alcian blue staining of the cartilage in repaired group A–E and sham group respectively; (M–R) Safranin O staining of the cartilage in repaired groups A–E and sham group, respectively; (S–X) Toluidine blue staining of the cartilage in repaired groups A–E and sham group, respectively. Scale bar =100 μm.
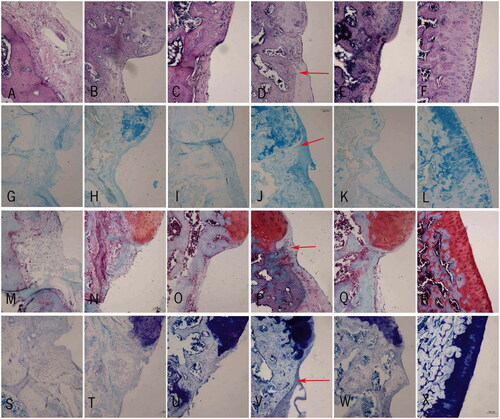
In group A, there was an obvious defect. Four layers of cartilage could not be distinguished. The chondrocytes had different sizes with a disorder distribution. The tidal line was twisted irregularly and blood vessels passed through the tidal line in partial cartilage. The subchondral bone was irregular. All kinds of dyeing were uneven. Safranin O, toluidine blue, and alcian blue were almost absent ().
In group B, the defect started to become shallow. The scaffold started to degrade. Newborn cartilage and chondrocytes could be found between the scaffold and normal cartilage, but the four layers could not be distinguished. The chondrocytes were distributed sporadically in clusters. The subchondral bone was still irregular. Although the scaffold repaired the defect in part, all kinds of dyeing were still uneven and safranin O, toluidine blue, and alcian blue staining were weak ().
In group C, the defect became shallow. The scaffold was partially degradative. There were more newborn cartilage and chondrocytes appearing in the repairing area. Although the chondrocytes distributed still disorder, we could distinguish partial layers of the cartilage. The tidal line was relatively complete. The subchondral bone was a little irregular. All kinds of dyeing started to become even. Safranin O, toluidine blue, and alcian blue staining were deeper ().
In groups D and E, the defects were the shallowest and the scaffolds degraded most. There were a large number of newborn cartilage and chondrocytes in the repairing area. The size of chondrocytes was moderate with a rule distribution. The structure of surface layer, transitional layer, radiation layer, and calcified layer could be distinguished. The tidal line was complete without blood vessels passing through. The subchondral bone was regular. Safranin O, toluidine blue, and alcian blue staining were almost normal. And compared with group E, group D (with 114.28 μmol/L resveratrol) had a better repair effect for the cartilage defects ().
Discussions
It is difficult to repair the defects entirely when articular cartilage suffer damage or degeneration, furthermore, the surrounding cartilages will also change sequentially and destruct by degeneration with time [Citation27]. In recent years, the technology of tissue engineering (TE) has become a hot spot for the repair of articular cartilage defects and a multitude of innovative study including supporting scaffold, biological factor, and seed cells has been conducted and used in the transformation and treatment of associated diseases.
The material and preparation method of tissue engineering scaffold is of great significance to promote the repair effects of articular cartilage defects. The majority of former researches put their attentions on the artificial materials or biological material individually and very few of them try to fabricate the composite scaffold with both types of materials [Citation28,Citation29]. In our study, we established a synthetic polymer (PLA) and biopolymer (Gelatine) composite 3D nano-scaffolds by electrospinning technology, lyophilisation, and crosslinking technology, which overcame the deficiencies of synthetic polymer with low affinity of cells and biopolymer with poor mechanical stability, and integrated the advantages of synthetic polymer with good mechanical stability and biopolymer with good biocompatibility. It showed that our scaffold sufficiently satisfied the basic properties [Citation30] of tissues repair including biocompatibility and mechanical stability. Moreover, the degradable peculiarity in our scaffold and biomimic 3D nano structure with a uniformly distributed and interconnected porous structure on the one hand support the adequate space for the growth and migration of chondrocytes, on the other hand will not generate remnant residue after repair.
To further promote the repair of articular cartilage defects, we added an analogous biological factor named resveratrol in the PLA and Gelatine composite 3D nano-scaffolds. Resveratrol is a kind of strong natural polyphenols that have a lot of benefits for the growth of chondrocytes including anti-senescence, anti-inflammation, and reducing the damage of extracellular matrix, which has been proved in many studies [Citation18–20]. But, how to seek out the appropriate concentrations of resveratrol for the chondrocytes and carry into the scaffold is an awkward problem. The method of using conventional static 96-well plate for cell culture and drug screening is complicated and inaccurate. In our study, we designed and fabricated a microfluidic device screen the proper concentrations of resveratrol for the regeneration of chondrocytes. The results showed that our microfluidic device is an accurate, efficient, and high throughput platform for the drug screening. With the microfluidic device, we successfully obtained eight concentrations of resveratrol for the growth of chondrocytes in the eight downstream parallel cell culture chambers, respectively, and found resveratrol was beneficial to the growth of chondrocytes in a certain extent but not endless. According to the screening results, we selected four concentrations of resveratrol (0, 57.14, 114.28, and 200 μmol/L) to load in the fabrication of PLA and Gelatine composite 3D nano-scaffold. Our microfluidic device could continuously provide cells with fresh nutrition and oxygen by a controlled fluid culture medium, and multiple concentration gradients of resveratrol could be screened effectively.
Seed cell is also playing an important role in the tissue-engineering repair; however, we did not add any seed cells in this study. For the reason that we consider an amount of activated cells including chondrocytes, haemocytes, bone mesenchymal stem cells (BMSCs), etc. will be released around the defects in a short time after the microfracture induced by the articular cartilage defects. Some studies [Citation31,Citation32] reported to use microfracture of joint to stimulate the release of BMSCs and chondrocytes to promote the repair of articular cartilage defects and we adopt the same concept in our study.
In the parts of animal experiments, we compared the repair of cartilage defects from different direction and perspective by biodegradable different concentrations of resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds. After 3 months, the evaluative results through general observation showed that the defects of articular cartilage in groups B, C, D, and E were better than group A, and group D (with 114.28 μmol/L resveratrol) had the optimal luster and colour and more close to sham group, which largely suggested that PLA/Gelatine 3D nanofibre scaffolds could promote the repair of cartilage defects and resveratrol loading in the scaffold could optimize the treatment effects, 114.28 μmol/L was an appropriate concentration of resveratrol. After 3 months, the further evaluative results through microCT reconstruction showed that the cartilage collapse defects in groups B, C, D, and E were lighter, the bone trabecula structures were less disorder, the cartilage surfaces were more smooth, and the trabecular structure were more neat than group A. But in group D, the defect collapses were shallowest, the cartilage surface and scaffold was well integrated, the bone trabecular was generated, and the defect was almost flat and completely fused compared with surrounding cartilage, which supported that PLA/Gelatine 3D nanofibre scaffolds could promote the repair of cartilage defects, resveratrol loading in the scaffold could optimize the treatment effects, and 114.28 μmol/L could be an appropriate concentration of resveratrol. After 3 months, the further evaluation was carried out through immunohistochemistry. HE staining and safranin O- fast green staining could distinguish the structure of each layer in cartilage tissue. Safranin O- fast green staining, toluidine blue staining, and alcian blue staining could better show the changes in cartilage. When the cartilage tissue damaged, safranin O, toluidine blue, and alcian blue were shallow or even loss staining. In our study, the results of immunohistochemistry also showed that PLA/Gelatine 3D nanofibre scaffolds could promote the repair of cartilage defects, resveratrol loading in the scaffold could optimize the treatment effects, and 114.28 μmol/L could be an appropriate concentration of resveratrol.
Conclusions
In summary, we designed and fabricated a practical, high efficient, and accurate microfluidic device for the drug screening in the treatment of articular cartilage defects. With this platform, we successfully obtained the appropriate concentrations of resveratrol for the growth of chondrocytes. In addition, we loaded different concentrations of resveratrol in the construction of biodegradable resveratrol-loading synthetic polymer (PLA) and biopolymer (Gelatine) composite 3D nano-scaffolds, and evaluated the characteristics. At last, we verified the repair effects of articular cartilage by the biodegradable resveratrol-loading PLA/Gelatine 3D nanofibre scaffolds from different direction, and established the optimal resveratrol-loading tissue engineering repair method for articular cartilage defects. It would be possible for materials and medical researchers to explore individualized pharmacotherapy and drug-loading tissue engineering scaffold for the repair of tissue defects in future.
Disclosure statement
The authors declare no conflicts of interest in this paper and are responsible alone for the content and writing.
Additional information
Funding
References
- Zhu W, Guo D, Peng L, et al. Repair of rabbit cartilage defect based on the fusion of rabbit bone marrow stromal cells and Nano-HA/PLLA composite material. Artif Cells Nanomed Biotechnol. 2017;45:115–119.
- Mumme M, Barbero A, Miot S, et al. Nasal chondrocyte-based engineered autologous cartilage tissue for repair of articular cartilage defects: an observational first-in-human trial. Lancet. 2016;388:1985–1994.
- Sun X, Wang J, Wang Y, et al. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artif Cells Nanomed Biotechnol. 2017;6:1–10.
- Schagemann JC, Rudert N, Taylor ME, et al. Bilayer implants: electromechanical assessment of regenerated articular cartilage in a sheep model. Cartilage. 2016;7:346–360.
- Bahney CS, Jacobs L, Tamai R, et al. Promoting endochondral bone repair using human osteoarthritic articular chondrocytes. Tissue Eng Part A. 2016;22:427–435.
- Muiños-López E, Hermida-Gómez T, Fuentes-Boquete I, et al. Human amniotic mesenchymal stromal cells as favorable source for cartilage repair. Tissue Eng Part A. 2017;23:901–912.
- Armiento AR, Stoddart MJ, Alini M, et al. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2017;65:1–20.
- Brittberg M, Gomoll AH, Canseco JA, et al. Cartilage repair in the degenerative ageing knee. Acta Orthop. 2016;87:26–38.
- Christensen BB, Foldager CB, Olesen ML, et al. Experimental articular cartilage repair in the Göttingen minipig: the influence of multiple defects per knee. J Exp ORTOP. 2015;2:13.
- De Windt TS, Vonk LA, Brittberg M, et al. Treatment and prevention of (Early) osteoarthritis using articular cartilage repair-fact or fiction? A systematic review. Cartilage. 2013;4:5S–12S.
- Yodmuang S, Mcnamara SL, Nover AB, et al. Silk microfiber-reinforced silk hydrogel composites for functional cartilage tissue repair. Acta Biomater. 2015;11:27.
- Zhang F, He C, Cao L, et al. Fabrication of gelatin-hyaluronic acid hybrid scaffolds with tunable porous structures for soft tissue engineering. Int J Biol Macromol. 2011;48:474–481.
- Shi W, Sun M, Hu X, et al. Structurally and functionally optimized silk‐fibroin–gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater. 2017;29:1701089.
- Si Y, Yu J, Tang X, et al. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat Commun. 2014;5:5802.
- Xu T, Miszuk JM, Zhao Y, et al. Electrospun polycaprolactone 3D nanofibrous scaffold with interconnected and hierarchically structured pores for bone tissue engineering. Adv Healthc Mater. 2015;4:2238.
- Grémare A, Guduric V, Bareille R, et al. Characterization of printed PLA scaffolds for bone tissue engineering. J Biomed Mater Res A. 2017. DOI:https://doi.org/10.1002/jbm.a.36289
- Pal A, Pathak C, Vernon B. Synthesis, characterization and application of biodegradable polymer grafted novel bioprosthetic tissue. J Biomater Sci Polym Ed. 2017;29:217–235.
- Oh SH1, Lee JH. Hydrophilization of synthetic biodegradable polymer scaffolds for improved cell/tissue compatibility. Biomed Mater. 2013;8:014101.
- Bertram U, Steiner D, Poppitz B, et al. Vascular tissue engineering: effects of integrating collagen into a PCL based nanofiber material. Biomed Res Int. 2017;2017:9616939.
- Lo KW, Ulery BD, Kan HM, et al. Evaluating the feasibility of utilizing the small molecule phenamil as a novel biofactor for bone regenerative engineering. J Tissue Eng Regen Med. 2014;8:728–736.
- Buhrmann C, Popper B, Aggarwal BB, et al. Resveratrol downregulates inflammatory pathway activated by lymphotoxin α (TNF-β) in articular chondrocytes: comparison with TNF-α. PLoS One. 2017;12:e0186993.
- Wu G, Wang L, Li H, et al. Function of sustained released resveratrol on IL-1β-induced hBMSC MMP13 secretion inhibition and chondrogenic differentiation promotion. J Biomater Appl. 2016;30:930–939.
- Qin N, Wei L, Li W, et al. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J Pharmacol Sci. 2017;134:166–174.
- Zitzmann FD, Jahnke HG, Pfeiffer S, et al. Microfluidic free flow electrophoresis based solvent exchanger for continuously operating lab-on-chip applications. Anal Chem. 2017;89:13550–13558.
- Liu BM, Li M, Yin BS, et al. Effects of incorporating carboxymethyl chitosan into pmma bone cement containing methotrexate. PLoS One. 2015;10:e0144407.
- Chen W, Chen S, Morsi Y, et al. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl Mater Interfaces. 2016;8:24415–24425.
- Chen S, Fu P, Wu H, et al. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell Tissue Res. 2017;370:53–70.
- Almqvist KF, Dhollander AA, Verdonk PC, et al. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am J Sports Med. 2009;37:1920–1929.
- Wang SQ, Xia J, Chen J, et al. Influence of biological scaffold regulation on the proliferation of chondrocytes and the repair of articular cartilage. Am J Transl Res. 2016;8:4564–4573.
- Lazzeri L, Cascone MG, Danti S, et al. Gelatine/PLLA sponge-like scaffolds: morphological and biological characterization. J Mater Sci: Mater Med. 2006;17:1211–1217.
- Shi W, Sun M, Hu X, et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv Mater. 2017;29:1701089.
- Meng Q, Hu X, Huang H, et al. Microfracture combined with functional pig peritoneum-derived acellular matrix for cartilage repair in rabbit models. Acta Biomater. 2017;53:279–292.