Abstract
Literature search revealed no systematic report on iron oxide nanoparticle-incorporating calcium phosphate cement scaffolds (IONP-CPC). The objectives of this study were to: (1) use γFe2O3 nanoparticles (γIONPs) and αFe2O3 nanoparticles (αIONPs) to develop novel IONP-CPC scaffolds, and (2) investigate human dental pulp stem cells (hDPSCs) seeding on IONP-CPC for bone tissue engineering for the first time. IONP-CPC scaffolds were fabricated. Physiochemical properties of IONP-CPC scaffolds were characterized. hDPSC seeding on scaffolds, cell proliferation, osteogenic differentiation and bone matrix mineral synthesis by cells were measured. Our data demonstrated that the osteogenic differentiation of hDPSCs was markedly enhanced via IONP incorporation into CPC. Substantial increases (about three folds) in ALP activity and osteogenic gene expressions were achieved over those without IONPs. Bone matrix mineral synthesis by the cells was increased by two- to three folds over that without IONPs. The enhanced cellular osteogenesis was attributed to: (1) the surface nanotopography of IONP-CPC scaffold, and (2) the cell internalization of IONPs released from IONP-CPC scaffold. Our results demonstrate that the novel CPC functionalized with IONPs is promising to promote osteoinduction and bone regeneration. In conclusion, it is highly promising to incorporate γIONPs and αIONPs into CPC scaffold for bone tissue engineering, yielding substantially better stem cell attachment, spreading and osteogenic differentiation, and much greater bone mineral synthesis by the seeded cells. Therefore, novel CPC scaffolds containing γIONPs and αIONPs are promising for dental, craniofacial and orthopaedic applications to substantially enhance bone regeneration.
Introduction
Bone defects and non-unions are a significant worldwide problem. The annual healthcare costs plus lost wages for people in the USA with musculoskeletal diseases reached $849 billion in 2004, or 7.7% of the gross domestic product [Citation1]. Furthermore, the cost is increasing dramatically as the population ages [Citation2]. Tissue engineering approaches offer immense potential to enhance the repair and regeneration efficacy and reduce the costs [Citation3–9]. Bone tissue engineering involves the use of biomaterials and cells. Iron oxide nanoparticles (IONPs) are safe for in vivo applications and are of great importance in biomedical and technological fields [Citation10,Citation11]. Among the various forms of iron oxides, maghemite (γFe2O3) and hematite (αFe2O3) are the most commonly used [Citation12]. γFe2O3 and αFe2O3 are different in many aspects. The unit cell of γFe2O3 is cubic, with both octahedrally and tetrahedrally coordinated Fe3+ sites (defect spinel structure). In contrast, αFe2O3 has a hexagonal unit cell and entirely octahedrally coordinated Fe3+ atoms (corundum structure). In addition, γFe2O3 is magnetic, while αFe2O3 is not [Citation12]. γFe2O3 nanoparticles (γIONPs) have numerous applications including serving as contrast agents for magnetic resonance imaging (MRI), magnetically-guided drug or gene delivery, cell targeting, cancer therapy [Citation13] and combinations of multiple applications. On the other hand, αFe2O3 nanoparticles (αIONPs) exhibit a high resistance to corrosion and have been used in many fields, including photo-anode for photo-assisted electrolysis of water. αIONPs are active components of gas sensors, catalyst, lithium ion battery, pigments and oxidizer in thermite composition [Citation14,Citation15].
There have been several reports on the effects of γIONPs on bone repair [Citation16–19]. Electrospun scaffolds containing γIONPs and hydroxyapatite nanoparticles (nHA) in poly (lactide acid) (PLA) enhanced bone repair [Citation16,Citation17]. An in vitro study suggested that magnetic field-induced assemblies of γIONPs promoted the differentiation of primary bone marrow cells into osteoblasts, indicating an improved bone repair should be achieved due to the accelerated cellular osteogenesis [Citation18]. A recent study investigated the direct biologic effects of γIONPs on cellular osteogenesis [Citation19]. A surprising osteogenic induction ability of γIONPs was detected even without the application of a magnetic field. However, little has been reported on the effects of αIONPs on bone tissue engineering.
Calcium phosphate cements are promising scaffolds for use in craniofacial and orthopaedic repairs, including the reconstruction of frontal sinus, augmentation of craniofacial skeletal defects, use in endodontics and the repair of periodontal bone defects [Citation20,Citation21]. The powder phase can be mixed with an aqueous liquid to form a paste that can be sculpted during surgery to conform to the defects in hard tissues. The paste self-hardens to form resorbable hydroxyapatite (HA) [Citation22]. Calcium phosphate cements have many advantages, including self-setting in vivo without producing heat, conformation to osseous defects with complex shapes, inexpensive and relatively easy to fabricate. The first calcium phosphate cement to win the approval of the Food and Drug Administration (FDA) was based on tetracalcium phosphate [TTCP: Ca4(PO4)2 O] and dicalcium phosphate anhydrous [DCPA: CaHPO4] (referred to as CPC) [Citation23]. In addition, there were other calcium phosphate cements with different compositions [Citation24,Citation25].
Efforts were made to enhance the osteoinduction of CPC via the incorporation of bioactive agents including drugs [Citation26], growth factors [Citation27], nanoparticles and nanostructures [Citation28]. Nanomaterials have great potential for bone regeneration [Citation29]. There were only a few reports on incorporating αIONPs and γIONPs into calcium phosphate cements [Citation30,Citation31]. The injectability and compressive strength of the cements were improved by αIONP powder incorporation, without adversely affecting the physicochemical setting reactions and cytocompatibility [Citation30]. However, there has been no report on the osteogenic behavior of this αIONP-modified calcium phosphate cement. The addition of γIONP powder into disodium hydrogen phosphate (Na2HPO4) solution and then mixing with α-TCP powder enhanced the mechanical strength and cellular behavior including cell adhesion, proliferation and osteogenic differentiation [Citation31]. The improved biological performance in that study was attributed to the reduced crystal size, without referring to any magnetic effects. There was no mention of the release from the scaffold and the intake by the cells of IONPs, and the cell internalization of magnetic nanoparticles inside the osteogenic cells.
Human dental pulp stem cells (hDPSCs) are postnatal stem cells with similar gene expressions and differentiation to that of bone marrow mesenchymal stem cells (BMSCs), which require an invasive procedure to obtain [Citation32]. hDPSCs can differentiate into odontoblast-like and osteoblast-like cells by a variety of inducing reagents such as dexamethasone (Dex), β-glycerophosphate (β-GP) and 1,25-dihydroxyvitamin D, and can help repair the dentin and bone after injury [Citation33]. hDPSCs are a promising cell source for bone regeneration due to the following advantages: its collection site is easy and produces very low morbidity; the extraction of stem cells from pulp tissues is highly efficient; and they have extensive differentiation ability and can attach to biomaterials, which makes them ideal for tissue reconstruction [Citation34].
Therefore, the objectives of this study were to develop a novel CPC scaffold containing αIONPs and γIONPs (IONP-CPC) by using a well-dispersed hydrophilic IONP solution as the CPC liquid, and investigate the osteogenic differentiation of hDPSCs seeded on IONP-CPC for bone tissue engineering for the first time. The following hypotheses were tested: (1) the addition of IONPs would improve the properties of CPC, thus enhancing the bone repair efficacy; (2) the osteogenic gene expressions and alkaline phosphatase (ALP) protein synthesis of hDPSCs would be greatly enhanced via IONP incorporation; and (3) the bone mineral synthesis by stem cells would be substantially increased by adding IONPs into the CPC scaffold.
Materials and methods
Preparation of CPC powder
TTCP was synthesized from a solid-state reaction between DCPA and CaCO3 (J.T. Baker, Philipsburg, NJ, USA), then ground in a blender to obtain particle sizes of 1–80 μm (median = 5 µm), as previously reported [Citation21]. DCPA was ground to obtain particle sizes of 0.4–3.0 μm (median = 1.0 µm). The TTCP and DCPA powders were mixed at a molar ratio of 1:3 to form CPC powder.
Preparation and characterization of IONPs
γIONPs was synthesized via a modified chemical co-precipitation method using ferrous chloride hexahydrate and ferrous chloride tetra-hydrate (Sigma-Aldrich, St. Louis, MO, USA) as precursors [Citation35]. Briefly, 0.2 g of polyglucose-sorbitol-carboxymethylether (PSC) was dissolved in 10 mL deionized water, then a mixture of 0.06 g of FeCl3 and 0.03 g of FeCl2 in 15 mL deionized water was added. This mixture was cooled to 5 °C, and 1 g of 28% ammonium hydroxide was added with stirring for 2 min. The mixture was heated at 80 °C for 1 h and purified with five cycles of ultrafiltration against deionized water using a 100 kDa membrane. This yielded PSC-coated γIONPs. αIONPs were obtained from Aladdin Chemicals (Shanghai, China) and then surface-modified by dimercaptosuccinic acid (DMSA; Aladdin Chemicals, Shanghai, China). Generally, the pH and concentration of αIONP solution were adjusted to 2.7 and 2 mg/mL, respectively. Then, DMSA solution was added into the system to react for 5 h. Finally, the impurity was removed by dialysis and centrifugation [Citation36]. This produced DMSA-coated αIONPs. The colloidal suspension of IONPs was examined with transmission electron microscope (TEM; JEOL-7100, Tokyo, Japan). The hydrodynamic diameters of nanoparticles were measured by dynamic light scattering (DLS) with a particle size analyzer (Malvern Zetasizer Nano ZS90, Malvern, UK). The magnetic properties were measured by a vibrating sample magnetometer (VSM; Lakeshore 7470, Lake Shore Cryotronics, Lake Shore, CA, USA) in an applied magnetic field of up to 20 kOe, to determine saturation magnetization and hysteresis loops. VSM was calibrated using a standard reference (high-purity nickel sphere), supplied with the instrument.
Preparation and characterization of IONP-CPC scaffold
The IONP liquid was used as the CPC liquid. The scaffold was prepared at a CPC powder to liquid mass ratio of 2:1. Three groups were fabricated:
CPC control: CPC powder and distilled water as the CPC liquid;
γIONP-CPC: CPC powder with γIONPs solution as the CPC liquid (24 mg/mL);
αIONP-CPC: CPC powder with αIONPs solution as the CPC liquid (2.55 mg/mL).
Unless otherwise specified, the specimen was incubated for one day in water at 37 °C. Scanning electron microscopy (SEM; JEOL JSM-840, Tokyo, Japan) with energy dispersive X-ray spectrometry (EDS; EX-250, HORIBA, Tokyo, Japan) was used to investigate the microstructure of the IONP-CPC scaffold. The samples were sputtered with platinum prior to analysis. The phase composition was evaluated via X-ray diffraction (XRD; X'TRA, ARL Ltd, Bern, Switzerland) with Ni-filtered Cu Kα radiation. Step-scanning was performed at an integration time of 50 s with intervals of 0.02° (2θ). Peak indexing was carried out by means of ICSD 64599 for Fe2O3, ICSD 22059 for HA.
An in vitro degradation test was performed as previously reported [Citation37]. Briefly, CPC samples were prepared (6 mm in diameter and 1 mm in thickness), dried and weighed. After soaking in a demineralizing solution (1.15 mmol/L Ca, 1.2 mmol/L P, 133 mmol/L NaCl, pH adjusted to 3–5 by adding HCl or NaOH) for a certain time, the samples were taken out, dried and weighed again. Nine specimens were prepared for each pH (pH = 5.5 and 4). The mass loss of each sample was calculated as follows: Mass loss = (sample weight before immersion – sample weight after immersion)/sample weight before immersion. Released iron content was determined by spectrophotometry. The iron content in IONP-CPC and that released after four weeks of immersion in 1 × phosphate buffered saline (PBS) was detected by atomic absorption spectroscopy (AAS; 180-80, Hitachi, Tokyo, Japan).
Measurements of surface contact angles
Surface contact angles were investigated using a contact angle meter SL200B (Solon Technology Science, Shanghai, China) [Citation38]. To detect water spreading area on CPC disks, a neutral red solution (Sigma-Aldrich, St. Louis, MO, USA) was used. An equal amount of neutral red solution was dropped on each disk, and the water spreading area was measured using Image-Pro Plus 6.0 software (Media Cybernetics, Sliver Spring, MD, USA).
Protein adsorption test
Protein adsorption on CPC scaffolds was measured following previous reports [Citation39]. Scaffold samples were immersed in 1 × PBS for 2 h. The samples then were immersed in a bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO, USA) solution at 37 °C for 12 h, which contained BSA at a concentration of 4.5 g/L. The disks were then rinsed with fresh PBS and immersed in 1% sodium dodecyl sulfate (SDS; Sigma-Aldrich, St. Louis, MO, USA) in PBS. A Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, MA, USA) was used to determine the BSA concentration in the SDS solution. Nine CPC disks were evaluated for each group.
Isolation and culture of hDPSCs
Human dental pulp stem cells (hDPSCs) were obtained from the pulps of healthy teeth from a 14-year-old patient who had his teeth extracted due to orthodontic treatment. This was performed with proper consent and without patient identification, and was approved by the Institutional Review Board of the University of Maryland at Baltimore (HP-00040437). Cells were isolated as described previously [Citation40]. Briefly, the pulp tissues were minced and digested in a solution of 3 mg/mL of collagenase type I and 4 mg/mL dispase for 30–60 min at 37 °C. Cell suspension was obtained by passing the digested tissue through a 70 μm cell strainer. The cells were pelleted and seeded in culture dishes, and incubated with DMEM growth medium (DMEM +10% fetal bovine serum +1% penicillin/streptomycin, Gibco, Grand Island, NY, USA) in a humidified atmosphere of 5% CO2 and 95% air. Non-adherent cells were removed at 48 h after the initial plating. The medium was replaced every three days. When the primary culture became subconfluent after approximately one to two weeks, cells were collected by trypsinization and subcultured at 5000 cells/cm2 in a growth medium. The fourth passage of hDPSCs was used in the following experiments, to ensure the high purity and bioactivity of the stem cells.
Attachment and spreading of hDPSCs on CPC scaffolds
Cellular imaging on CPC after seeding was performed by immersing the scaffold in a live/dead staining solution (Invitrogen, Carlsbad, CA, USA). Cells were examined via epifluorescence microscopy (Eclipse TE-2000 S, Nikon, Tokyo, Japan). Three images were taken at random locations for each sample, with six samples yielding 18 images for each group at each time point. The images were analysed by Image-Pro Plus 6.0 software. Live cell spreading area was calculated as: S = Stotal/NLive, where Stotal is the total cell spreading area in the image, NLive is the number of live cells [Citation39]. A cell counting kit (CCK-8, Enzo Biochem, New York, NY, USA) was used to evaluate the adhered cell ratio normalized by culture well control at 4 h after seeding. After incubating with 10% CCK-8/media in the dark for 2 h, optical density (OD) was read at a wavelength of 450 nm. Cell adhesion ratio = OD value of scaffold group/OD value of the culture well control [Citation31].
Cell proliferation assay
The same CCK-8 kit was used to evaluate cell proliferation at 1, 4, 7 and 14 days [Citation41]. The cell proliferative rate was determined via the absorbance at OD 450 nm using a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA, USA).
ALP activity assay
At 4, 7 and 14 days, the cells were lysed and assayed for ALP activity using a Wako ALP kit (Wako Pure Chemical, Osaka, Japan) [Citation41]. ALP activity (n = 6) was normalized by the total protein from each sample, which was quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) [Citation41].
Quantitative reverse transcription–polymerase chain reaction (qRT-PCR)
qRT-PCR was used to examine the expression of osteogenic genes, including alkaline phosphatase (ALP), collagen type Iα (COLIα), runt-related transcription factor 2 (RUNX2), and osteocalcin (OCN). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene. Primer sequences for the above genes are listed in Table S1. At 7 days and 14 days [Citation42], the total RNA of the collected cells was extracted with TRIzol reagent and PureLink RNA Mini Kit (Invitrogen, Carlsbad, CA, USA), and reverse-transcribed into cDNA using high-capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) in a thermal cycler (GenAmp PCR 2720, Applied Biosystems, CA, USA). qRT-PCR was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) in the ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Relative expression was evaluated using the 2-ΔΔCt method and normalized by the Ct of the housekeeping gene GAPDH [Citation42]. Ct of hDPSCs cultured on CPC control served as their own calibrator at each time period.
Alizarin red S (ARS) staining for mineralization by the cells
After 7, 14 and 21 days [Citation41], the cell-scaffold constructs were washed with PBS, fixed in 10% formaldehyde, and staining with 2% (mass/volume) ARS (Millipore, Billerica, MA, USA). Quantification of cell mineralization was performed by measuring the absorbance at 550 nm after eluting the ARS deposit with 10% (mass/volume) cetylpyridinium chloride (Sigma-Aldrich, St. Louis, MO, USA) [Citation43]. The amount of cell-synthesized minerals on CPC control served as their own calibrator at each time period.
TEM examination
To detect whether there were IONPs internalized by the hDPSCs, the cell samples were prepared seven days after seeding. They were observed by TEM (Tecnai™ G2 Spirit Twin, FEI, Hillsboro, OR, USA). Quantitative measurement of iron content inside the cells was done by inductively coupled plasma optical emission spectrometry (ICP-OES; Optima 5300DV, PerkinElmer, Waltham, MA, USA).
Statistical analysis
All quantitative data were expressed as mean value and standard deviation (SD). A SPSS statistical package (version 22.0; IBM, Armonk, New York, NY, USA) was used for statistical analysis. Group comparisons were conducted by one-way ANOVA with Bonferroni post-hoc tests. Differences were considered significant if p < .05 and highly significant if p < .01.
Results
Characterization of IONPs and IONP-CPC
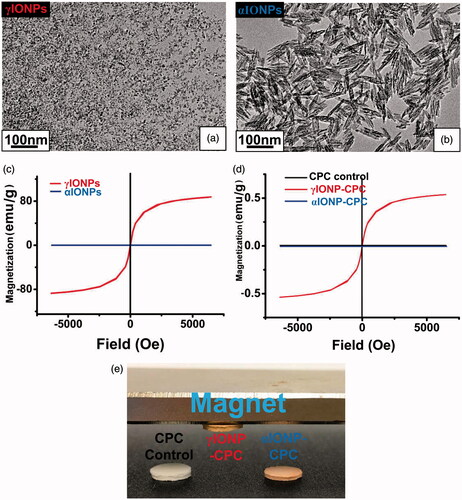
γIONPs were spherical and 7–8 nm in average diameter (). αIONPs were spindle-shaped, with average dimensions of 10 × 90 nm (). The main parameters of IONPs are shown in Table S2. The two groups both had low polydispersity index (PDI), indicating narrow size distributions. These results are consistent with the TEM examination. In addition, the nanoparticles were negatively-charged from the coating on the particle surfaces. The magnetic properties of IONPs were investigated by observing the magnetization curve of samples according to variations in the magnetic field. While αIONPs showed no magnetization behavior, γIONPs exhibited typical magnetization behavior, confirming its super-paramagnetic properties (). The incorporation of γIONPs endowed super-paramagnetic properties to the scaffolds, as γIONP-CPC was attracted by the magnet, while CPC control and αIONP-CPC were not (). This was further confirmed by the VSM results, presenting as overlap magnetic hysteresis loop, which indicates that γIONP-CPC was super-paramagnetic ().
Figure 1. Fabrication of iron oxide nanoparticle-containing CPC (IONP-CPC). (a) TEM image of γIONPs. (b) TEM of αIONPs. (c) Magnetic hysteresis loops of γIONPs and αIONPs, showing that γIONPs were super-paramagnetic, while αIONPs were not. (d) The overlap magnetic hysteresis loop of VSM results confirmed that γIONP-CPC was super-paramagnetic, while CPC control and αIONP-CPC were not super-paramagnetic. (e) CPC control, γIONP-CPC and αIONP-CPC. Only γIONP-CPC was attracted by a magnet.
The surface morphology of the scaffolds was examined by SEM. CPC control had particulates, which consisted of small crystallites and numerous pores (Figure S1(a)). IONP-CPC had more micropores (Figure S1(b, c)). γIONP-CPC appeared to be more porous than αIONP-CPC. EDS detected iron content in γIONP-CPC, but not αIONP-CPC (Figure S2). AAS was used to determine the iron content in IONP-CPC scaffold, which was about 1% in γIONP-CPC and 0.05% in αIONP-CPC.
According to the XRD spectrum, the characteristic peaks of Fe2O3 were detected in γIONP-CPC (marked by * in Figure S1(e)). However, the peaks were not very sharp. This was consistent with the low-iron content in γIONP-CPC and even lower iron content in αIONP-CPC. The incorporation of IONPs into CPC neither increased nor decreased the mechanical strength, as no significant changes in mechanical properties of CPC scaffolds were detected (p > .05, Figure S3).
The degradation and iron release from CPC scaffolds were measured. No differences were found when the three groups were tested in pH 5.5 physiological-like solution (Figure S4(a)). However, when immersed in pH 4 physiological-like solution, the degradation rate was different. IONP-CPC degraded significantly faster than CPC control (p < .01, Figure S1(f)). No differences were found between γIONP-CPC and αIONP-CPC (p > .05).
More iron was released from γIONP-CPC than from αIONP-CPC (Figure S4(b,c,d)). The iron release increased with a longer immersion time and a lower pH value. Even in 1 × PBS at pH 7.4, the iron release was still detectable (Figure S4(d)).
Surface properties of IONP-CPC scaffold
The results of water contact angle are shown in Figure S5. All three groups are hydrophilic with water contact angles near 0 (Figure S5(a,b,c), but differences were found in water spreading areas. The drop spreading area on γIONP-CPC and αIONP-CPC surfaces was larger than that on CPC control, and quantitative analysis proved significant area increases in IONP-CPC (p < .01, Figure S5(d)). γIONP-CPC had a larger spreading area than αIONP-CPC (p < .01). Protein adsorption was examined after soaking the scaffolds in a protein medium for 12 h, as shown in Figure S5(e). The amount of protein adsorbed was also significantly increased in IONP-CPC (p < .01). γIONP-CPC adsorbed more protein than αIONP-CPC (p < .05).
Biological properties of IONP-CPC
hDPSCs adhesion and spreading were examined via fluorescence microscopy at 4 h (). While cells on CPC control showed limited spreading, those on γIONP-CPC had highly extended cytoskeletal processes. It was also proved by SEM cell morphology on the scaffolds four days after seeding (data not shown). Cell spreading area, quantified from fluorescence images, showed significant improvement on γIONP-CPC (more than two-fold increase), compared to CPC control (). The ratio of adhered cells normalized by culture well control was calculated. The results showed that more cells adhered to γIONP-CPC (p < .01) and αIONP-CPC (p < .05) than CPC control (). Cell spreading was greater (p < .01) and appeared to adhere better (p < .05) on γIONP-CPC than on αIONP-CPC.
Figure 2. IONP-CPC promoted the adhesion, spreading and proliferation of hDPSCs. (a) Images of live cells stained with Calcein AM (green) at different time points after seeding: 4 h and 14 days. (b) Cell spreading area on the scaffold. (c) Cell adhesion ratio normalized by culture well control. (d) Cell proliferation on the scaffold by CCK-8 (n = 4). Cells on γIONP-CPC were significantly more than CPC control. Cell spreading on γIONP-CPC was significantly greater than that on CPC control. In each plot, bars indicated by different letters are significantly different from each other (p < .05).
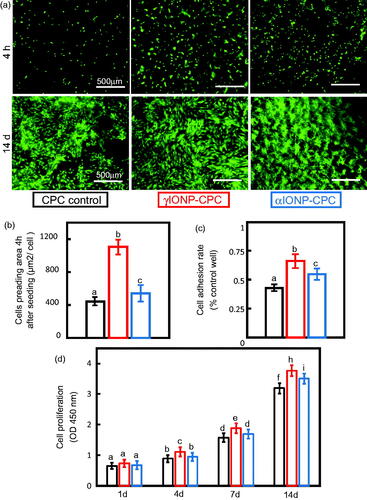
The proliferation of hDPSCs was examined (). The OD value increased with time. γIONP-CPC started to show an advantage in promoting cell proliferation as early as day 4 (p < .05), and even more at day 14 (p < .01). αIONP-CPC did not enhance cell proliferation till day 14 (p < .05). Live/dead staining revealed almost no dead cells. Typical images at day 14 were presented, and they were consistent with the quantitative CCK-8 results, showing continuous increases in cell numbers and more and thicker cells on IONP-CPC than on CPC control ().
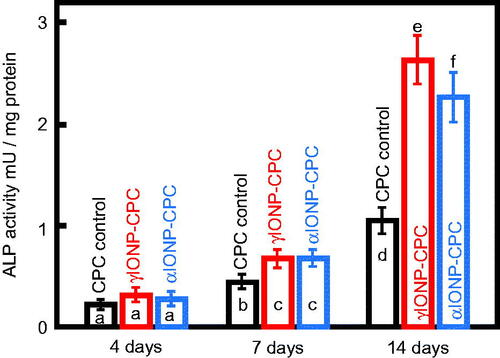
ALP activity was tested up to 14 days (). All three groups had steady increases from day 4 to 7, and a greater increase from day 7 to 14. IONP-CPC showed greater increases at day 7 (p < .05) and day 14 (p < .01) than CPC control. γIONP-CPC showed greater increases than αIONP-CPC at 14 days (p < .05).
Figure 3. ALP activity of hDPSCs cultured on CPC control, γIONP-CPC and αIONP-CPC at 4, 7 and 14 days (n = 6). ALP activity of hDPSCs on γIONP-CPC was significantly higher than that on CPC control at 7 and 14 days. In each plot, bars indicated by different letters are significantly different from each other (p < .05).
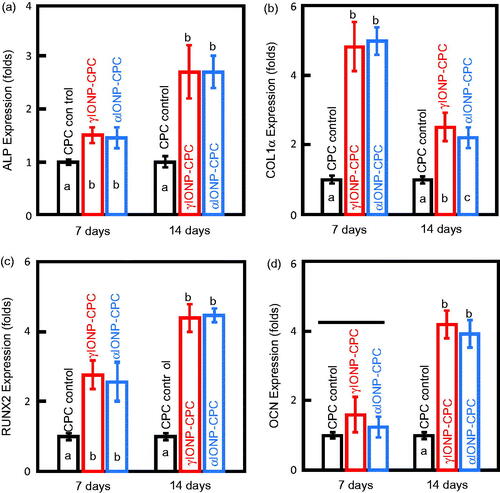
The osteoblast-specific mRNA expressions, including ALP, COLIα, RUNX2 and OCN genes, were compared to CPC control at 7 days and 14 days (). IONP-CPC exhibited significantly higher expressions of ALP, COLIα and RUNX2 at 7 days than CPC control (p < .01). However, the expression of OCN was similar among the three groups at 7 days (p > .05). At 14 days, higher levels of ALP, RUNX2 and OCN genes were detected in IONP-CPC. The expression of OCN was increased significantly (p < .01), by almost four-folds. Comparatively, the fold of increase for COLIα was less than that at seven days, although it was still greater than CPC control.
Figure 4. The mRNA expression levels of osteogenic genes in hDPSCs at 7 days and 14 days, with all data relative to hDPSCs on CPC control. (a) Expression levels of ALP. (b) Expression levels of COLIα. (c) Expression levels of RUNX2. (d) Expression levels of OCN (n = 3). In each plot, bars with different letters are significantly different (p < .05).
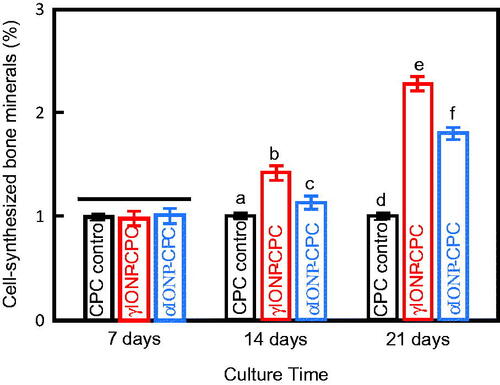
Mineral synthesis by the cells was measured. From 7 to 21 days, the red staining of the synthesized bone mineral on the scaffold surface became denser and a darker red. The mineralization was increased on IONP-CPC. The quantitative results are plotted (). No significant differences among the three groups were found at seven days (p > .05). IONP-CPC showed more mineral synthesis at 14 days (p < .01 in γIONP-CPC, and p < .05 in αIONP-CPC), and became more significant at 21 days (p < .01), with nearly two-fold increase over CPC control. γIONP-CPC showed more mineral synthesis than αIONP-CPC at 14 days and 21 days (p < .01). These results indicate that IONP in CPC promoted the mineral synthesis of hDPSCs.
IONP internalization by hDPSCs
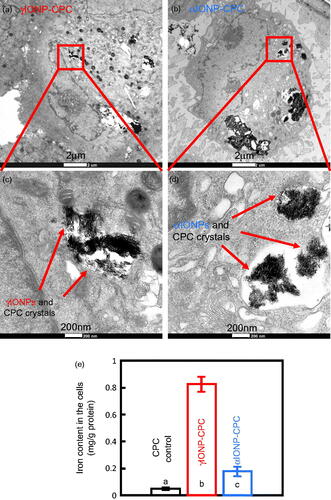
TEM was done to detect the endocytosed IONPs by hDPSCs on IONP-CPC. The images showed that the nanostructured aggregates were taken up by the hDPSCs (). The endocytotic aggregates were in perinuclear vesicular structures close to the cell nucleus. Most of the internalized aggregates were detected in cytosol in the endosomal vesicles. To confirm that there were IONPs in these aggregates inside the cells, quantitative measurement of iron content inside the cells was performed via ICP-OES (). Indeed, more iron was detected in the cells on γIONP-CPC and αIONP-CPC (p < .01).
Figure 6. High-magnification TEM images inside the cells, showing hDPSCs with endocytic IONPs. (a) Cells seeded on γIONP-CPC contained internalized nanoaggregates inside the cells. (b) Cells seeded on αIONP-CPC had internalized nanoaggregates inside the cell. (c) Quantitative measurement of iron content by ICP-OES. Data = mean ± SD (n = 4). Bars indicated by different letters are significantly different from each other (p < .05).
Effects of magnetism in enhancing cellular osteogenic differentiation
To detect whether magnetic effects from γIONP-CPC scaffold contributed to the enhanced cellular effects, a group of demagnetized γIONP-CPC were prepared by heating at 120 °C for over 8 h (marked as DγIONP-CPC). It is well known that this high temperature annealing demagnetizes the material [Citation18]. The cellular behaviours on DγIONP-CPC were tested in the same way as γIONP-CPC. No difference was found between DγIONP-CPC and γIONP-CPC (Figures S6 and S7). Further tests were done to confirm that demagnetization did not cause changes in γIONP-CPC microstructure. SEM (Figure S1(d)), AFM (Figure S8(a–d)) and XRD (Figure S8(e)) were performed to confirm that there were no noticeable changes in the microstructure and crystalline phases in γIONP-CPC and DγIONP-CPC. Therefore, the only difference between γIONP-CPC and DγIONP-CPC was magnetism. Therefore, it seemed that magnetism did not give significant influence here without an external magnetic field.
In addition, gene expression of an exogenous magnetoreceptor, an iron-sulfur cluster assembly protein 1 (ISCA1), was examined to confirm the results. An up-regulated expression of ISCA1 would be found if magnetism had significant effects [Citation44]. However, there were no significant differences in its expression among CPC control, γIONP-CPC and DγIONP-CPC (p > .05, Figure S7(e)). These results demonstrate that magnetism had little effect on the hDPSC behaviour on the scaffolds.
Discussion
In this study, we added stable colloidal solutions of IONPs as CPC liquid, including γIONPs and αIONPs, to improve the injectable CPC scaffold for bone tissue engineering. This represents the first report on incorporating IONPs into CPC scaffold, yielding substantial enhancements in stem cell spreading, osteogenic differentiation and bone mineral synthesis by the cells. The hypotheses were proven that the addition of IONPs improve the properties of CPC such as wetting and protein adsorption as well as cell attachment and spreading; and the osteogenic differentiation of hDPSCs were greatly improved via IONPs incorporation in CPC, demonstrating substantial increases in ALP activity and osteogenic gene expressions, and a two- to three-fold increase in bone matrix mineral synthesis than those without IONPs.
Efforts were made to explore the underlying mechanisms. First, the altered surface morphology, i.e. the change in surface crystal shape and size and surface topography, may have contributed to the cellular behavior. As confirmed by XRD spectrum, the incorporation of IONPs neither hampered the crystallization process, nor changed the crystal phase, compared to CPC control. However, the microstructure was changed due to IONP addition. It was obvious that the crystal size was decreased, which could increase the surface area thus providing more space where protein molecules could adhere. These proteins may involve in cell adhesion, possibly increasing the cell recognition sites and adhesion process [Citation45]. The surface-bound protein layer is also influenced by geometrical features besides physical and chemical surface properties of the biomaterial [Citation46]. The proteins may adapt their conformation to the nanostructured surface, which may have a direct impact on the availability of epitopes and thereby on their recognition by specific cell surface receptors [Citation47]. Therefore, the nanotopography of IONP-CPC could be an influencing factor in cellular attachment.
Second, IONPs released from IONP-CPC and internalized by the cells may affect the biological behavior of the cells. The incorporated IONPs in calcium phosphate cements could be released. The released nanoparticles can interact with the cells by membrane adsorption and subsequently be internalized through endocytosis. This could induce the osteogenic differentiation by activating the pathways such as the classical MAPK pathway to regulate downstream events that were closely related to osteogenesis [Citation19]. Mechanical stress signals in promoting osteogenic differentiation may be produced in the beginning of the interactions between the cell membrane and the IONPs [Citation48]. Thus, IONPs can promote osteogenic differentiation of stem cells like BMSCs. Internalized nanoaggregates were seen in cells on both γIONP-CPC and αIONP-CPC. These aggregates could possibly be a mixture of IONPs and CPC crystals. A higher iron content in the cells indicates the presence of IONPs inside the cells. In addition, after the internalization of IONPs into the cells, the IONPs could be transferred to lysosomes, where degradation may occur and free iron ions could be released into the cytoplasm. The free iron ions were reported to be able to increase cell growth [Citation49].
Our results excluded the effects from magnetism. We wanted to confirm that the main effects in enhancing cellular osteogenic differentiation were from the change in the microstructure and nanotopography of the CPC scaffold. The expression levels of osteopontin (OPN) gene were examined. The increased OPN expression on a nanotopographical structure should be higher than the increase in the OCN expression. This is because the dual role of OPN as a protein containing the pro-adhesive tripeptide motif-RGD, and as a calcium sequestering component of the extracellular matrix (ECM) [Citation50]. Figure S7(f) showed a significant (almost six-fold) increase in OPN expression in γIONP-CPC and αIONP-CPC, compared to CPC control (p < .01). This was greater than the OCN expression in , with a four-fold increase. These data indicated that the nanotopography indeed played a main role.
However, nearly all the biological effects of nanomaterials on the cells were systematic and multifactorial [Citation51,Citation52], suggesting that other physical and biochemical stimuli may also exist. Nevertheless, the IONP-CPC scaffold nanotopography and the internalization of IONPs by the cells appeared to be the two main factors in the present study.
The performance of αIONP-CPC was not as good as γIONP-CPC in osteogenic differentiation. Since the effect of magnetism was excluded, the possible reasons are: the larger size and spindle shape of αIONPs compared to the smaller γIONPs; the differences in the microstructure of the resulting IONP-CPC scaffold; and there was more internalization of γIONPs than αIONPs, which may result in more free iron ions. Little has been reported on the application of αIONPs in bone engineering; the literature focus has been on γIONPs, which is magnetic. There was only one report on αIONP incorporation into a calcium phosphate cement, which did not report cell response [Citation30]. The present study for the first time provided a systemic study on the cell assays and osteogenic abilities of αIONP incorporation in CPC. Further studies are needed to investigate the biological effects of αIONPs on stem cells. αIONPs could be a good alternative to γIONPs in improving the CPC properties and osteogenesis in applications where magnetism should be avoided, e.g. in cases such as when artefacts from γIONPs should be avoided during MRI follow-up after CPC injection in the bone defect [Citation30,Citation31]. In addition, animal studies are also needed to investigate the effects of αIONP and γIONP incorporation in CPC scaffolds on bone regeneration in vivo.
Conclusions
Novel IONP-CPC scaffolds were developed and substantial enhancements in osteogenic differentiation of hDPSCs on the IONP-CPC scaffolds were obtained for the first time. The addition of IONPs improved the properties of CPC, including better wetting, more protein adsorption and greater cell attachment and spreading. The osteogenic differentiation of hDPSCs was markedly enhanced via IONP incorporation in CPC. Substantial increases in ALP activity and osteogenic gene expressions were achieved. The bone matrix mineral synthesis by cells was increased by two- to three-folds, compared to that without IONPs. The enhancement in cell functions was attributed to the nanotopography of IONP-CPC scaffold and the release and intake of IONPs inside the hDPSCs. The magnetic effect was not an influencing factor as this study did not use an external magnetic field. Future studies will apply an external magnetic field to investigate the magnetic effects from superparamagnetic γIONPs in CPC. The present study showing substantial enhancements in osteogenesis indicate that the novel IONP-CPC scaffolds are highly promising for bone tissue engineering and regenerative medicine applications.
Yang_Xia_et_al._Supplementary_information.pdf
Download PDF (1.6 MB)Acknowledgements
We thank Dr Ashraf F. Fouad for providing the hDPSCs, Peng Wang for discussions, and Zhichao Lou for AFM experiment.
Disclosure statement
The authors declare no conflict of interests.
Additional information
Funding
References
- United States Bone and Joint Decade. The Burden of Musculoskeletal Diseases in the United States. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2008.
- Mikos AG, Herring SW, Ochareon P, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339.
- Shi S, Gronthos S, Chen S, et al. Bone formation by human postnatal bone marrow stromal stem cells is enhanced by telomerase expression. Nat Biotechnol. 2002;20:587–591.
- Vural AC, Odabas S, Korkusuz P, et al. Cranial bone regeneration via BMP-2 encoding mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2017;45:544–550.
- Karp JM, Ferreira LS, Khademhosseini A, et al. Cultivation of human embryonic stem cells without the embryoid body step enhances osteogenesis in vitro. Stem Cells. 2006;24:835–843.
- Eğri S, Eczacıoğlu N. Sequential VEGF and BMP-2 releasing PLA-PEG-PLA scaffolds for bone tissue engineering: I. Design and in vitro tests. Artif Cells Nanomed Biotechnol. 2017;45:321–329.
- Casagrande S, Tiribuzi R, Cassetti E, et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif Cells Nanomed Biotechnol. 2017;21:1–11.
- Cheng T, Qu H, Zhang G, et al. Osteogenic and antibacterial properties of vancomycin-laden mesoporous bioglass/PLGA composite scaffolds for bone regeneration in infected bone defects. Artif Cells Nanomed Biotechnol. 2017;7:1–13.
- Askari F, Solouk A, Shafieian M, et al. Stem cells for tissue engineered vascular bypass grafts. Artif Cells Nanomed Biotechnol. 2017;45:999–1010.
- Mahmoudi M, Hofmann H, Rothen-Rutishauser B, et al. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2012;112:2323–2338.
- Soenen SJ, De Cuyper M. Assessing iron oxide nanoparticle toxicity in vitro: current status and future prospects. Nanomedicine (Lond). 2010;5:1261–1275.
- Sahoo SK, Agarwal K, Singh AK, et al. Characterization of γ-and α-Fe2O3 nano powders synthesized by emulsion precipitation-calcination route and rheological behaviour of α-Fe2O3. Inter J Eng SciTech. 2010;2:118–126.
- Shabestari KS, Farshbaf M, Akbarzadeh A, et al. Magnetic nanoparticles: preparation methods, applications in cancer diagnosis and cancer therapy. Artif Cells Nanomed Biotechnol. 2017;45:6–17.
- Figuerola A, Di Corato R, Manna L, et al. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res. 2010;62:126–143.
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
- Meng J, Xiao B, Zhang Y, et al. Super-paramagnetic responsive nanofibrous scaffolds under static magnetic field enhance osteogenesis for bone repair in vivo. Sci Rep. 2013;3:2655.
- Meng J, Zhang Y, Qi X, et al. Paramagnetic nanofibrous composite films enhance the osteogenic responses of pre-osteoblast cells. Nanoscale. 2010;2:2565–2569.
- Sun J, Liu X, Huang J, et al. Magnetic assembly-mediated enhancement of differentiation of mouse bone marrow cells cultured on magnetic colloidal assemblies. Sci Rep. 2014;4:5125.
- Wang Q, Chen B, Cao M, et al. Response of MAPK pathway to iron oxide nanoparticles in vitro treatment promotes osteogenic differentiation of hBMSCs. Biomaterials. 2016;86:11–20.
- Wang P, Zhao L, Liu J, et al. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014;2:14017
- TheinHan W, Liu J, Tang M, et al. Induced pluripotent stem cell-derived mesenchymal stem cell seeding on biofunctionalized calcium phosphate cements. Bone Res. 2013;4:371–384.
- Xu HH, Quinn JB, Takagi S, et al. Processing and properties of strong and non-rigid calcium phosphate cement. J Dent Res. 2002;81:219–224.
- Chow LC. Next generation calcium phosphate-based biomaterials. Dent Mater J. 2009;28:1–10.
- O'Neill R, McCarthy HO, Montufar EB, et al. Critical review: injectability of calcium phosphate pastes and cements. Acta Biomater. 2017;50:1–19.
- Zhang J, Liu W, Schnitzler V, et al. Calcium phosphate cements for bone substitution: chemistry, handling and mechanical properties. Acta Biomater. 2014;10:1035–1049.
- Ginebra MP, Canal C, Espanol M, et al. Calcium phosphate cements as drug delivery materials. Adv Drug Deliv Rev. 2012;64:1090–1110.
- Zhao L, Tang M, Weir MD, et al. Osteogenic media and rhBMP-2-induced differentiation of umbilical cord mesenchymal stem cells encapsulated in alginate microbeads and integrated in an injectable calcium phosphate-chitosan fibrous scaffold. Tissue Eng Part A. 2011;17:969–979.
- Gong T, Xie J, Liao J, et al. Nanomaterials and bone regeneration. Bone Res. 2015;3:15029
- Adabi M, Naghibzadeh M, Adabi M, et al. Biocompatibility and nanostructured materials: applications in nanomedicine. Artif Cells Nanomed Biotechnol. 2017;45:833–842.
- Vlad MD, del Valle LJ, Barracó M, et al. Iron oxide nanoparticles significantly enhances the injectability of apatitic bone cement for vertebroplasty. Spine (Phila Pa 1976). 2008;33:2290–2298.
- Perez RA, Patel KD, Kim HW. Novel magnetic nanocomposite injectables: calcium phosphate cements impregnated with ultrafine magnetic nanoparticles for bone regeneration. RSC Adv. 2015;5:13411–13419.
- Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000;97:13625–13630.
- Tonomura A, Sumita Y, Ando Y, et al. Differential inducibility of human and porcine dental pulp-derived cells into odontoblasts. Connect Tissue Res. 2007;48:229–238.
- Graziano A, d'Aquino R, Laino G, et al. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–26.
- Chen B, Li Y, Zhang X, et al. An efficient synthesis of ferumoxytol induced by alternating-current magnetic field. Mater Lett. 2016;170:93–96.
- Sun J, Su Y, Wang C, et al. The investigation of frequency response for the magnetic nanoparticulate assembly induced by time-varied magnetic field. Nanoscale Res Lett. 2011;6:453.
- Sun L, Xu HH, Takagi S, et al. Fast setting calcium phosphate cement-chitosan composite: mechanical properties and dissolution rates. J Biomater Appl. 2007;21:299–315.
- Khaledian M, Jiroudhashemi F, Biazar E. Chitosan- and polypropylene-oriented surface modification using excimer laser and their biocompatibility study. Artif Cells Nanomed Biotechnol. 2017;45:135–138.
- Zhang N, Weir MD, Chen C, et al. Orthodontic cement with protein-repellent and antibacterial properties and the release of calcium and phosphate ions. J Dent. 2016;50:51–59.
- Wang L, Zhang C, Li C, et al. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater Sci Eng C Mater Biol Appl. 2016;69:1125–1136.
- Gurel Pekozer G, Ramazanoglu M, Schlegel KA, et al. Role of STRO-1 sorting of porcine dental germ stem cells in dental stem cell-mediated bone tissue engineering. Artif Cells Nanomed Biotechnol. 2017;31:1–12.
- Le Pape F, Richard G, Porchet E, et al. Adhesion, proliferation and osteogenic differentiation of human MSCs cultured under perfusion with a marine oxygen carrier on an allogenic bone substitute. Artif Cells Nanomed Biotechnol. 2017;22:1–13.
- Gregory CA, Gunn WG, Peister A, et al. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal Biochem. 2004;329:77–84.
- Liu X, Zhang J, Tang S, et al. Growth enhancing effect of LBL-assembled magnetic nanoparticles on primary bone marrow cells. Sci China Mater. 2016;59:901–910.
- Hess C, Schwenke A, Wagener P, et al. Dose-dependent surface endothelialization and biocompatibility of polyurethane noble metal nanocomposites. J Biomed Mater Res. 2014;102:1909–1920.
- Kasemo B. Biological surface science. Surf Sci. 2002; 500:656–677.
- Rattier BD, Hoffman AS, Schoen FJ, et al. Biomaterials science: an introduction to materials in medicine. J Clinic Eng. 1997;22:26.
- Yi C, Liu D, Fong CC, et al. Gold nanoparticles promote osteogenic differentiation of mesenchymal stem cells through p38 MAPK pathway. ACS Nano. 2010;4:6439–6448.
- Huang DM, Hsiao JK, Chen YC, et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645–3351.
- Yang J, McNamara LE, Gadegaard N, et al. Nanotopographical induction of osteogenesis through adhesion, bone morphogenic protein cosignaling, and regulation of microRNAs. ACS Nano. 2014;8:9941–9953.
- Harisa GI, Badran MM, Alanazi FK, et al. An overview of nanosomes delivery mechanisms: trafficking, orders, barriers and cellular effects. Artif Cells Nanomed Biotechnol. 2017;13:1–11.
- Prabha S, Arya G, Chandra R, et al. Effect of size on biological properties of nanoparticles employed in gene delivery. Artif Cells Nanomed Biotechnol. 2016;44:83–91.