Abstract
The current study was aimed (1) to study the green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract, (2) to investigate the induction of apoptosis by biologically synthesized silver nanoparticles in gastric cancer cell line (AGS) and (3) to compare their anti-cancer potential with commercial silver nanoparticles. The specification and morphology of the phytosynthesized AgNPs were evaluated using transmission electron microscopy (TEM), scanning electron microscopy (SEM), UV–visible spectroscopy, X-ray diffraction and Fourier transform infrared spectroscopy (FTIR). The nanoparticles synthesized were of an average size of 22 nm. The cytotoxicity of biological and commercial nanoparticles was investigated in gastric cancer cells (AGS) as well as normal fibroblast cells (L–929) by MTT assay. By increasing the concentration of phytosynthesized and commercial silver nanoparticles, a decrease was observed in the cell viability. Increased apoptosis was observed in the cells treated with biological silver nanoparticles compared to untreated cells (p < .001). Based on these findings, it was inferred that biologically synthesized silver nanoparticles induced apoptosis, and showed a cytotoxic and anti-cancer effect against gastric cancer cell lines in a dose- and time-dependent manner. Biologically synthesized nanoparticles may possess higher anti-cancer properties than commercial silver nanoparticles.
Introduction
According to World Health Organization estimates in 2011, the magnitude of mortality associated with cancer is higher than coronary heart disease [Citation1]. Among the most common types of cancers, gastric cancer ranks fifth with 952,000 newly diagnosed cases in 2012. It is the third leading cause of deaths in the world. The most fatal form of cancer is lung cancer (1.6 million deaths), followed by liver cancer (745,000 deaths) and stomach cancer (723,000 deaths) [Citation2].
Due to the genetic and epigenetic changes, two most important inducers of cancer are the inactivation of tumour suppressor genes and the activation of oncogenes. Non-genetic factors associated with gastric cancer can relate to the type of nutrition, alcohol consumption, smoking and Helicobacter pylori infection. The appearance of cancer often results in the loss of healthy cells, which can subsequently cause various toxic and side effects in the patients. Therefore, it is the need of the hour to find novel and effective alternatives to cure this disease [Citation3].
Significant advances have been made in the application of nanotechnology and novel methods of preparing and using nanomaterials. With the advent of new methods of chemical synthesis of nanoparticles, the concern of environmental pollution and consequent hazardous side-effects are increasing. Therefore, it is imperative to use certain methods based on green chemistry, since they are non-toxic and eco-friendly approaches for the environment [Citation4].
Earlier studies have shown that 65% of the nanoparticles used across the world in biological applications are silver nanoparticles. Also, the nanoscale dimensions of these particles make it easy to pass through the biological membrane and influence the cellular physiology. As the diameter decreases, the contact surface increases, which has a direct effect on their penetration and effectiveness [Citation5]. Silver in larger form has a lower activity, but when it is converted into nanoparticles, it exhibits different effects. It also increases its anti-microbial properties and affects the metabolism, respiration and reproduction of microorganisms. The bactericidal properties of silver nanoparticles are used in various industrial applications [Citation6].
Silver nanoparticles have several important implications, including medical diagnostics [Citation7] therapeutics, anti-oxidant [Citation8], anti-bacterial [Citation9] and cytotoxic [Citation10]. They can be produced by physical, chemical and biological methods [Citation11].
Recently, researchers have used bacteria, fungi, yeast and plant sources for biological synthesis of nanoparticles [Citation12]. The synthesis of nanoparticles using plant extracts (green synthesis) has many advantages over microbial synthesis because microbial synthesis employs the processes that can cause the environmental hazards (biohazard) [Citation13].
Plants are considered as common and inexpensive sources of nanomaterials [Citation14]. Plant extracts including secondary metabolites, such as phenolic acids, flavonoids, alkaloids and terpenoids, play a major role in revitalizing metal ions to form nanoparticles in an eco-friendly reaction [Citation15].
Plant-mediated synthesis is easy, cost-effective, safe and rapid, and does not require reducing and stabilizing agents. Biological production of nanoparticles can significantly conserve the environment and nature, reduce the risk factors in humans, air and the ecosystem [Citation16].
At present, several herbs and herbal compounds are used to treat various diseases around the world. The use of plants has long been considered in the traditional medicinal system [Citation17].
Artemisia belongs to Asteraceae (Compositae) family. This plant is found in Europe, Asia and North America. There are over 250 species of Artemisia in the world, with about 34 species grown in Iran [Citation18].
Artemisia species are widely used in the treatment of certain diseases such as malaria, hepatitis, cancer, tumours, neuritis, fever, swelling and wound healing due to their antibacterial, antifungal and disinfectant properties. Besides, many species are also used to treat stomach ulcers [Citation19].
There have been published several reports regarding the cytotoxic and anticancer properties of the extracts of various Artemisia species [Citation20–22]. However, only a few studies have been conducted regarding the synthesis of nanoparticles using Artemisia species and exploring its cytotoxic effects and apoptosis induction. Basavegowda et al. [Citation23] and Vijayakumar et al. [Citation24] produced nanoparticles from Artemisia nilagirica and Artemisia annua extracts, respectively, and investigated their antibacterial activity. Salehi et al. used Artemisia marschalliana to study the cytotoxic properties and induction of apoptosis in AGS cells [Citation25].
Regarding the medicinal and therapeutic properties of Artemisia plant, the present study is aimed to achieve the biological synthesis of silver nanoparticles using Artemisia turcomanica leaf extract for the first time. The cytotoxic effect and apoptosis were also studied in AGS cell line of gastric cancer.
Methods
Extraction and synthesis of green nanoparticles
A. turcomanica was procured from Iranian Biological Resources Center Herbarium, Tehran, Iran (Voucher specimens No. IBRC P1006115). The leaves were dried in air and ground to a powdered form. A total of 20 g of dried leaf powder was added to 500 mL of ethanol and then boiled for 20 min. The crude extract was filtered using Whatman No. 1 filter paper (Whatman plc, Kent, UK). Finally, the solvent was eliminated using rotary evaporator (Rv10 digital, Germany). In order to synthesize bio-silver nanoparticles with high-purity, the sedimentation method was employed with the regeneration of silver ions (AgNO3) (Merck, Germany) by A. turcomanica leaf extract.
AgNPs were synthesized by adding 3 mL of extract at a concentration of 0.01 mM of AgNO3. The compounds present in the herbal extract resulted in the reduction of silver nitrate salt into AgNPs as exhibited by a change in colour after 1 h of reaction. The sediment obtained was washed three times with distilled water at 10,000 rpm for 30 min. The final wash was performed with ethanol, and the product was kept at 37 °C for 4 h. Commercial nanoparticles of 20 nm were also purchased (CAS # 7440-22-4, US Research Nanomaterials, Inc., Houston, TX, USA).
This study was approved by the ethics committee of Islamic Azad University and was performed based on the principles outlined in Declaration of Helsinki.
Structure confirmation of nanoparticles
UV–visible spectroscopic characterization of AgNPs
The spectroscopic analysis of silver nanoparticles synthesized using A. turcomanica extract was performed using a UV–Vis PerkinElmer device in the range of 200–800 nm.
X-ray diffraction analysis
Crystallographic structures of the phytosynthesized AgNPs were performed by X-ray diffraction (XRD) method. XRD test was carried out with Cu Ka radiation in the range of 2θ = 10°–80°.
Fourier-transform infrared spectroscopic studies
Fourier-transform infrared (FT-IR) spectroscopy studies were performed using a spectrum RX 1 instrument. The FT-IR spectra were scanned between 4000 and 400 cm−1 at a resolution of 4 cm−1 in the transmittance mode.
Field-emission scanning electron microscopy and transmission electron microscopy (TEM)
Morphological studies and size examination of the synthesized AgNPs were conducted by a field-emission scanning electron microscope (FE-SEM, SIGMA; Carl Zeiss Meditec AG; Jena, Germany) and a transmission electron microscopy (Leo 906, Zeiss100KV model, Germany).
Cytotoxicity assays
Cell culture
The AGS (Human Gastric Adenocarcinoma, IBRC C10071) and normal L-929 (IBRC C10102) cell lines were provided by the Iranian Biological Resource Center (IBRC). The AGS and L-929 cell lines were cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS), 1% penicillin–streptomycin at 37 °C in a humid atmosphere with 5% CO2. To study morphology, health and the number, the cell was checked using inverted microscope. When the cells reached at least 70% cell growth, they were detached from the bottom of the flask by 0.05% trypsin and centrifuged at 1500 rpm for 5 min. The resulting precipitate was produced as suspension and the percentage of living cells was determined by Neubauer chamber and Trypan blue dye by optical microscopy. After ensuring no contamination, cells with viability above 90% were used for the investigation.
MTT assay
MTT test was used to evaluate the cytotoxic effect of AGS and L-929 cells. A 100 μL culture medium containing 10,000 AGS and L-929 cells were seeded into 96-well plates separately and incubated for 24 h. Then different concentrations of AgNPs (3, 5, 12.5, 25, 50 and 100 μg/mL) were added to the wells separately and the plates were incubated for 24 h. Then, 100 μL of MTT (3,4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (Sigma, Germany) was added to each well at 0.5 mg/mL concentration, and they were incubated for 4 h at 37 °C in a humidified incubator with a 5% CO2 environment. The purple crystals of formazan formed in cell cytoplasm were dissolved by adding pure DMSO solution to the wells. Optical absorbance was determined using an ELISA reader at 570 nm. The results were reported as viability percentage and IC50 value.
Annexin V-FITC assay for apoptosis analysis
Evaluation of apoptosis induction was performed in accordance with the Annexin V-FITC kit (eBioscience, San Diego, CA, USA). For this purpose, the cells were treated with IC50 concentration of AgNPs (4.48 μg/mL) for 24 h. Cellular analysis was performed by flow cytometry device (Biocompare, South San Francisco, CA, USA).
Expression of BAX and Bcl2 genes with real time-PCR
At first, AGS cells were treated with IC50 (4.48 µg/mL) concentration of commercial and phytosynthesized AgNPs for 24 h. RNA extraction and cDNA synthesis were performed according to CinnaGen (Iran) and Revert AidTM First Strand cDNA Synthesis Kits (Fermentas, Germany) protocols, respectively. Briefly, the cells were lysed by RNX solution (Trizol reagent) (200 μL of chloroform was added to 1 mL of RNX solution). Samples were centrifuged at 12,000 rpm. Then three phases were formed. The RNA molecule was placed in the upper phase and the protein and DNA were placed in the middle and lower phases. The high transparent phase was transferred to the new vial. A volume of isopropanol the same as the supernatant phase volume was added then centrifuged for 15 min at 12,000 rpm. The supernatant was discarded and pellet was dissolved in DEPC water.
After confirming the quality and quantity of RNA, cDNA synthesis was performed according to the protocol of the Revert AidTM First Strand cDNA Synthesis Kit (Fermentas, Germany). In summary, 1–2 μg of the RNA, 1 μL of DNAse and 1 μL of 10× buffer were mixed and incubated at 37 °C for 30 min. A 1 μL of EDTA was added and incubated at 65 °C for 10 min to inactivate the DNAse enzyme activity. Then, 4 μL of 5× buffer, 0.5 μL of RNasin (40 Unit/μL), 2 μL of dNTP mixture (10 mM), 2 μL of dT oligo, 1.5 μL of MgCl2 and 1 μL of Revers Transcriptase enzyme were added. The reaction mixture was placed in thermocycler for 60 min at 42 °C. Samples were put in thermocycler for 10 min at 70 °C to deactivate RT enzyme. The reaction product was kept at −20 °C.
SYBER Green method was used to determine the expression of BAX and Bcl2 genes in AGS cells treated with AgNPs after 24 h. The beta-actin gene was used as internal control. The final volume of each reaction was considered 25 μL as follows; 12.5 μL of Mater mix (Bioneer, Korea), (0.1–1 μg) 1 μL of synthesized cDNA of β-actin, BAX and Bcl2 genes, (10 mM) 1 μL of forward and reverse primers for each gene (Takapuzist company, Tehran, Iran), 9.5 μL of DEPC water. The sequence of primers is presented in . The PCR for each gene was performed separately and repeated three times. The Real Time PCR was performed with the Bioneer exicycler 96 according to the following protocol. The temperature program was optimized as follows: pre-denaturation at 95 °C for 10 min; 40 cycles of denaturation at 95 °C for 20 s, annealing at 56 °C for 40 s and extension at 72 °C for 40 s.
Table 1. Specific primer sequences used for real-time PCR.
After the reaction, data were extracted from the device as ct and gene expression was measured using ΔΔCt method by Rest software. Then, the gene expression was plotted using the SPSS version 19 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 6.04 (La Jolla, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 19 software (SPSS Inc., Chicago, IL, USA) and the results were analyzed by one way ANOVA. The expression level of target genes between the treated samples and control group was measured by Tukey's HSD post hoc test. Data were presented as mean ± standard deviation (SD) and p < .05 considered as statistically significant.
Results
Biosynthesis of silver nanoparticles using A. turcomanica
The change in the colour of the solution from clear to yellow and then brown is indicative of conversion of silver ions into silver nanoparticles ().
UV–visible spectroscopic analysis
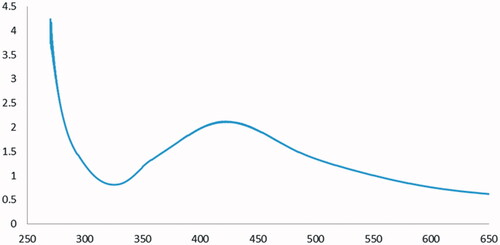
The absorption peak at 430 nm in the UV–Vis region confirmed the synthesis of silver nanoparticles (AgNPs) (). The appearance of this peak is the result of the Surface Plasmon Resonance (SPR) of silver nanoparticles. In the UV–Vis spectrum of silver nanoparticles, the presence of a peak at 430 nm represents the complete synthesis of silver nanoparticles ().
X-ray diffraction (XRD) analysis
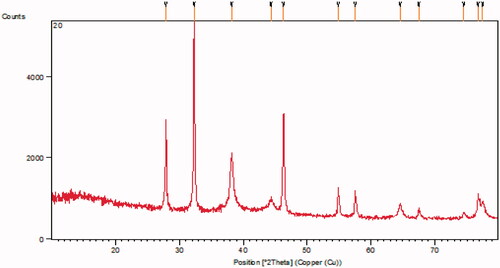
The X-ray diffraction patterns of silver nanoparticles are presented in . The X-ray patterns showed diffraction peaks at about 38.183°, 46.287°, 64.530° and 77.43° corresponding to (1 1 1), (2 0 0), (2 2 0) and (3 1 1) planes of the face-centred cubic (fcc) silver crystal, respectively.
Fourier transform infrared spectroscopy (FT-IR) analysis
An analysis of the FT-IR spectrum of the extract confirmed the presence of biomolecules. The presence of a broad and strong peak in 13,429 cm−1 is related to the stretching vibration of the phenolic and alcoholic O–H groups in the extract. The absorption peaks at 3029 and 2929 cm−1 belong to the stretching vibrations of the aromatic group C–H of benzene rings and aliphatic groups, respectively. Strong absorption peak was observed at 1635 cm−1, associated with stretching vibrations of the C=O bond of the carbonyl amide protein group. The peak at 1459 cm−1 refers to the bending vibration of the CH2 group. The peaks of C–O–C phenolic stretching vibration at 1273 cm−1 and the peaks of C–O–C and C–O–H stretching vibrations appeared at 1064, 1119, 1168 and 1201 cm−1. The bending vibrations of C–H bonds in the C=C–H groups appeared in the region below 1000 cm−1 ().
Figure 4. FT-IR spectra of Artemisia turcomanica leaf extract (A) and phytosynthesized nanoparticles (B).
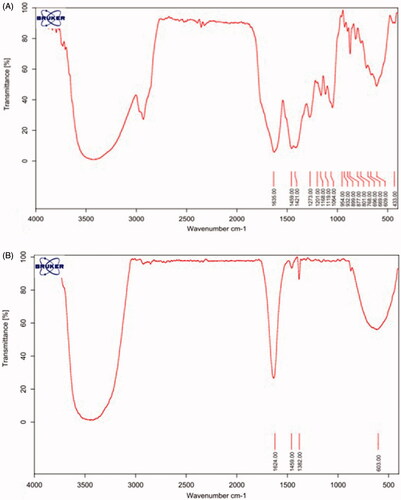
The remaining part of the spectrum of the plant extract showed characteristic features belonging to silver nanoparticles. The O–H peak transfer from 3429 to 3473 cm−1 is due to the bonding of O–H groups to silver nanoparticles. The reduction in the absorption frequency of the stretching vibration of the carbonyl amide group from 1635 to 1624 cm−1 indicates the bonding of silver to this group. The peak at 1382 cm−1 is related to the stretching vibration of the nitro group N=O, which is formed by the oxidation of the NH2 group of amine and reduction of silver cation into metal silver ().
TEM and SEM analysis
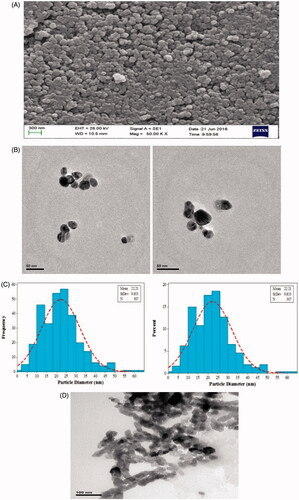
The nanoparticles had a spherical structure ranging from 20 to 60 nm as confirmed by SEM and TEM (). The silver nanoparticles were photographed by an EM10C–100 KV Zeiss camera. The average particle size was estimated as 21.22 nm. The histogram is shown in .
Identification of silver nanoparticles using an X-ray diffraction spectrum (EDX)
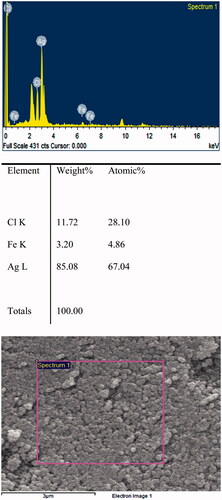
In the mentioned spectrum, the presence of the silver signal confirms the existence of silver nanoparticles ().
Cell viability
AGS cells were treated with 3, 5, 12.5, 25, 50, 100 μg/mL of phytosynthesized AgNPs and commercial AgNPs for 24 h. The highest activity against AGS cells (95% and 84%) was observed at 100 μg/mL concentration of phytosynthesized and commercial nanoparticles, respectively, which was statistically significant compared to the control cells (p < .001). However, the concentration of 3 μg/mL showed the least anti-proliferation effect (30% and 24% for biological and commercial nanoparticles, respectively), which was comparable to the control group (p < .05) (). The IC50 value of the biological and commercial silver nanoparticles was 4.88 and 6.37 μg/mL, respectively.
Figure 7. The results of the MTT assay in AGS (A) and L-929 cells (B) treated with biological nanoparticles and commercial AgNPs after 24 h (results are reported as viability in comparison with control samples ***p ≤ .001, **p ≤ .01, *p ≤ .05) (n = 3).
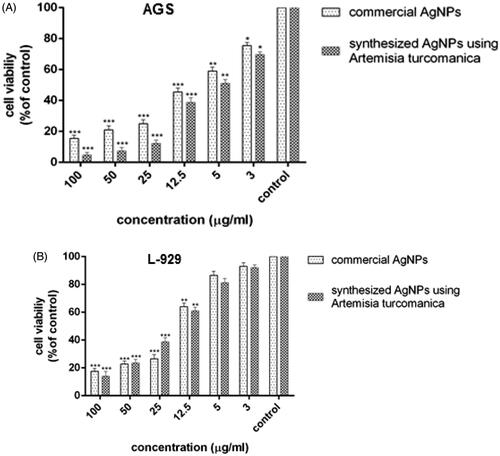
The L-929 cells were treated with different concentrations (3, 5, 12.5, 25, 50, 100 μg/mL) of biological and commercial silver nanoparticles for 24 h. The highest inhibitory effect was observed on L-929 cell proliferation at 100 μg/mL concentration, which was statistically significant compared to the control group (p < .001). There was no significant difference in the bioavailability percentage of 3 and 5 μg/mL of phytosynthesized and commercial silver nanoparticles compared to the untreated cells (p > .05) (). The bioavailability for 3 μg/mL of biological and commercial nanoparticles was 92% and 94%, respectively.
The IC50 value was 14.56 and 15.43 μg/mL for phytosynthesized and commercial silver nanoparticles, respectively, against the L-929 normal cell line in 24 h, which was higher than the IC50 for the AGS cell line. This indicates that higher concentration of nanoparticles is required to inhibit 50% of normal cells.
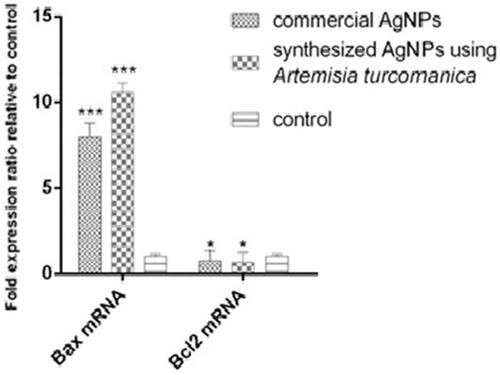
Comparison of BAX and Bcl2 mRNA expression in AGS cells treated with phytosynthesized AgNPs and commercial AgNPs
The dose of IC50 (4.88 μg/mL) was used to evaluate the expression of BAX and Bcl2 genes after 24 h, the results are presented in . The expression of BAX mRNA in AGS cancer cells treated with phytosynthesized AgNPs and commercial AgNPs increased 10.58-fold and 8.1-fold, respectively, compared to the control group (untreated cells). The inhibition of Bcl2 expression in the cells was statistically significant (p < .05). Bax expression in cells treated with phytosynthesized AgNPs was 1.5-fold higher than commercial AgNPs, which was statistically significant (p < .05, p=.02).
Comparison of induction of apoptosis in AGS cells treated with phytosynthesized AgNPs and commercial AgNPs with Annexin V-FITC
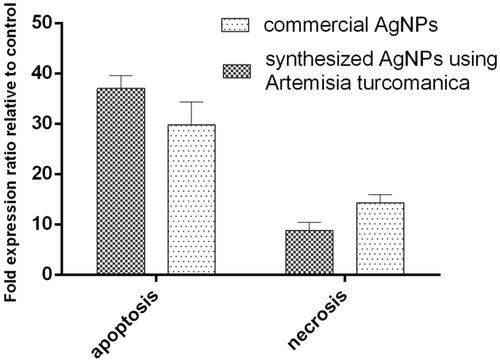
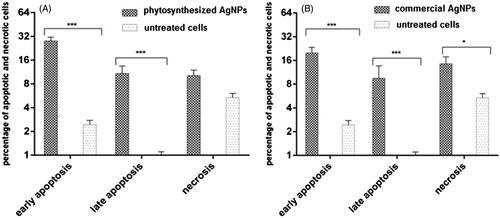
The results of flow cytometry are shown in . The early apoptosis in AGS cells treated with phytosynthesized AgNPs and commercial AgNPs was 28.55% and 20%, respectively, which was significantly higher than the control cells (p=.007, p < .001) ()). The rate of late apoptosis induced by both the silver nanoparticles also increased significantly (p < .001). The late apoptosis induced by phytosynthesized AgNPs was 10.39%, compared to 9.78% for commercial silver nanoparticles. There was no significant increase in the percentage of necrotized cells by phytosynthesized AgNPs compared to the untreated cells (p > .05, p=.07) ()).
Figure 9. Flow cytometry charts for evaluating apoptosis induced by AgNPs in 24 h. (A) Control cells (untreated cells); (B, C) IC50 concentration of phytosynthesized and commercial AgNPs, respectively. Q1 represents early apoptotic cells with Annexin-FITC + and PI− staining index, Q2 represents late apoptotic cells with Annexin-FITC + and PI+, Q3 represents healthy cells with Annexin-FITC− and PI− staining index and Q4 is necrotic cells with Annexin-FITC− and PI+ staining index.
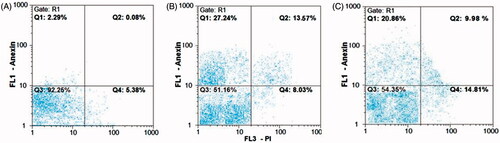
Figure 10. Comparison of apoptosis induced by silver nanoparticles synthesized by Artemisia turcomanica (A) and commercial silver nanoparticles (B) compared to the control group in 24 h.
In another analysis, in the AGS cell lines treated with IC50 concentration of bio-nanoparticles, the total percentage of cells located in Q1 and Q2 regions was considered as the percentage of total apoptosis. The cells were compared with those located in Q4 regions that represent necrotic cells. The percentage of all apoptotic and necrotized cells for phytosynthesized nanoparticles was 38.94% and 9.24%, respectively. A high rate of the apoptosis than necrosis was recorded, which was statistically significant (p=.00073, p < .001). Besides, in the cell lines treated with commercial nanoparticles at the IC50 concentration, the total apoptotic and necrotized cells were detected as 29.93% and 14.72%, respectively. The apoptosis was significantly increased in these cells (p < .001). A higher apoptosis was observed in the biologically synthesized nanoparticles compared to the commercial nanoparticles, but it was not statistically significant (p = .08, p > .05). Moreover, the necrosis observed for commercial nanoparticles was also not statistically significant (p=.95, p > .05), as compared to the biological nanoparticles ().
Discussion
The prophylaxis and treatment of cancer are two of the most important concerns in the field of medicine. In recent years, a plenty of natural products have been investigated for their therapeutic effects. The research is still going on toward identifying plants with different medicinal properties and plant-derived compounds that may have potential for the prevention or treatment of malignancies [Citation26].
Plants contain various bioactive compounds showing anti-oxidant, anti-inflammatory and anti-tumour activity. Many herbal extracts have shown positive effects on cancer cells compared with chemotherapy or hormonal drugs [Citation27]. Many researchers are investigating novel and effective compounds from plants for their anti-cancer properties.
At present, with the development of new methods, a major biotechnological revolution is going on. The applications of various fields of biological science, for example, traditional and herbal medicine, along with innovative and efficient biotechnological tools for the production of green-synthesized AgNPs, have provided a new strategy and perspective for the prevention and treatment of different diseases, including cancer.
There are different methods available for synthesizing nanoparticles. However, unfortunately, the use of hazardous materials is a necessity in most of the methods for the production of nanoparticles. The other limitations of these methods are the low production of nanoparticles, high energy dissipation and a defective purification system [Citation28].
Hence, there is an urgent need to find low-cost and non-toxic material with high efficiency in the medical field. One of the various methods of producing nanoparticles is by biologicals means such as plants, algae, fungi, yeasts and bacteria [Citation12]. The biosynthesis of nanoparticles using plants also has other advantages such as the safety and the higher capabilities of herbals over the other biological methods, because they are more reliable and healthier than bacteria, fungi and yeast. In addition, the activities mentioned earlier are improved by binding the plant molecules to the surface of the nanoparticles during the biosynthesis. Due to this reason, the use of plants as a sustainable source of bio-compatible nanoparticles has attracted the attention of several researchers in recent years [Citation29].
Primary and secondary metabolites of plants such as phenolic acids, flavonoids, alkaloids and terpenes play a major role in the reduction of metal ions to produce nanoparticles. During biosynthesis, these compounds are involved in reducing, capping and stabilizing of nanoparticles [Citation15,Citation30].
The exploration of phytosynthesized AgNPs is a new revolution in the treatment of malignancies without damaging healthy and normal cells [Citation31]. Recently, many studies have shown that the nanoparticles produced from plants have a higher potential for controlling the growth of tumour cells. Enhanced cytotoxic effects can be attributed to secondary metabolites and other non-metallic compounds involved in synthesis [Citation32–36].
The cytotoxic and anticancer effects of the extracts obtained from various species of Artemisia have been reported against several cancer cells [Citation20–22,Citation37,Citation38]. The IC50 value of the extracts of various species is higher than that of pure nanoparticles. Thus, high concentrations of the extract can inhibit cell growth, in contrast to the nanoparticle.
However, there are limited studies on the synthesis of nanoparticles using Artemisia species and the evaluation of cytotoxic effects and the induction of apoptosis. It is the first time when nanoparticles are synthesized using A. turcomanica leaf extract and their cytotoxic, apoptotic and anti-cancer effects have been reported against AGS cell line of gastric cancer.
In the present study, the complete conversion of Ag+ ions into silver nanoparticles was confirmed by a colour change in the spectroscopic analysis. The synthesized nanoparticles were of an average diameter of 22 nm, which was similar to the commercial silver nanoparticles used in this study.
Basavegowda et al. synthesized silver nanoparticles of 30–50 nm diameter using A. annua extract and evaluated their antibacterial effects [Citation23]. Vijaya Kumar and his colleagues, synthesized nanoparticles of about 70–90 nm using A. nilagirica extracts and examined their antibacterial effects [Citation24]. They used silver nitrate as a metal precursor and hydrazine as a revitalizing agent. The nanoparticles of 5–50 nm size synthesized using A. marschalliana were able to inhibit cancer cell growth and induce apoptosis in AGS cell line [Citation25]. The nanoparticles synthesized using Artemisia pallens were spherical in shape with an average size of 28 nm [Citation39]. However, the nanoparticles synthesized using the leaf extract of A. turcomanica in the present study were smaller than those reported by Arde and Vijayakumar [Citation24,Citation39].
Various factors influence the shape and size of nanoparticles, such as the concentration of hydrogen ions, temperature and the resuscitation reaction time [Citation40–42].
The results of MTT assay and biomass analysis showed that the increase in the concentrations of phytosynthesized, as well as commercial AgNPs, enhanced the inhibitory effect on the cell proliferation in AGS and L-929 cell lines. The comparative analysis of the IC50 value of biological and commercial nanoparticles (4.88 and 6.37 μg/mL, respectively) demonstrated that a lower concentration of phytosynthesized AgNPs was required to inhibit cell growth than the commercial nanoparticles. The highest bioavailability of L-929 cells at a concentration of 3 μg/mL was observed in phytosynthesized as well as commercial AgNPs (92% and 94%, respectively).
Likewise, the IC50 values of phytosynthesized and commercial silver nanoparticles on normal L-929 cells were 14.56 and 15.43 μg/mL, respectively, which was higher than those of AGS cells in 24 h (4.88 and 6.37 μg/mL respectively). This indicates that a low concentration of nanoparticles is needed to inhibit the proliferation of cancer cells. In other words, threefold higher concentration of phytosynthesized AgNPs is needed for executing the anti-proliferation effect in normal fibroblast cells compared to the cancer cells.
This variation is likely to be associated with the difference in nanosilver absorption by cancerous and normal cells. It seems that nanosilver absorption by the cancerous cells is more than that of normal cells and fewer side effects are found in normal cells. Based on these results, the rest of the tests were performed only on cancer cells.
The upregulation of BAX mRNA and the downregulation of Bcl2 were observed in AGS cells treated with both types of nanoparticles compared to the untreated cells. Thus, the BAX mRNA level in AGS cells treated with phytosynthesized AgNPs was 10.5-fold higher than the untreated cells (p < .001). The BAX mRNA levels were 1.3-fold higher in the AGS cells treated with the phytosynthesized AgNPs than commercial silver nanoparticles (p < .05, p = .02).
The increase in the expression of Bax/Bcl2 mRNA observed in AGS cancer cells treated with phytosynthesized and commercial silver nanoparticles compared to the untreated cells was 36- and 30-fold higher, which was statistically significant (p < .001).
The comparative account of apoptosis and induced necrosis showed that early apoptosis (26%) and late apoptosis (10%) increased significantly as compared to untreated cells as revealed by flow-cytometric data in the cells treated at an IC50 concentration of phytosynthesized AgNPs. Similarly, the commercial AgNPs also increased the early and late apoptosis in cancer cells by 18% and 9.7%, respectively compared to untreated cells.
So far there has not been published any report about the synthesis of silver nanoparticles by A. turcomanica and it is the first investigation in Iran. Therefore, the findings of this study are in accordance with silver nanoparticles synthesized with different species of Artemisia.
Salehi and his colleagues [Citation25] synthesized nanoparticles of 5–50 nm using A. marschalliana extract, which was almost similar to the particle size of the present study. However, in this study, the IC50 value of the nanoparticle was 21.55 μg/mL against AGS cells after 24 h compared with 4.88 μg/mL in our study. An IC50 value 4.5 times higher than that reported in the present study indicates a stronger effect of nanoparticles synthesized with A. turcomanica than A. marschalliana. Similarly, about 7% and 30% increase was reported in early and delayed apoptosis in the cells treated at an IC50 concentration of nanoparticles [Citation24], while in our study, this was 26% and 10%, respectively. The evaluation of pro-apoptotic and anti-apoptotic genes in AGS cells treated with nanoparticles by real-time PCR indicated an increase in BAX expression and a decrease in Bcl2 expression. Based on the findings of MTT assay, real time-PCR and flow-cytometric analysis, it is presumed that the nanoparticles synthesized by A. turcomanica leaf extract have a higher toxic effect than those synthesized by A. marschalliana extract.
Vijayakumar et al. and Arde et al. showed that the synthesized nanoparticles possess antibacterial properties against pathogens; however, they did not investigate the cytotoxic and anti-carcinogenic properties of nanoparticles [Citation24,Citation39].
Nanoparticles generate free radicals that can damage the target cells and tissues through the mechanism of oxidative stress [Citation43,Citation44]. The mechanism of action of nanoparticles has not yet been elucidated. However, several in vitro, as well as in vivo studies suggest that silver nanoparticles can inhibit cell growth by interacting with membrane proteins and activating signalling pathways. Nanoparticles enter a cell via diffusion or endocytosis and disturb the electron transfer chain in the mitochondria. Reactive oxygen species (ROS) can also affect the concentration of intracellular calcium and activate transcription factors and alter cytokines. The ROS have been associated with various processes such as DNA damage, interference with cellular signalling pathways, changes in the gene expression, and finally the pathways involved in inflammatory and apoptotic processes that can stop cell growth including MAPK/ERK Kinase, MIP-2, Caspase 3, Caspase 9, BCl2 and BAX [Citation43,Citation45–48].
Hsin et al. showed that the application of silver nanoparticles causes the release of cytochrome C into the cytosol and transfer BAX gene to mitochondria. They also reported that the silver nanoparticles through ROS and JNK could induce apoptosis in the mitochondrial pathway. The interaction of nanosilver with DNA was also demonstrated by arresting the cell cycle in the G2/M phase [Citation49].
Rashmezad and his team evaluated the toxic effects of commercial as well as bio-synthesized silver nanoparticles from Eucalyptus leaf extract on gastric cancer cell lines (AGS) and lung fibroblasts (MRC-5). The nanoparticles ranged between 30 and 70 nm with an average size of about 63.7 nm. The results showed that the concentration of nanoparticles had a direct fatal effect on AGS cancer cell line. The IC50 value for commercial and biological silver nanoparticles was 23.88 and 9.17, respectively, after 24 h in AGS cells. While the IC50 value for commercial and biological silver nanoparticles on normal MRC-5 fibroblast cells was 38.86 and 35.44, respectively [Citation50]. In our study, the synthesized nanoparticles were smaller. As per the IC50 value reported in the present study, the synthesized nanoparticle showed higher toxic effects on AGS cancer cells compared to the nanoparticle synthesized with the Eucalyptus. Therefore, the nanoparticles synthesized using A. turcomanica have a twofold potential of reducing the cancer cell growth than nanoparticle synthesized with Eucalyptus.
Conclusion
The size of nanoparticles is highly dependent on the method of synthesis, plant genus and the species. The comparative analysis of the size of biosynthesized silver nanoparticles in the current study (22 nm) with those reported earlier suggests that the method and the type of plant used considerably influence this parameter. The nanoparticles synthesized in this study were smaller than previously reported ones and showed a strong cytotoxic and anti-carcinogenic potential. The anti-proliferation effect of A. turcomanica entirely depended on the concentration of synthesized silver nanoparticles on AGS cancer cells. As shown by the flow cytometric data, the induction of apoptosis was more than that of necrosis.
The comparative analysis of the IC50 value of the Artemisia extract alone and the synthesized silver nanoparticles using the Artemisia extract indicated that the synthesized silver nanoparticles are more effecting in preventing cancer cell growth compared to the plant extracts alone. Given that green synthesis is an eco-friendly, safe, low-cost method, it is suggested that nanoparticles synthesized with this plant species can be used instead of extracts to inhibit or prevent the growth of cancer cells. There are certain advantages of this method such as cost-effectiveness, time savings, synthesis of the small-size particles for inhibiting the proliferation of AGS cancer cells and inducing apoptosis. Therefore, we hope that this method would be useful in the fields of therapeutics and targeted drug delivery.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- World Health Organization. Global Health Observatory Data Repository. Number of deaths (World) by cause; 2011 [cited 2013 Oct 31]. Available from: http://apps.who.int/gho/data/node.main.CODWORLD?lang=en.
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359–386
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40:250–260.
- Nagati V, Koyyati R. Green Synthesis and characterization of Silver nanoparticles from Cajanus cajan leaf extract and its antibacterial activity. J Nanomater. 2012;3(2):39–43.
- Martirosyan A, Bazes A, Schneider YJ. In vitro toxicity assessment of silver nanoparticles in the presence of phenolic compounds\textemdash preventive agents against the harmful effect? Nanotoxicology. 2014;8:573–582.
- Foladband F. Health and nano-technology. Health Magazine. 2008;197:16, 9.
- El-Sayed IH, Huang X, El-Sayed MA. Surface plasmon resonance scattering and absorption of anti-EGFR antibody conjugated gold nanoparticles in cancer diagnostics: applications in oral cancer. Nano Lett. 2005;5:829–834.
- Lim YY, Murtijaya J. Antioxidant properties of Phyllanthus amarus extracts as affected by different dryingmethods. Leb + Technol. 2007;40:1664–1669.
- Sathishkumar M, Sneha K, Won SW, et al. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces. 2009;73:332–338.
- Safaepour M, Shahverdi AR, Shahverdi HR, et al. Green synthesis of small silver nanoparticles using geraniol and its cytotoxicity against fibrosarcoma-Wehi 164. Avicenna J Med Biotechnol. 2009;1:111–115.
- Warheit DB, Borm PJ, Hennes C, et al. Testing strategies to establish the safety of nanomaterials conclusions of an ECETOC workshop. Inhal Toxicol. 2007;19:631–643.
- Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomedicine. 2010;6:257–262.
- Shankar SS, Rai A, Ahmad A, et al. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;275:496–502.
- Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;10:105104–1051014.
- Aswathy Aromal S, Philip D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;97:1–5.
- Hutchison JE. Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano. 2008;2:395–402.
- Romero MR, Serrano MA, Vallejo M, et al. Antiviral effect of artemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhoea virus (BVDV). Planta Med. 2006;72:1169–1174.
- Mozaffarian V 3rd, editor. A dictionary of Iranian plant names. Tehran: Farhang Mo’aser; 1996. p. 56. 32.
- Carvalho IS, Cavaco T, Brodelius M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind Crops Prod. 2011;33:382–388.
- Taghizadeh Rabe SZ, Mahmoudi M, Ahi A, et al. Antiproliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharm Biol. 2011;49:962–969.
- Mahmoudi M, Rabe SZT, Ahi A, et al. Evaluation of the cytotoxic activity of different Artemisia khorassanica samples on cancer cell lines. Pharmacol Online. 2009;2:778–786.
- Emami SA, Vahdati-Mashhadian N, Oghazian M, et al. The anticancer activity of five species of Artemisia on Hep2 and HepG2 cell lines. Pharmacol Online. 2009;3:327–339.
- Basavegowda N, Idhayadhulla A, Lee YR. Preparation of Au and Ag nanoparticles using Artemisia annua and their in vitro antibacterial and tyrosinase inhibitory activities. Mater Sci Eng C. 2014;43:58–64.
- Vijayakumar M, Priya K, Nancy FT, et al. Biosynthesis, characterization and antibacterial effect of plant-mediated silver nanoparticles using Artemisia nilagirica. Ind Crops Prod. 2013;41:235–240.
- Salehi S, Shandiz SA, Ghanbar F, et al. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomed. 2016;11:1835–1846.
- Rao CV, Newmark HL, Reddy BS. Chemopreventive effect of farnesol and lanosterol on colon carcinogenesis. Cancer Detect Prev. 2002;26:419–425.
- Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53:121–133.
- Mathur P, Jha S, Ramteke S, et al. Pharmaceutical aspects of silver nanoparticles. Artif Cells Nanomed Biotechnol. 2017;12:1–12.
- Arunachalam R, Dhanasingh S, Kalimuthu B, et al. Phytosynthesis of silver nanoparticles using Coccinia grandis leaf extract and its application in the photocatalytic degradation. Colloids Surf B Biointerfaces. 2012;94:226–230.
- Vanaja M, Rajeshkumar S, Paulkumar K, et al. Phytosynthesis and characterization of silver nanoparticles using stem extract of Coleus aromaticus. Int J Mater Biomat. 2013;3:1–4.
- Suman TY, Radhika Rajasree SR, Kanchana A, et al. Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B Biointerfaces. 2013;106:74–78.
- Raghunandan D, Ravishankar B, Sharanbasava G, et al. Anti-cancer studies of noble metal nanoparticles synthesized using different plant extracts. Cancer Nanotechnol. 2011;2:57–65.
- Das S, Das J, Samadder A, et al. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Colloids Surf B Biointerfaces. 2013;101:325–336.
- Alavi M, Karimi N. Characterization, antibacterial, total antioxidant, scavenging, reducing power and ion chelating activities of green synthesized silver, copper and titanium dioxide nanoparticles using Artemisia haussknechtii leaf extract. Artif Cells Nanomed Biotechnol. 2017;12:1–16.
- Singh P, Ahn S, Kang JP, et al. In vitro antiinflammatory activity of spherical silver nanoparticles and monodispersehexagonal gold nanoparticles by fruit extract of Prunus serrulata: a green syntheticapproach. Artif Cells Nanomed Biotechnol. 2017;30:1–11.
- Dehghanizade S, Arasteh J, Mirzaie A. Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol. 2018;46:160–168.
- Feridberg R. An investigation into the antimicrobial and anticancer activities of Geranium incanum, Artemisia afra and Artemisia absinthium [dissertation]. Faculty of Health Science, Nelson Mandela Metropolitan University; 2009. p. 1–245.
- Nikbakht MR, Sharifi S, Emami SA, et al. Chemical composition and antiprolifrative activity of Artemisia persica Boiss. and Artemisia turcomanica G and essential oils. Res Pharm Sci. 2014;9:155–163.
- Arde SM, Salokhe PR, Mane AH, et al. Facile green synthesis of silver nanoparticles by Artemisia pallens leaves extract and evaluation of antimicrobial activity. Chem Sci Rev Lett. 2014;3(11):557–562.
- Shankar SS, Ahmad A, Sastry M. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol Prog. 2003;19:1627–1631.
- Marchiol L. Synthesis of metal nanoparticles in living plants. Ital J Agronomy. 2012;7:37–44.
- Rai A, Singh A, Ahmad A, et al. Role of halide ions and temperature on the morphology of biologically synthesized gold nanotriangles. Langmuir. 2006;22:736–741.
- Akradi L, Sohrabi Haghdoost I, Djeddi AN. Histopathologic and apoptotic effect of nanosilver in liver of broiler chickens. African J Biotechnol. 2012;11:6207–6211.
- Bressan E, Ferroni L, Gardin C, et al. Silver nanoparticles and mitochondrial interaction. Int J Dent. 2013;2013:312747.
- Cristina B, Ivan I, Pache C, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointer Phases. 2007;2:4.
- Asharani PV, Lian Wu Y, Gong Z, et al. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19:255102
- He Y, Du Z, Ma S, et al. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus longan Lour. Nanoscale Res Lett. 2016;11:300–309.
- Nayak D, Pradhan S, Ashe S, et al. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 2015;457:329–338.
- Hsin YH, Chen CF, Huang S, et al. The apoptotic effect of nanosilver is mediated by a ROS- and JNK-dependent mechanism involving the mitochondrial pathway in NIH3 T3 cells. Toxicol Lett. 2008;179:130–139.
- Rashmezad MA, Ali Asgary E, Tafvizi F, et al. Comparative study on cytotoxicity effect of biological and commercial synthesized nanosilver on human gastric carcinoma and normal lung fibroblast cell lines. Tehran Univ Med J. 2015;72:799–807.