?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
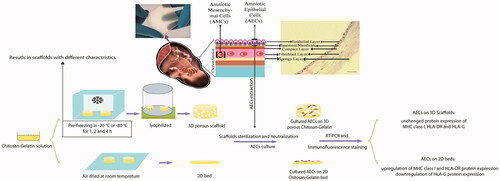
Placenta-derived amniotic epithelial cells (AECs), a great cell source for tissue engineering and stem cell therapy, are immunologically inert in their native state; however, immunological changes in these cells after culture and differentiation have challenged their applications. The aim of this study was to investigate the effect of 2D and 3D scaffolds on human lymphocyte antigens (HLA) expression by AECs. The effect of different preparation parameters including pre-freezing time and temperature was evaluated on 3D chitosan–gelatine scaffolds properties. Evaluation of MHC class I, HLA-DR and HLA-G expression in AECs after 7 d culture on 2D bed and 3D scaffold of chitosan–gelatine showed that culture of AECs on the 2D substrate up-regulated MHC class I and HLA-DR protein markers on AECs surface and down-regulated HLA-G protein. In contrast, 3D scaffold did not increase protein expression of MHC class I and HLA-DR. Moreover, HLA-G protein expression remained unchanged in 3D culture. These results confirm that 3D scaffold can remain AECs in their native immunological state and modification of physical properties of the scaffold is a key regulator of immunological markers at the gene and protein expression levels; a strategy which circumvents rejection challenge of amniotic stem cells to be translated into the clinic.
Graphical Abstract
Introduction
Cell therapy and regenerative medicine are the modern approaches to restoration and maintenance of tissue function. These fields of study are partially based on using stem cells to generate biological substitutes [Citation1]. The amniotic membrane (AM), which is the innermost layer of foetal membranes, is an enriched source of stem cells. AM is consist of five layers including an epithelium, a basement membrane, a compact layer, a fibroblast layer and a spongy layer [Citation2]. AM’s potential in healing skin wounds and burn [Citation3], reconstruction of cornea [Citation4], engineering vascular tissues [Citation5] and antibacterial activities [Citation1] have made it as a suitable biomaterial for being used in clinical applications. Amniotic epithelial cells (AECs) have a great potential for use in cell therapy and tissue engineering. These cells are directly in contact with amniotic fluid during the pregnancy and display anti-cancer properties [Citation6]. This characteristic is due to the induction of apoptosis and cell cycle arrest in cancer cells alongside the inhibition of angiogenesis. It has been hypothesized that the inhibition of heat shock protein 90 (HSP90) is responsible for anti-cancer properties of AECs [Citation7]. Also, it has been shown that cell cycle is arrested in G1/S phase in cancer cells after treatment with AECs’ condition medium. Furthermore, the expression of angio-modulatory factors, such as thrombospondin-1, endostatin and heparan sulphate and all tissue inhibitors of metalloproteinase (TIMP-1, 2, 3 and 4) result in inhibition of angiogenesis by AECs [Citation8,Citation9], which is an effective way to treat cancer.
AECs are able to differentiate into the three germ layers (ectoderm, mesoderm and endoderm) and express the cell surface markers associated with embryonic stem cells, such as SSEA-3, SSEA-4, TRA-1–60, TRA-1–81 as well as transcriptional factors Oct-4 and Nanog [Citation10,Citation11].
While AECs express the non-polymorphic, non-classical human leukocyte antigen (HLA-G) on their surfaces [Citation12], they lack the polymorphic antigens HLA-A, HLA-B and HLA-DR [Citation13]. Therefore, these cells have been deemed non-immunogenic [Citation14]. However, some studies have shown that the immunogenicity of AECs increased after culture or differentiation [Citation12,Citation15,Citation16], potentially affecting the transplant fate of these cells.
Since, the interaction of cells and the extracellular matrix (ECM) can affect many cell behaviours, such as attachment, migration, proliferation and induction of immune response [Citation17,Citation18], the use of culture condition near to in vivo, for example using three-dimensional matrices (scaffold), would help the cells stay in their native status [Citation19]. Scaffolds are made of common biocompatible and biodegradable materials in form of porous matrices to help cells attach better. The pore size, porosity and biodegradation rate are the most important factors in designing a suitable scaffold. The degradation rate of scaffold should be proportional to the new tissue formation rate in order to remove the need for removing the scaffold by surgery. Moreover, not only the physical and chemical properties of scaffolds determine its success, but also the mechanical properties play a crucial role in scaffolds’ biocompatibility [Citation20,Citation21]. In this regard, numerous natural and synthetic polymers have been used to date for scaffold fabrication and many investigations have been carried out to optimize their properties for specific applications [Citation22].
Gelatine is a biopolymer derived from collagen. It possesses desirable properties, such as low antigenic property, lack of harmful by-products in enzymatic degradation, cost-effectiveness and high contents of arginine-glycine-aspartic acid (RGD) motifs and functional groups in its structure, which mediate cell attachment. The main concern for using gelatine in biomedical applications is its low strength and stability in the body. Thus, it is usually composited with other materials [Citation23,Citation24,Citation25].
Chitosan is a linear polysaccharide derived from deacetylation of chitin. It has an analogous structure of glycosaminoglycans (GAGs) which are abundant in ECM. Due to its biocompatibility and antibacterial properties, it has achieved a high scientific popularity in various biomedical applications [Citation26,Citation27].
It has been shown that fabricating composite porous scaffolds based on gelatine and chitosan, enhances their biological and mechanical properties [Citation28–30]. The aim of this study was to investigate the effect of physicochemical properties of 2D and 3D scaffolds, based on gelatine and chitosan, on HLAs expression in AECs.
Materials and methods
Preparation of chitosan–gelatine scaffolds
To prepare the 2D bed and 3D scaffolds, chitosan (LMW, Sigma, St. Louis, MO) and gelatine (type A, Sigma, St. Louis, MO) were dissolved in 1% (w/v) acetic acid in distilled water at 37 °C with a ratio of 1:3. In order to evaluate the effect of freezing conditions on 3D matrices’ properties, 350 µl of homogeneous suspension was poured into 24 well tissue culture plates and placed in −20 and −80 °C freezers for 1, 2 and 4 h. The frozen samples were lyophilized (ZIRBUS, Bad Grund, Germany) for 12 h. To form the 2D membrane, 24 well plates were completely covered by the chitosan–gelatine mixture and dried at room temperature.
Scaffolds characterizations
In order to evaluate the effects of preparation conditions on scaffolds structure and to select the most appropriate scaffold for cell seeding, different characteristics of 3D scaffolds were investigated as described below.
Morphology analysis
The scaffolds’ morphology was evaluated by a scanning electron microscope (AIS2100, Seron Technologies, Uiwang-si, Korea). The mean pore size of each scaffold was measured using ImageJ software (National Institutes of Health, Bethesda, MD).
Porosity measurement
The liquid displacement method was used to measure the porosity of scaffolds [Citation31]. Briefly, the scaffolds were placed in a known volume of absolute ethanol (V1) for 5 min. The volume after scaffold immersion and the remaining volume after removing the scaffolds were recorded as V2 and V3, respectively. The porosity of scaffolds was calculated using the following equation:
Evaluation of scaffolds swelling behaviour
To determine the swelling behaviour of scaffolds, the dried samples of scaffolds were weighed (Wd) and then immersed in phosphate buffer saline (PBS) at 37 °C for 24 h. The samples were weighed again after removing their surface water (Ww) and then centrifuged at 500 rpm for 3 min to record the final weight (W′w). The swelling and water retaining capacity of scaffolds were measured using the following equation [Citation32]:
Contact angle measurement
OCA PLUS 15 instrument (Dataphysics, San Jose, CA) was used to evaluate the degree of hydrophilicity of scaffolds. Three water droplets with a volume of 0.5 µl were put on each sample to measure the water contact angle.
Evaluation of scaffolds biodegradability
Scaffolds were weighed (W0) and placed in 10 ml PBS at 37 °C to evaluate their biodegradability properties for 21 d. Samples were weighed again (Wt) after being dried in 80 °C oven at distinct points during this period. The biodegradability rate of scaffolds was determined by the following equation [Citation33]:
Scaffold sterilization and neutralization
All samples were sterilized using ethylene oxide gas and vacuumed by covers. Before cell seeding, sterile scaffolds were neutralized with absolute ethanol for 30 min and then rinsed with sterile PBS several times before being soaked in Dulbecco’s modified eagle medium (DMEM) for 30 min [Citation29].
Amniotic epithelial cells isolation and culture
All Methods and experimental procedures of this study were in accordance with the experimental guidelines and regulations and approved by the research ethics committee of Shahid Beheshti University of Medical Sciences. Human placentas were obtained from healthy mothers having elective caesarean and transported to the laboratory under sterile conditions in cold PBS. The written consent for the use of these tissues was obtained from the parents. The AM was mechanically dissected from the chorion and washed several times with cold PBS to remove blood. To extract AECs, the AM was divided into 3–4 pieces and transferred into tubes containing 30 ml 0.15% trypsin-EDTA (Sigma) and incubated at 37 °C for 10 min. The first digestion was discarded to avoid debris and blood contamination. The extracted cells from the second and third digestions (20 min each) were poured over mesh (100 µm) and suspended in PBS containing 10% foetal bovine serum (FBS, Gibco, Carlsbad, CA). AECs were counted and their viability was determined by trypan blue exclusion. DMEM/F12 (Gibco, Carlsbad, CA) containing 10% FBS to which 100 U/ml penicillin-streptomycin and 10 ng/ml epithelial growth factor (EGF, Sigma, St. Louis, MO) was used as cell culture medium. The cells were seeded on the 2D and 3D scaffolds, respectively, following by incubating (37 °C, 5% CO2) for 1 h to achieve the initial cell attachment, and then the medium was added to each well to a final volume of 0.5 ml [Citation34].
Cell attachment
Cell attachment to the scaffolds was evaluated by SEM and MTT (methylthiazol tetrazolium) assay. 3D scaffolds were washed with PBS to remove suspended cells 24 h after cell seeding. Medium containing 10% MTT was added and incubated for 4 h at 37 °C. Then MTT was replaced with dimethyl sulfoxide (DMSO) (30 min). The scaffolds without cells were considered as controls.
Purification of human macrophages
In this study, macrophages served as an HLA class I and HLA-DR expression control, purified by Histopaque (Sigma-Aldrich, St. Louis, MO) gradient. To isolate monocyte/macrophage cells, peripheral blood mononuclear cells (PBMCs) were cultured in tissue culture plate (TCP) with complete medium (RPMI +10% FBS +1% penicillin/streptomycin, Gibco, Carlsbad, CA) for 4 h. Then, non-adherent cells (i.e. lymphocytes) were removed and the attached cells (i.e. macrophages) were used as a positive control for immunocytochemistry assays [Citation35].
Immunofluorescence staining
HLAs expression in native and cultured AECs was analysed by immunocytochemistry. All assessments were performed by multiple blinded observers. AECs were detached from the 2D and 3D matrices using trypsin-EDTA after a week and fixed on glass slides in cold acetone. The cells were blocked for 30 min with 1% bovine serum albumin (BSA), then incubated with the following specific antibodies: primary antibodies Pan-cytokeratin (1:100, Sigma-Aldrich, St. Louis, MO), MHC class I conjugated to FITC (1:100, Sigma-Aldrich, St. Louis, MO), HLA- DR (1:50, ab20718, Abcam, Cambridge, UK), HLA-G (1:100) (ab7758, Abcam, Cambridge, UK). Labelled antibodies with phycoerythrin (PE) (1:100, ab7002) and fluorescein isothiocyanate (FITC) (1:100, Sigma-Aldrich, St. Louis, MO) were used as secondary antibodies. Isotype immunoglobulin (Sigma-Aldrich, St. Louis, MO) was used as a negative control for each antibody and HLA-G-positive human choriocarcinoma cell JEG-3 and macrophage cells were used as positive controls. After the incubation period, the cells were rinsed with PBS and cell nuclei were stained with DAPI for 1 min. Samples were analysed using a fluorescence microscope (Nikon, Tokyo, Japan).
RT-PCR analysis
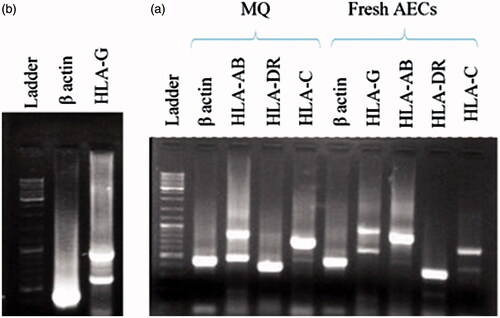
Total RNA was isolated (RNeasy Mini Kit; Qiagen, Hilden, Germany) from AECs immediately after isolation and after 7 d of culture on the 2D membrane and 3D scaffolds according to the manufacturer’s instructions. RNA concentration was measured with the Biophotometer (Eppendorf, Hamburg, Germany) at 260 nm. Of 2 µg RNA was used as template for One-Step RT-PCR Kit (Qiagen, Hilden, Germany) and reactions were carried out at 39 cycles of 50 °C (30 min), 95 °C (15 min), 94 °C (45 s), 62–68 °C (45 s), 72 °C (1 min), followed with 72 °C (10 min). PCR products were visualized in 1.5% agarose gels. β-Actin was used as internal control and macrophage cells (HLA class I & HLA-DR) as well as JEG-3 cell line (HLA-G) were used as transcription controls. Primer sequences and annealing temperatures are listed in .
Table 1. Primer sequences and annealing temperatures for the evaluated markers.
Statistical analysis
All experiments were performed at least three times and data were assessed as the mean ± SD using GraphPad Prism software version 7.01 (La Jolla, CA). Statistical significance was determined by one-way analysis of variance (ANOVA) followed by Tukey’s post-test. A p values less than .05 was considered statistically significant.
Results
SEM observations
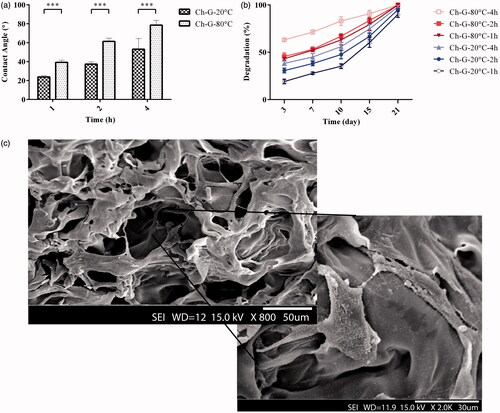
shows the microscopic images of 3D chitosan–gelatine scaffolds prepared in different pre-freezing conditions and presents their mean pore size. It can be seen that increasing the freezing time increases the pore size in all pre-freezing temperatures. On the other hand, pore size is smaller when the scaffold is frozen in the lower temperature. It may be due to the increase in freezing rate. The scaffold with freezing temperature and time of −20 °C and 1 h has a mean pore size of approximately 80 µm which could be a suitable scaffold for cell culture.
Figure 1. SEM images of chitosan–gelatine scaffolds prepared at (a) − 20 °C for 1 h, (b) − 20 °C for 2 h, (c) − 20 °C for 4 h, (d) − 80 °C for 1 h, (e) − 80 °C for 2 h and (f) − 80 °C for 4 h.
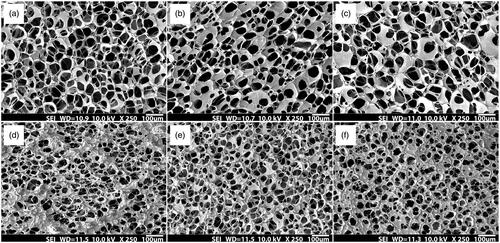
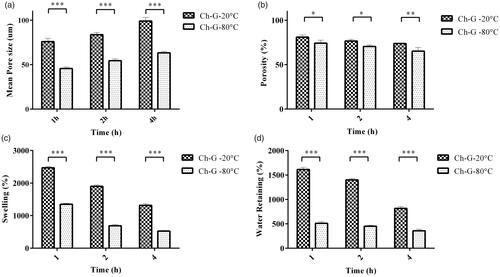
Figure 2. (a) The mean pore size of chitosan–gelatine scaffolds prepared at different pre-freezing temperatures and times. The pore size increased by increasing the pre-freezing time. Also, preparation at lower temperature yields smaller pores than higher temperature in the similar freezing time. (b) The results of porosity measurement of chitosan–gelatine scaffolds prepared at different pre-freezing temperatures and times. The results indicated that decreasing the pre-freezing temperature significantly decreases the porosity of scaffold in all preparation times. (c) Swelling characteristics and (d) Water retaining of chitosan–gelatine scaffolds prepared at different pre-freezing temperatures and times. Increasing the pre-freezing temperature increased both properties significantly. Furthermore, the swelling ratio and water retaining of scaffolds are higher when frozen for shorter times (*p < .05, **p < .01 and ***p < .001).
Porosity of scaffolds
shows the porosity of scaffolds prepared in different pre-freezing conditions. It can be perceived that increasing the freezing time from 1 to 4 h reduces the porosity of scaffolds, independent of the freezing temperature. Although, increasing the time produces larger pores, they have thick walls inhibiting the interconnection and porosity. Moreover, preparation in higher temperature leads to an increased porosity in scaffolds because they have been formed in a lower freezing rate and have larger pores with thin walls which makes interconnection between pores. The maximum porosity of 80% belonged to chitosan–gelatine scaffold frozen in −20 °C for 1 h which makes it a suitable scaffold for cell seeding.
Swelling behaviour of scaffolds
As displayed in ), the longer freezing time and lower freezing temperature both decrease the swelling ratio and water retaining of scaffolds since the less porous scaffolds have been formed in these conditions. Due to the importance of water absorption for a scaffold to provide cells with a suitable environment, the chitosan–gelatine scaffold prepared at −20 °C for 1 h with 24.71% swelling and 16.14% water retaining could be a great candidate to evaluate its impacts on immunological states on AECs.
Scaffolds hydrophilicity
Water contact angle measurements showed that increasing the freezing time and decreasing the freezing temperature both yield a higher contact angle meaning more hydrophobicity of the structure (). Water contact angle for chitosan–gelatine (−20 °C and 1 h) was 23.8°, the most hydrophilic scaffold among all samples.
Figure 3. (a) The results of contact angle measurement of chitosan–gelatine scaffolds prepared at different pre-freezing temperatures and times. When prepared in a determined time, decreasing the temperature increased the contact angle which indicates a more hydrophobic surface. (b) The degradation profiles of chitosan–gelatine scaffolds prepared at different pre-freezing temperatures and times. (c) SEM image of amniotic epithelial cells attached to the chitosan–gelatine scaffold (***p < .001).
Biodegradation of scaffolds
As shown in , increasing the freezing time and decreasing the freezing temperature yield higher rates of scaffold degradation. In these preparation conditions, the scaffolds have either smaller partially interconnected pores or pores with thick walls so a larger area is exposed to the degrading agent and the scaffold degrades faster.
Cell attachment
The SEM image of attached AECs to the 3D chitosan–gelatine scaffold prepared at −20 °C for 1 h is depicted in . The scaffold was able to provide a suitable bioenvironment to support cell attachment.
HLA expression
Expression of HLA class I, HLA-DR, HLA-G molecules in AECs was evaluated by immunofluorescence staining. The pan-cytokeratin specific antibody was used to detect AECs and confirm no contamination with amnion mesenchymal cells during isolation process. Results showed that fresh AECs express HLA-G molecules with high intensity and MHC class I molecules with very low intensity, whereas, these cells were not reactive with the anti-HLA-DR antibody. After 7 d of culture on the 2D and 3D matrices, HLA-G expression did not show significant alteration in AECs cultured on both matrices as compared to fresh AECs, but HLA- DR expression was up-regulated after culture on the 2D and 3D matrices with the same intensity. MHC class I was up-regulated in AECs cultured on 2D bed, whereas, there were no significant differences between fresh AECs and those of embedded in the 3D scaffold ( and , ).
Figure 4. Immunofluorescence staining of fresh AECs (a) and control groups (b). (a) AECs staining with specific antibodies conjugated with FITC (green) and phycoerythrin (red), (b) Representative image of negative and positive controls; from the left: negative control in all immunocytochemical experiments in which the primary antibody was deleted; macrophage (MQ) cells as positive control for HLA-DR; macrophage (MQ) cells as positive control for HLA class I; and choriocarcinoma (JEG-3) cell line as positive control for HLA-G antibody, respectively. Inset shows nuclear staining by DAPI for the same images. (Scale bar = 100 µm).
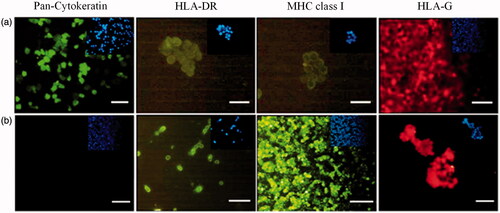
Figure 5. Up: fluorescence staining of AECs by specific antibodies after 7 d culture on 2D bed. Down: fluorescence staining of AECs by specific antibodies after 7 d culture on 3D scaffolds. Inset shows nuclear staining by DAPI for the same images (scale bar = 100 µm).
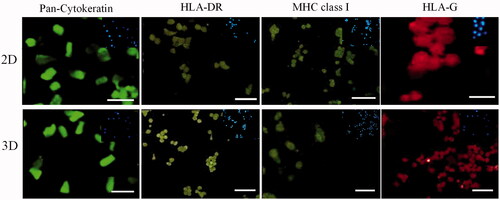
Table 2. The marker expression of fresh (0) and cultured AECs (for 7 d) on 2D and 3D scaffolds.
RT-PCR
One step RT-PCR was conducted to investigate HLAs expression in fresh and cultured AECs. Because of gene overlapping between HLA-A and HLA-B, a common primer was designed for two genes. The expression of HLA-AB, HLA-DR, HLA-G and at a lower intensity, HLA-C were detected in fresh AECs. After 7 d culture, a significant decrease was observed in expression of HLA-AB, HLA-C and HLA-G. MHC class I was expressed with lower intensity in AECs cultured on the 3D scaffold in comparison with the cells cultured on the 2D membranes. There was not any observable band for HLA-DR expression after culture under both 2D and 3D condition ().
Discussion
Structural differences between 2D cell culture and in vivo cellular niches led to efforts to develop 3D culture models, which are more similar to the body’s microenvironment. 3D models affect many cellular mechanisms such as adhesion, migration and differentiation properties. Several factors contribute to cell fate cultured on 2D and 3D matrices, for example, different ligand distribution (especially for integrins), can modify intracellular signalling pathways which affects cell surface molecule expression, growth, differentiation and cell apoptosis [Citation36,Citation37].
In this study, we used chitosan–gelatine scaffolds as 3D matrices. Chitosan was mixed with gelatine to take advantage of biocompatibility, bioabsorbability and non-immunogenic properties [Citation38]. The 3D scaffolds and 2D membranes were prepared and the scaffold pore size was determined for cell seeding. The optimal results were achieved when the 3D scaffolds were pre-frozen at −20 °C for 1 h. Based on these results, a mean pore size of 80 µm and 80% porosity are optimal to support the growth of AECs. The results were consistent with the study of Kang et al. in which demonstrated that freezing gelatine scaffolds in lower temperatures caused the formation of smaller pores because of increasing freezing rate [Citation39]. Moreover, Huang et al. reported the similar results about chitosan–gelatine scaffolds in which the pores are larger at −20 than −80 °C [Citation29]. One of the characteristics which can affect the adhesion of cells to the scaffolds is hydrophilicity which was achieved by pre-freezing at −20 °C for 1 h. Using the SEM images and MTT assay to identify cell attachment showed that the chitosan–gelatine scaffolds provided an effective attachment condition for AEC culture.
There is a scientific consensus on low immunogenicity of AECs [Citation2]; however, there are some controversial reports in some studies on HLA expression by these cells. Therefore, we measured HLA molecule expression in fresh AECs. From the results of immunocytochemistry assay, we found that fresh AECs express membrane HLA-G with high intensity, MHC class I (HLA-AB and HLA-C) with very low intensity and are negative for HLA-DR. mRNA expression for all of the mentioned markers in fresh AECs was consistent with protein markers except for HLA-DR mRNA which was positive. These findings suggest that mRNA expression by native AECs refer to the nature of these cells. AECs arise out of epiblast [Citation10], therefore, similar to three germ cell lineages, these cells tend to express MHC class I and HLA-DR mRNA, but it is inhibited because of suppressive mechanisms in amniotic fluid such as transforming growth factor beta-1 and beta-2 (TGF-β1 and TGF-β2), and soluble HLA-G [Citation40,Citation41]. Therefore, it is rational to accept that omission of inhibitory signals would be resulted in expression of MHC class I and non-classical MHC on AECs after isolation and culture of these cells. Consistent with this idea, culture of AECs on 2D scaffold resulted in upregulation of MHC class I and HLA-DR protein markers on the AECs surface and downregulation of HLA-G protein. In comparison to 2D culture, 3D scaffold did not increase protein expression of MHC class I and HLA-DR. Moreover, HLA-G protein expression remained unchanged in 3D culture. These results confirm that 3D structure can regulate expression of markers in gene and protein levels and there is a capability to affect the immunological state of stem cells with modification of physical properties of the scaffold. This data was consistent with the report of Hudson et al. in which showed that immunological profile of human stem cells could be manipulated using the modification of scaffold compositions [Citation42]. Due to immunoprotective features of the encapsulation technique [Citation43,Citation44], it seems 3D encapsulation of AECs within chitosan–gelatine hydrogel would be a proper strategy to circumvent immunological response of the cells which needs to be evaluated in the future studies.
Conclusions
According to the results of this study, 3D chitosan–gelatine scaffolds can preserve the AECs in their native immunological state. While culturing these cells on the 2D substrate resulted in higher levels of MHC class I and HLA-DR expression, AECs cultured on the 3D scaffold showing the expression levels similar to fresh cells. These results alongside the results of previous related studies suggest that manipulation of the physical and chemical structure of scaffolds could affect the immunological response of cells and enable us to overcome the rejection problems in stem cell therapy. Further studies would be required for the design of biomaterials specifically engineered to optimize the modification of MHC class receptor expressions in the culture process.
Acknowledgements
The authors would like to thank the operation room personnel of Erfan hospital for their assistance in this research.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Tehrani FA, Modaresifar K, Azizian S, et al. Induction of antimicrobial peptides secretion by IL-1β enhances human amniotic membrane for regenerative medicine. Sci Rep. 2017;7:17022.
- Niknejad H, Peirovi H, Jorjani M, et al. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88–99.
- Mohammadi AA, Sabet B, Riazi H, et al. Human amniotic membrane dressing: an excellent method for outpatient management of burn wounds. Iran J Med Sci. 2015;34:61–64.
- Deihim T, Yazdanpanah G, Niknejad H. Different light transmittance of placental and reflected regions of human amniotic membrane that could be crucial for corneal tissue engineering. Cornea. 2016;35:997–1003.
- Kakavand M, Yazdanpanah G, Ahmadiani A, et al. Blood compatibility of human amniotic membrane compared with heparin‐coated ePTFE for vascular tissue engineering. J Tissue Eng Regen Med. 2015;11:1701–1709.
- Niknejad H, Yazdanpanah G. Anticancer effects of human amniotic membrane and its epithelial cells. Med Hypotheses. 2014;82:488–489.
- Niknejad H, Yazdanpanah G, Mirmasoumi M, et al. Inhibition of HSP90 could be possible mechanism for anti-cancer property of amniotic membrane. Med Hypotheses. 2013;81:862–865.
- Modaresifar K, Azizian S, Zolghadr M, et al. The effect of cryopreservation on anti-cancer activity of human amniotic membrane. Cryobiology. 2017;74:61–67.
- Yazdanpanah G, Paeini-Vayghan G, Asadi S, et al. The effects of cryopreservation on angiogenesis modulation activity of human amniotic membrane. Cryobiology. 2015;71:413–418.
- Miki T, Lehmann T, Cai H, et al. Stem cell characteristics of amniotic epithelial cells. Stem Cells. 2005;23:1549–1559.
- Parolini O, Hess DC, Borlongan CV. Human amniotic epithelial cells and amniotic mesenchymal cells express the embruonic stem cell marker Oct-4 in vitro and promote behavioral and histological benefits when transplanted in ischemic stroke rats. J Am Heart Assoc. 2008;39:657–657.
- Lefebvre S, Adrian F, Moreau P, et al. Modulation of HLA-G expression in human thymic and amniotic epithelial cells. Hum Immunol. 2000;61:1095–1101.
- Niknejad H, Yazdanpanah G, Kakavand M. Extract of fetal membrane would inhibit thrombosis and hemolysis. Med Hypothese. 2015;85:197–202.
- Hori J, Wang M, Kamiya K, et al. Immunological characteristics of amniotic epithelium. Cornea. 2006;25:53–58.
- Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363:599–608.
- Ilancheran S, Michalska A, Peh G, et al. Stem cells derived from human fetal membranes display multilineage differentiation potential. Biol Reprod. 2007;77:577–588.
- Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573.
- Methe H, Hess S, Edelman ER. The effect of three-dimensional matrix-embedding of endothelial cells on the humoral and cellular immune response. Semin Immunol. 2008;20:117–122.
- Abandansari HS, Nabid MR, Rezaei SJT, et al. PH-sensitive nanogels based on Boltorn® H40 and poly(vinylpyridine) using mini-emulsion polymerization for delivery of hydrophobic anticancer drugs. Polymer. 2014;55:3579–3590.
- Gonsalves K, Halberstadt C, Laurencin CT, et al. Biomedical Nanostructures. Hoboken (NJ): John Wiley & Sons; 2007.
- Tabatabaei Rezaei SJ, Amini V, Nabid MR, et al. Folate-decorated polymeric Pt(ii) prodrug micelles for targeted intracellular delivery and cytosolic glutathione-triggered release of platinum anticancer drugs. Polym Chem. 2015;6:2844–2853.
- Rahmani Del Bakhshayesh A, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells Nanomed Biotechnol. 2017 [Jul 12]; [15 p.]. DOI:https://doi.org/10.1080/21691401.2017.1349778
- Hoque ME, Nuge T, Yeow TK, et al. Gelatin based scaffolds for tissue engineering-a review. J Polym Res. 2015;9:15.
- Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–1408.
- Mahnama H, Dadbin S, Frounchi M, et al. Preparation of biodegradable gelatin/PVA porous scaffolds for skin regeneration. Artif Cells Nanomed Biotechnol. 2017;45:928–935.
- Dutta P, Rinki K, Dutta J. Chitosan: a promising biomaterial for tissue engineering scaffolds. In: Jayakumar R, Prabaharan M, Muzzarelli RAA, edtors. Chitosan for biomaterials II. Berlin (Germany): Springer; 2011. p. 45–79.
- Karahaliloglu Z, Kilicay E, Denkbas EB. Antibacterial chitosan/silk sericin 3D porous scaffolds as a wound dressing material. Artif Cells Nanomed Biotechnol. 2017;45:1172–1185.
- Han F, Dong Y, Su Z, et al. Preparation, characteristics and assessment of a novel gelatin–chitosan sponge scaffold as skin tissue engineering material. Int J Pharm. 2014;476:124–133.
- Huang Y, Onyeri S, Siewe M, et al. In vitro characterization of chitosan–gelatin scaffolds for tissue engineering. Biomaterials. 2005;26:7616–7627.
- Hajiabbas M, Mashayekhan S, Nazaripouya A, et al. Chitosan–gelatin sheets as scaffolds for muscle tissue engineering. Artif Cells Nanomed Biotechnol. 2015;43:124–132.
- Nazarov R, Jin H-J, Kaplan DL. Porous 3-D scaffolds from regenerated silk fibroin. Biomacromolecules. 2004;5:718–726.
- Alizadeh M, Abbasi F, Khoshfetrat A, et al. Microstructure and characteristic properties of gelatin/chitosan scaffold prepared by a combined freeze-drying/leaching method. Mater Sci Eng C. 2013;33:3958–3967.
- Guan S, Zhang X-L, Lin X-M, et al. Chitosan/gelatin porous scaffolds containing hyaluronic acid and heparan sulfate for neural tissue engineering. J Biomater Sci Polym Ed. 2013;24:999–1014.
- Niknejad H, Deihim T, Peirovi H, et al. Serum-free cryopreservation of human amniotic epithelial cells before and after isolation from their natural scaffold. Cryobiology. 2013;67:56–63.
- Ross DW. Current protocols in cytometry. Arch Pathol Lab Med. 1998;122:660.
- Ross AM, Jiang Z, Bastmeyer M, et al. Physical aspects of cell culture substrates: topography, roughness, and elasticity. Small. 2012;8:336–355.
- Santos E, Hernández RM, Pedraz JL, et al. Novel advances in the design of three-dimensional bio-scaffolds to control cell fate: translation from 2D to 3D. Trends Biotechnol. 2012;30:331–341.
- Gorgieva S, Kokol V. Collagen-vs. gelatine-based biomaterials and their biocompatibility: review and perspectives. In: Rosario Pignatello, editor. Biomaterials applications for nanomedicine. Westminster (London): Intech; 2011.
- Kang H-W, Tabata Y, Ikada Y. Fabrication of porous gelatin scaffolds for tissue engineering. Biomaterials. 1999;20:1339–1344.
- Insausti CL, Blanquer M, García-Hernández AM, et al. Amniotic membrane-derived stem cells: immunomodulatory properties and potential clinical application. Stem Cells Cloning. 2014;7:53.
- Kopaczka K, Skowron K, Kolanko E, et al. The relationship between amniotic epithelial cells and their microenvironment. J Appl Biomed. 2016;14:1–17.
- Hudson KD, Rodriguez M-A, Bonassar LJ. Differential MHC class receptor expression in in vitro human mesenchymal stem cells is mediated by scaffold material. Orlando (FL): Orthopaedic Research Society; 2016.
- Mehrabi M, Dounighi NM, Rezayat Sorkhabadi SM, et al. Development and physicochemical, toxicity and immunogenicity assessments of recombinant hepatitis B surface antigen (rHBsAg) entrapped in chitosan and mannosylated chitosan nanoparticles: as a novel vaccine delivery system and adjuvant. Artif Cells Nanomed Biotechnol. 2017;43:124–132.
- Abandansari HS, Aghaghafari E, Nabid MR, et al. Preparation of injectable and thermoresponsive hydrogel based on penta-block copolymer with improved sol stability and mechanical properties. Polymer. 2013;54:1329–1340.