Abstract
The pathogenicity of Candida species in human is dependent on a variety of virulence factor such as adhesion factors, germ tube and hyphal formation, secretion of hydrolytic phospholipases and proteinases and drug resistance biofilm. ZnO NPs have been synthesized by using leaf extract of Crinum latifolium and were characterized by UV–Vis spectrophotometer, FTIR, SEM, EDX and TEM. In this study for the first time, potent inhibitory effects of ZnO NPs on principal virulence factors of Candida albicans and non-albicans such as germ tube formation, secretion of hydrolytic phospholipases and proteinases and biofilm formation has been investigated. ZnO NPs remarkably reduced the germ tube formation of C. albicans at 1 (86.4%), 0.5 (75.0%), 0.25 (61.4%), 0.125 (34.1%) and 0.062 mg/ml (11.4%). ZnO NPs significantly lowered the phospholipase and proteinase secretion by 58.8 and 95.2% at 0.25 mg/ml, respectively. CSLM results showed that ZnO NPs suppressed biofilm formation up to 85% at 0.25 mg/ml. SEM and TEM micrograph showed that ZnO NPs penetrated inside the cell and causes extensive damaged in cell wall and cell membrane. Inhibition of Candida growth and various virulent factors by ZnO NPs provides an insight towards their therapeutic application for the treatment of Candida-associated infections.
Introduction
Several species of Candida have been reported as etiological agents of human infection and 90% of infections are mainly caused by Candida albicans, C. tropicalis, C. glabrata, C. dubliniensis, C. parapsilosis and C. krusei [Citation1,Citation2]. C. albicans remains the most widespread yeast pathogen and is responsible for ∼50% of candidiasis, including life-threatening nosocomial candidemias that have mortality rates reaching half of infected patients [Citation3]. The pathogenicity of Candida species is dependent on a variety of determinants such as adhesion factors, germ tube and hyphal formation, biofilm formation and secretion of various hydrolytic enzymes [Citation4,Citation5]. The germ tube formation is a transition state from budding to hyphal cells and is known to facilitate yeast adherence to epithelial cells and promote the aggregation of yeast cells [Citation6]. Germ tubes also impart resistance to phagocytic killing compared with the blastoconidia form [Citation7]. Among various extracellular enzymes; phospholipases and proteinases play a vital role in Candida species growth because these enzymes assist in adherence, tissue penetration and invasion to the host [Citation4]. Phospholipases act by cleaving the phospholipids, invading host cells and believed to be associated with germ tubes production, transition into hyphal forms and tissue damage by rupturing the epithelial cell membranes [Citation8,Citation9]. Production of proteinases assists certain organisms to colonize and penetrate host tissue and degrades human proteins such as immunoglobulins, complement proteins and cytokines [Citation9–11]. The ability of Candida spp. to form biofilms on medical devices such as dentures, catheters, contact lenses, voice prostheses has been reported [Citation12,Citation13]. Biofilm formation by Candida species play an important role in pathogenesis because generally they do not respond to conventional antifungal drugs and that may lead to life threatening systemic infections [Citation14–16]. Candida infections associated with medical devices tend to be more problematic than bacterial infections [Citation17]. The leaf of the Crinum latifolium has been chosen for the green synthesis of ZnO NPs because of the presence of various phytochemicals active substances such as alkaloids (e.g. hydroxycrinamidine, epoxyambelline), tannins, phenolic, anthraquinone glycosides, proteins, carbohydrates, gums and mucilages [Citation18–21] which might possibly act as reducing and stabilizing agents during the biosynthesis of zinc oxide nanoparticles (ZnO NPs). C. latifolium are also traditionally used to treat fistula, tumours, rheumatism, rubefacient earaches, whitlow, tubercle and allergic disorders [Citation18–21]. Mild antibacterial activity of aqueous leaf extract of this plant has also been investigated against E. coli, P. aeruginosa and Staphylococcus aureus by some authors [Citation22,Citation23]. Because of the increasing incidence of invasive Candida species infections and their high antifungal resistance, new strategies are needed to suppress the activity of various virulence factors involved in pathogenicity of Candida species. The objective of present work is (i) green synthesis of ZnO NPs using aqueous leaf extract of C. latifolium as bioreductant and stabilizing agents; (ii) characterization of as-synthesized ZnO NPs by UV–Vis spectrophotometer, FTIR, SEM, EDX and TEM; (iii) evaluation of anticandidal potential of ZnO NPs against C. albicans, C. tropicalis and C. dubliniensis using standard serial dilution and well diffusion methods; (iv) characterization of inhibitory effects of ZnO NPs on the germ tube formation; (v) investigation of potent inhibitory effects of ZnO NPs for the first time, on the production of extracellular hydrolytic enzymes, i.e. phospholipases and proteinases secreted by C. albicans, C. tropicalis and C. dubliniensis; (vi) antibiofilm potential of ZnO NPs against C. albicans isolates characterized by CLSM using dual staining (ConA-FITC and PI) method; and (vii) Investigation of morphological and ultrastructural changes in C. albicans after exposure to ZnO NPs by using SEM and TEM.
Materials and methods
Preparation of the leave extract
The collected fresh leaves of Crinum latifolium were washed thoroughly with double distilled water, incised into small pieces and air-dried. About 10 g of leaves were transferred into 250 ml beaker containing distilled water and boiled for 15 min. After cooling, the mixtures were filtered through Whatman No. 1 filter paper and stored at 4 °C for further for further studies.
Biosynthesis of ZnO NPs
For the synthesis of ZnO NPs, 20 ml of leaf extract was added in to 80 ml of aqueous solution of 0.02 M zinc acetate dihydrate (Zn(CH3COO)2.2H2O). The mixture was kept under constant stirring until zinc acetate completely dissolved. Then the flask containing the mixtures was heated at 80 °C for 10–15 min. Addition of 2 M NaOH (pH 12) resulted in a pale white aqueous solution. The precipitate was then washed several times with distilled water followed by ethanol to remove the impurities. The precipitate were further dried at 60 °C overnight to obtained the pale white powder of ZnO nanoparticles.
Characterization of ZnO NPs
The synthesized ZnO NPs were characterized by using UV–Vis spectroscopy, FT-IR, energy dispersive X-ray spectroscopy (EDS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The UV–Vis spectrum of ZnO NPs was recorded using LAMBDA 25-PERKIN ELMER spectrometer. The FT-IR spectra of ZnO NPs were recorded on SHIMADZU-8400 spectrometer using KBr pellets. The elemental composition of ZnO NPs was performed by EDS (JED-2300 Japan). The external morphology and size of the NPs were characterized by scanning electron microscope (SEM, JSM-6510LV, JEOL, JAPAN) and transmission electron microscope (TEM, 2100-JEOL).
Strains
The clinical isolates of Candida species (n = 115) used in this study were collected from oral mucosa specimens submitted to the Antiretroviral (ART) centre, JNMC, AMU, Aligarh. Identification and characterization of various Candida isolates spp. were carried out at department of Microbiology, JNMC as previously described [Citation24].
In vitro susceptibility testing
Fluconazole (25 µg), ketoconazole (10 µg), nystatin (100 U), itraconazole (10 µg), amphotericin B (100 U) and clotrimazole (10 µg) (Hi-Media, Mumbai India) disk were used for susceptibility testing of Candida species as per CLSI [Citation25].
Antifungal activity of ZnO NPs using agar diffusion method
Zone of inhibition test has been performed on SDA plates in an increasing concentration of ZnO NPs, i.e. 0.062, 0.125, 0.25, 0.5 and 1 mg/ml. The antifungal activity was evaluated by measuring the zone of inhibition in millimetres [Citation26].
Minimum inhibitory and minimum fungicidal concentration of ZnO NPs
MICs and MFCs values were assessed on SDA plates as described by Jalal et al. [Citation26] with slight modification.
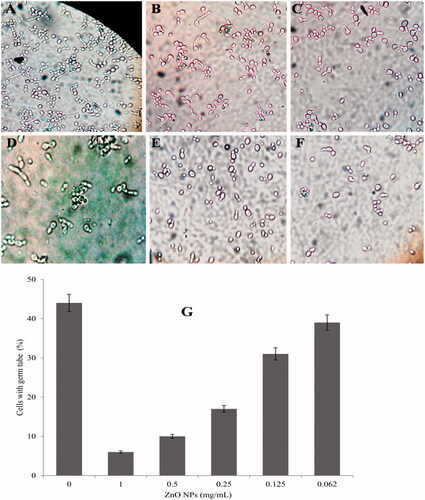
Effect of ZnO NPs on the germ tube formation
Effect of the biosynthesized ZnO NPs on the germ tube formation was studied using method of Mackenzie [Citation27] with modification. Briefly, 2 ml of sterile pooled sheep serum containing 1, 0.5, 0.25, 0.125 and 0.062 mg/ml of ZnO NPS was inoculated with 10 µl of culture and incubate at 37 °C. After 3 h of incubation, smears of each culture were prepared. Sheep serum without NPs was taken as a control. Per smear, fifty cells were randomly selected and the number of cells with germ tube was recorded. Cells were considered germ tube positive, if germ tube was at least twice the length of the cell.
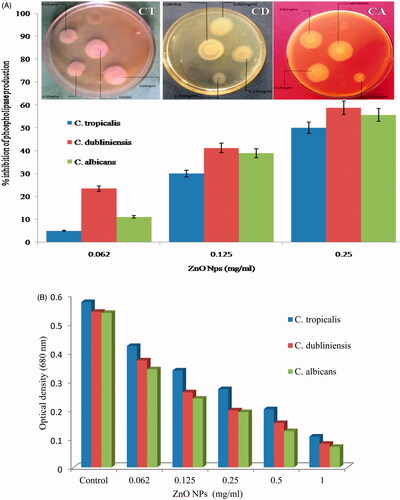
Effect of ZnO NPs on hydrolytic enzyme secretion in Candida isolates
Phospholipase assay. Phospholipase activity of control and treated Candida isolates was performed using the egg-yolk agar plate as described by Price et al. [Citation28]. Briefly, the culture suspensions of each isolates were incubated at 37 °C for overnight, centrifuged at 3000 rpm for 5 min and then pellet was washed with PBS to remove the residual culture medium. The suspensions of C. albicans, C. tropicalis and C. dubliniensis were exposed for 1 h at 0.062, 0.125 and 0.25 mg/ml of ZnO NPs. After that, 10 μl suspension of each strain treated or untreated with ZnO NPs were spotted at equidistant points on egg-yolk agar plate and incubated at 37 °C for 4 days. The secretion of phospholipases was determined by the formation of opaque zones due to precipitation around the yeast colonies. Pz values were calculated as follows: The colony diameter (a) and the diameter of the colony plus the precipitation zone (b). Further, the phospholipase activity of Candida isolates treated with different concentration of ZnO NPs was assessed using spectrophotometer by reading OD at 680 nm as described by Ravichandran and Muthuraman [Citation29].
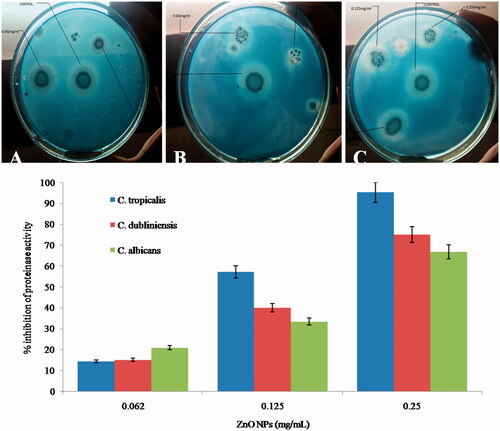
Proteinase assay. Proteinase positive C. albicans, C. tropicalis and C. dubliniensis were transferred to 100 ml flasks containing 5 ml YEPD broth (Yeast extract–peptone–dextrose) and incubated at 37 °C for overnight. After incubation, the culture was centrifuged at 3000 rpm, for 5 min and the pellet were washed by phosphate buffer saline (PBS) to remove the residual medium [Citation30]. The suspensions of C. albicans, C. tropicalis and C. dubliniensis were exposed for 1 h at 0.062, 0.125 and 0.25 mg/ml of ZnO NPs. After that, 10 μl suspension of each strain treated or untreated with ZnO NPs were spotted at equidistant points on yeast carbon base (YCB) medium (1.17%) supplemented with 0.2% bovine serum albumin (BSA) and incubated at 37 °C for 3 days. Secreted proteinase enzyme will degrade BSA and form zone of clearance around the yeast colonies. An index (Pz value) was used to represent the extent of proteinase activity by different strains of C. albicans. A Pz value of 1.0 depicts no enzyme activity. It was calculated as follows: The diameter of colony (a) and the diameter of colony (a) plus the diameter of zone of degradation (b).
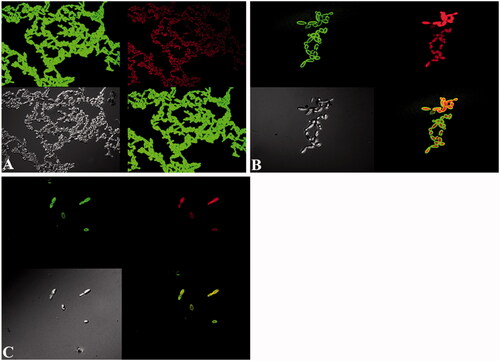
CLSM analysis of C. albicans biofilm. The effect of ZnO NPs on biofilms was qualitatively analysed by CLSM. Biofilms were formed on glass cover slips in 12-well cell culture plates. Biofilms were formed in the presence of 0, 0.125 and 0.25 mg/ml of ZnO NPs at 37 °C for 2 days. At the end of incubation cover slips were transferred to new 12-well plates and washed with PBS. For CLSM analysis, the cells adhered and grown on the surface of cover slips were stained as described previously [Citation31] with 15 mM propidium iodide (PI; Sigma–Aldrich) for 15 min. PI, is a red-fluorescent nucleic acid stain and penetrates only the cells with damaged membranes, thus only the dead cells are visualized. After through washing with PBS, the biofilms were further stained with 50 µg/ml of concanavalin-A conjugated fluorescein isothiocyanate (ConA-FITC; Sigma–Aldrich) in dark for 20 min which stain live cells and exopolysaccharide matrix. Following incubation, images of stained biofilms were visualized with a CSLM system (Fluoview FV1000 Espectral, Olympus Latin America, Miami, FL) equipped with a UPlanSApo 100×/1.40 oil UIS2 Olympus oil immersion lens.
Electron microscopic analysis of effects of ZnO NPs on C. albicans
The effects of Zn NPs on the morphology and ultrastructure of Candida cells were investigated by using SEM (JSM-6510LV, JEOL, JAPAN) and TEM (2100-JEOL). The sample preparation and analysis methods for SEM and TEM were similar to those described previously [Citation26].
Results
Structural characterization of biosynthesized ZnO NPs
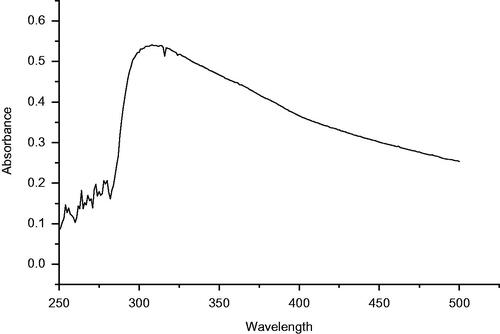
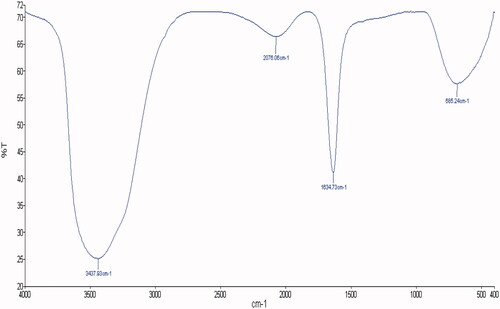
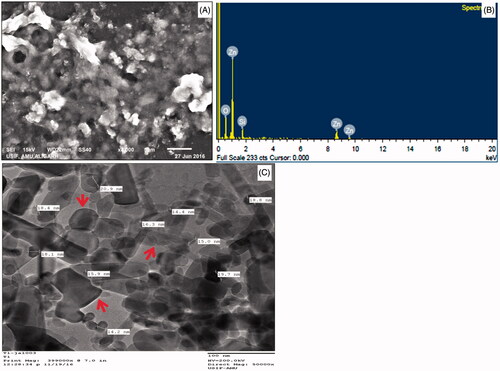
represents the UV–Vis spectra of synthesized ZnO NPs in aqueous suspension. An intensive absorption peak obtained at 320 nm clearly demonstrates the formation of ZnO NPs. The purity of as-prepared ZnO NPs confirmed by the non-existence of other absorption peaks in the spectrum (). FTIR analysis was performed in the 500–4000 cm−1 regions () to determine the functional groups and capping agents involved in the biosynthesis of ZnO NPs. The SEM image and EDX spectra of synthesized ZnO NPs are depicted in . The SEM image shows that the nanoparticles are hexagonal and spherical in shape (). EDX analysis of the synthesized ZnO NPs confirmed the presence of metallic ZnO NPs (). The horizontal axis displays energy in keV while vertical axis displays the number of X-ray counts (). The size of the ZnO NPs analysed by TEM was found to be in the range of 10–30 nm and the shape of the particles was pleomorphic ().
Prevalence and antifungal susceptibility pattern of Candida species
Among 115 Candida isolates evaluated, 52.2% (n = 60) isolates were C. albicans and 47.8% (n = 55) were non-C. albicans species. The non-C. albicans species included C. dubliniensis (24; 20.9%), C. glabrata (18; 15.7%), C. tropicalis (8; 6.9%) and C. krusei (5; 4.3%). The antifungal susceptibility result showed that all isolates were sensitive to nystatin, ketoconazole, itraconazole, amphotericin B and Clotrimazole, whereas 6.7% isolates of C. albicans (4/60) and 8.3% of C. dubliniensis (2/24) were resistant to fluconazole ().
Table 1. Susceptibility pattern of Candida species to antifungal drugs.
Extracellular enzymes, germ tubes and biofilm forming abilities of C. albicans isolates
In the present study, 91.7% (55/60) of C. albicans exhibited phospholipase activity, while proteinase activity was detected in 95% (57/60) of the C. albicans isolates. Germ tubes induction was positive in 66.7% (40/60) isolates of C. albicans. The biofilm forming ability was seen in 40% (24/60) of C. albicans isolates. The associations of GT and biofilm formation in C. albicans isolates with phospholipase and proteinase activity are shown in .
Table 2. The associations of GT and biofilm formation in C. albicans with phospholipase and proteinase activity.
Antifungal activity of ZnO NPs
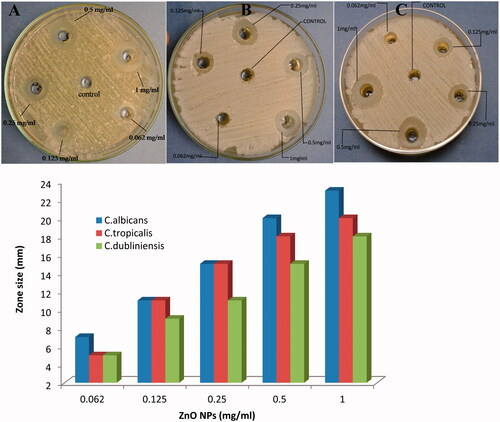
In the present study, antifungal activity of ZnO NPs against clinical isolates of Candida species was assessed on SDA plates by measuring the clear zone of inhibition around the wells supplemented with 0.062–1.0 mg/ml concentration of ZnO NPs. A significant reduction in the growth of Candida species (C. albicans, C. tropicalis and C. dubliniensis) was observed at highest concentration (1.0 mg/ml) (). A maximum zone of inhibition (23 mm) was recorded for C. albicans followed by C. tropicalis (20 mm) and C. dubliniensis (18 mm). Further, the anticandidal activity of ZnO NPs has been assessed using broth dilution method to determine the MICs and MFCs. The obtained MICs values showed that ZnO NPs inhibited the growth of all the tested Candida species. The recorded MICs for C. albicans, C. tropicalis and C. dubliniensis were 0.25, 0.5 and 0.5 mg/ml, respectively. While, the MFCs values for C. albicans, C. tropicalis and C. dubliniensis were 0.5, 1.0 and 1.0 mg/ml, respectively.
Effect of ZnO NPs on germ tube formation
In the present study, the effects of biosynthesized ZnO NPs on germ tube formation in clinical isolates of C. albicans have been investigated. Our results demonstrated that all tested concentration of ZnO NPs was able to inhibit the growth and germ tube formation (). The suppression of germ tube formation was significant in compared to the control. The decrease in the germ tube formation was 86.4, 75.0, 61.4, 34.1 and 11.4% at concentrations of 1.00, 0.5, 0.25, 0.125 and 0.062 mg/ml of ZnO NPs, respectively ().
Effects ZnO NPs on extracellular hydrolytic enzymes activity of C. albicans
We have investigated the effects of ZnO NPs on secretion of phospholipases activity against C. albicans, C. tropicalis and C. dubliniensis. Our results shows that exposure to 0.062, 0.125 and 0.25 mg/ml of ZnO NPs reduced the phospholipase production by 5, 30 and 50% in C. tropicalis; 23.5, 41.2 and 58.8% in C. dubliniensis and 11.1, 38.9 and 55.6% in C. albicans, respectively (). Spectroscopic analysis of change in phospholipase activity of C. albicans upon treatment of ZnO NPs has been shown in . As the concentration of ZnO NPs increases, the diameter of zone decreases indicating that the secretion of phospholipase lowered in various Candida species (). In the present study, we have also assessed the effects of ZnO NPs on the activity of extracellular proteinase enzymes and it was found that exposure to 0.062, 0.125, and 0.25 mg/ml concentration of ZnO NPs proteinase secretion has been suppressed by 14.3, 57.1 and 95.2% in C. tropicalis; 15, 40 and 75% in C. dubliniensis and 20.8, 33.3 and 66.7% in C. albicans, respectively (). A significant difference in precipitation zone with respect to control was observed. clearly shows that as the concentration increases the diameter of zone decreases indicating the proportional effect of ZnO NPs on the production of proteinase by Candida species tested.
Effect on C. albicans biofilm: confocal scanning laser microscopy analysis
In the present study, a significant reduction of biofilm formation abilities of C. albicans in the presence of ZnO NPs has been investigated (). It was demonstrated that ZnO NPs reduced the biofilm formation on cover slips in a concentration-dependent manner i.e. as the concentrations increased, inhibition of biofilm formation also increased. The control untreated biofilms had cells with normal structure and bound together with extracellular matrix (). However, no extracellular matrix was observed when the biofilms was grown in the presence of ZnO NPs and some of the cells had abnormal structure and were damaged (). The combined used of FITC-ConA and PI provided an effective labelling of yeast cells, exopolysaccharide matrix and the damaged cells. It has been found that ZnO NPs at concentration of 0.125 mg/ml result in the reduction of 62% biofilm formation () while the biofilm formation decreased up to 85% at 0.25 mg/ml concentration of ZnO NPs ().
Figure 8. CSLM image C. albicans biofilm. A. Control. (B and C) treated with 0.125 and 0.25 mg/ml of ZnO NPs. Biofilms were stained with ConA-FITC and P I. ConA-FITC stained C. albicans cells as well as exopolysaccharide matrix green. PI stained nucleic acid and fluorescent red. Obvious increase in cell shape and size can be seen in images B and C.
Effects of on the morphology of C. albicans: electron microscopy analysis
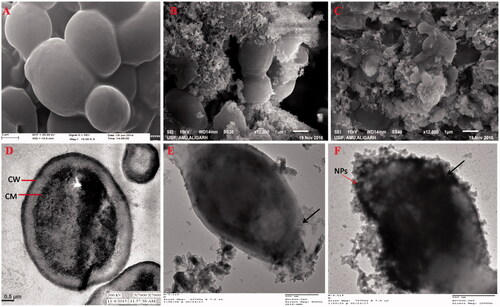
The effect of ZnO NPs on the morphology of C. albicans was monitored by SEM and TEM. The morphology of untreated control C. albicans examined under SEM exhibited normal, smooth, homogenous and regular cell surface (). Treated cells had a rough and abnormal appearance because ZnO NPs get attached and anchored on the cells surface (). ZnO NPs treated cells also exhibited obvious morphological alterations such as deformation and shrinkage (). TEM micrograph of untreated control C. albicans showed a well-conserved morphological characteristic, with a typical intact cell wall and membrane. The typical C. albicans cell wall is composed of an outer electron-dense layers and a low density intermediate space, the thin innermost layer of cell membrane (). Treatment of C. albicans with 0.25 and 0.5 mg/ml of ZnO NPs showed ultrastructural alterations in cell wall and cytoplasmic membrane. Treated cells were generally enlarged, with irregular shape and deformed cell walls. The rupturing and thinning of the cell wall and disintegration of cytoplasmic membrane and their detachment from the cell wall were evident of anticandidal potential of ZnO NPs ().
Figure 9. SEM (A–C) and TEM (D–F) of C. albicans. (A and D) Untreated control cells. (B and C) SEM images: cells treated with 0.25 and 0.5 mg/ml of ZnO NPs; illustrating structural deformities and irregular cell shape; (E and F) TEM images: cells treated 0.25 and 0.5 mg/ml of ZnO NPs; showing the attachment and penetration of NPs (red arrow), degradation and destruction of the outer most layers of cell wall and cytoplasmic membrane (black arrow).
Discussion
In the present study, it has been found that the aqueous leaf extract of C. latifolium, a traditional medicinal plant act as a reducing and stabilizing agents for the biosynthesis of ZnO NPs. The formation of ZnO NPs was confirmed by UV–Vis spectra in the range of 250–500 nm where the maximum absorbance was 320 nm. Similar results have been reported on the green synthesis of ZnO NPS using Aloe vera leaf extract [Citation32]. Nagarajan and Kuppusamy [Citation33] reported that zinc oxide exhibited a characteristics absorption peak in the range of 300–500 nm. The FTIR spectra showed broad peak at 3437.93 cm−1 indicate the hydroxyl group of phenolic component present in extarct (). A similar band pattern has been demonstrated by Ali et al. [Citation28] for the ZnO NPs synthesized by Aloe vera leaf extract. The sharp peak at 2076.06 cm−1 corresponds to stretching vibrations of C = C stretch of alkynes [Citation34]. The peak at 1634.73 cm−1 is due to the amide I region i.e. characteristics of proteins and enzymes present in the natural compound [Citation35]. The absorption peak at lower energy region, i.e. 685.24 cm−1 attributed to metal-oxygen bond and hexagonal phase ZnO [Citation36]. The presence of alkaloid, phenols, reducing carbohydrates, proteins and amino acids in leaf extract of C. latifolium could possibly act as reducing agent for the synthesis and stability of ZnO NPs [Citation37]. Furthermore, it is suggested that the presence of gums and mucilages in leaf extract of C. latifolium [Citation37] also acts as an effective stabilizing and capping agent for ZnO NPs [Citation38]. The shape and elemental composition of ZnO NPs were further confirmed by SEM and EDX. The shape of the as-synthesized ZnO NPs was hexagonal and spherical (). The elemental composition of ZnO NPs analysed by EDX was comprised of zinc (49.8%), oxygen (40.6%) and silicon (9.6%) (). Our EDX data is very similar to that of Nagarajan and Kuppusamy (2013). ZnO NPS usually showed absorption spectra in the range of 1–9.8 keV, similar results were also reported by Nagarajan and Kuppusamy [Citation33] and Ali et al. [Citation35]. In addition to Zn and O, the presence of single weak spectra of silicon (Si) at 1.8 keV () was attributed to the glass silica, which is often used in sample preparation for EDX analysis [Citation39]. The TEM micrographs shows that the shape of ZnO NPs was pleomorphic, i.e. hexagonal, rod, rectangle and spherical and the size were in the range of 10–30 nm (). The size of as-prepared ZnO NPs in the present study is in a good agreement of ZnO NPs synthesized from leaf extract of Azadirachta indica [Citation40,Citation41]. Furthermore, the TEM images clearly show a less intense layering at the periphery of the ZnO NPs (red arrows in ), which confer the presence of bioorganic compounds in plant leaf extract acts as a reducing and capping agents [Citation32,Citation34].
The prevalence of different Candida spp. in our study is in good agreement with previous reports on the distribution of Candida spp. in north Indian tertiary care hospital [Citation24,Citation42]. The virulence of the Candida species is attributed by biofilm and germ tube formation and secretion of extracellular enzymes, i.e. proteinases and phospholipases [Citation43–46]. In the present work, we found that 91.7% C. albicans showed phospholipase activity which are in concordance with the results of Lahkar et al. [Citation43] and de Paula Menezes et al. [Citation44]. However, 95% isolates of C. albicans produces proteinases. The proteinase production by C. albicans is in agreement with the results of de Paula Menezes et al. [Citation44] and Mane et al. [Citation47]. About 66.7% isolates of C. albicans were germ tube positive. In a previous study, 100% germ tube production was reported by Menezes et al. [Citation45]. However, only 40% isolates of C. albicans were able to produce biofilm formation. Our findings concur with the results of previous studies in which biofilm formation was observed in a similar manner [Citation43,Citation46].
The antimicrobial potential of ZnO NPs synthesized by different approach against bacteria [Citation32,Citation48,Citation49] and other fungal species [Citation34,Citation50–52] rather than Candida is well documented in the literature. However, the anticandidal activity of green synthesized ZnO NPs against clinical isolates of Candida species are limited [Citation53,Citation54]. In the present study, significant reduction in the growth of different Candida species has been investigated by well diffusion method (). Similar anticandidal activity of ZnO NPs synthesized by different methods has also been reported [Citation53,Citation55]. clearly shows that as the concentration of ZnO NPs increases, the growth inhibition of cells was also increased [Citation51]. The MICs for C. albicans, C. tropicalis and C. dubliniensis were found in the range of 0.25–0.5 mg/ml while MFCs were in the range of 0.5–1.0 mg/ml, respectively. The obtained MICs of ZnO NPs is in good agreement with the previous published reports [Citation54]. Lipovsky et al. [Citation56] reported that the ZnO NPs at 1.0 mg/ml killed 99.5% of C. albicans cells.
C. albicans is a pleomorphic opportunistic pathogen and germ tubes formation is a phenotypic characteristic of C. albicans which is essential for virulence [Citation57]. It is well known that C. albicans in germ tube form adhere more efficiently and competently to host cells. In the present study, ZnO NPs significantly inhibit the production of germ tube formation in C. albicans up to 86.4% at higher concentration of ZnO NPs, i.e. 1.00, mg/ml (). It was observed that as the concentration of ZnO NPs increased, the inhibitory effect on the germ tube formation also increased. Thus, the suppression of germ tube forming abilities in C. albicans by ZnO NPs could be employed to inhibit the adherence and virulence.
Further, we have investigated the inhibitory effects of biosynthesized ZnO NPs on the production of two important classes of virulence enzymes, i.e. phospholipases and proteinases. Phospholipase enzymes are associated with membrane damage of the host cells, adherence, and penetration [Citation58]. Early data suggest that phospholipases act by invading host cells, causing tissue damage, rupturing the epithelial cell membranes and allowing hyphae to penetrate the cytoplasm [Citation11]. Since phospholipase targets membrane phospholipids and degrades these components, leading to cell lysis; direct host cell damage and lysis has been proposed as a major mechanism contributing to microbial virulence [Citation58,Citation59]. In the present study, exposure to 0.25 mg/ml of ZnO NPs reduced the phospholipase production by 50% in C. tropicalis; 58.8% in C. dubliniensis and 55.6% in C. albicans, respectively in dose-dependent manner. The production of extracellular proteinases was recognized as one of the most important factors for pathogenicity of Candida [Citation60]. The secretion of proteinases is associated with tissue invasion and penetration which resulted degradation of many human proteins on the lesion site, such as secretory immunoglobulin A, albumin, haemoglobin and skin proteins [Citation9,Citation11]. It has also been reported that the expression of proteinase family genes is related to the co-regulation of other virulence factors, such as the adherence, filamentation, biofilm formation and interaction with the host’s immune system functions [Citation61]. In the present study, we found that exposure of 0.25 mg/ml of ZnO NPs proteinase secretion has been suppressed by 95.2% in C. tropicalis; 75% in C. dubliniensis and 66.7% in C. albicans, respectively. It has been found that the suppression of proteinase activity in non-albicans Candida isolates is more pronounced with compare to C. albicans isolates (). Till date, no data is available regarding the effect of ZnO NPs on proteinase and phospholipase activity of germ tube and biofilm forming clinical isolates of C. albicans. In this study, significant reduction in phospholipase and proteinase activity of biofilm forming clinical isolates of C. albicans and non-albicans was observed with green synthesized ZnO NPs. The result obtained on the inhibition of extracellular phospholipases and proteinases enzyme production by ZnO NPs shows that green synthesized ZnO NPs interferes by inhibiting one of the initial stages of the infection process, i.e. hydrolytic enzyme secretion. The lowered secretion of these extracellular hydrolytic enzymes in the presence of green synthesized ZnO NPs may indicate the less virulent nature of tested Candida species. Previously, mycogenic silver and triangular gold nanoparticles have been shown to exhibit significant inhibition of S aspartyl proteinase activity against C. albicans at 100 and 64 ppm, respectively [Citation62,Citation63].
Another important virulence factor involved in the pathogenesis is the biofilm forming abilities of C. albicans on medical devices and other surfaces. Biofilms are highly organized communities of cells and frequently associated with invasive fungal infections. These biofilms are major virulence factor and play an important role in C. albicans pathogenesis. The structural complexity and polymeric extracellular matrix make biofilms more resistant to antifungal drugs than planktonic cells [Citation64,Citation65]. It has been reported that C. albicans can form biofilms on almost any kind of medical device such as vascular and urinary catheters, joint prostheses, cardiac valves, artificial vascular bypass devices, pacemakers, ventricular assist devices and central nervous system shunts [Citation66]. The antibiofilm potential of ZnO NPs against C. albicans biofilm has not been explore yet. Though, the inhibition of C. albicans biofilm by other nanoparticles has been reported [Citation67–70]. In this study, biofilm formation ability of C. albicans was assessed by dual staining procedure using CLSM and found that biosynthesized ZnO NPs inhibit biofilm formation up to 85% at 0.25 mg/ml concentration. Recently, it has been reported that ZnO NPs synthesized using egg albumin as a biotemplate significantly inhibit biofilm formation in C. tropicalis at very low concentration [Citation71]. In a previous study, 55–86% reduction in biofilm formation was observed against C. albicans, C. tropicalis and C. parapsilosis biofilm after exposure to mycogenic synthesized AgNPs [Citation70]. Yu et al. [Citation69] reported that gold nanoparticles resulted in >80% decrease in biofilm formation in C. albicans at 20 ppm.
Finally, we have investigated the effects of green synthesized ZnO NPs on the morphology of the C. albicans by SEM and TEM to explore the ultrastructural changes and possible mechanism of action. SEM micrograph of control C. albicans was normal, smooth and regular while after exposure to ZnO NPs the surface of the cells were abnormal in appearance, deformated and NPs were anchored on the cells surface. TEM micrograph of ZnO NPs treated C. albicans exhibited ultrastructural changes such as rupturing and thinning of the cell wall, disintegration of cytoplasmic membrane and detachment of membrane from the cell wall. The results obtained indicate that the mode of action of ZnO NPs is disrupting cell wall and cytoplasmic membrane. In our previous study, a similar effect on C. albicans cell has been observed when exposed to Al2O3 NPs [Citation26]. In a recent study, it has been proposed that the increased fungicidal activity of ZnO NPs synthesized by leaf extract of Ziziphus nummularia against Candida spp. was attributed due to the small size ZnO NPs [Citation72]. Shoeb et al. [Citation73] explained that the anticandidal activity of ZnO NPs is principally due to the generation of reactive oxygen species (ROS). They demonstrated that the exposure of C. albicans cells to ZnO NPs increased the intracellular ROS production in a time- and dose-dependent manner. ROS-mediated cell injury and cell death in C. albicans has been reported by Lipovsky et al. [Citation56].
Conclusion
The urgency of new anticandidal compounds has been increased due to their therapeutic failure and development of drug resistant in clinical isolates. Germ tube and biofilm formation and the secretion hydrolytic enzymes especially proteinase and phospholipase are important interrelated virulence factors that play an important role in the pathogenicity of candidal infections. For the first time, we have demonstrated that the green synthesized ZnO NPs not only affects the growth of Candida cells but also modulates the pathogenicity of Candida by suppressing its primary virulence factor; germ tube and biofilm formation, secretion of hydrolytic enzymes proteinases and phospholipases. This study revealed the strong inhibitory effect of green synthesized ZnO NPs on pathogenic germ tube (86.4%) and biofilm formation (85%) and in this way; ZnO NPs prevent the invasion of Candida into host cells that may lead to the reducing of Candida species related infections. Further, the significant suppression of hydrolytic proteinase (95.2%) and phospholipase (58.8%) enzymes activity secreted by the Candida cells suggests that ZnO NPs could be a potent inhibitor of hydrolytic enzymes. In conclusion, the dose-dependent anticandidal and antivirulence potential of green synthesized ZnO NPs against clinical isolates of Candida spp. suggests that ZnO NPs could be used as promising anticandidal agents to inhibit the candidal adherence to medical devices and to prevent catheters related fungal infection by coating of catheters with ZnO NPs for biomedical applications.
Acknowledgements
Mr Mohammad Jalal is grateful to Maulana Azad National Fellowship, UGC, New Delhi, India for providing fellowship in the form of JRF. The authors are sincerely thankful to Ahmad Ali Khan and Sanjay Sharma Department of Microbiology, J.N. Medical College, Aligarh Muslim University, Aligarh, U.P India for their technical help and support. Authors thank to Aligarh Muslim University, Aligarh, India and IRMC, Imam Abdulrahman Bin Faisal University, Saudi Arabia for providing instruments facilities and other items used in this study.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Pfaller MA, Diekema DJ, Procop GW, et al. Multicenter comparison of the VITEK 2 antifungal susceptibility test with the CLSI broth microdilution reference method for testing amphotericin B, flucytosine, and voriconazole against Candida spp. J Clin Microbiol. 2007;45:3522–3528.
- Silva S, Negri M, Henriques M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305.
- Gudlaugsson O, Gillespie S, Lee K, et al. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003; 37:1172–1177.
- Schaller M, Borelli C, Korting HC, et al. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses. 2005;48:365–377.
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4:119–128.
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol. 2001;9:327–335.
- Trochin G, Bouchara JP, Robert R, et al. Adherence of Candida albicans germ tubes to plastic: ultrastructural and molecular studies of fibrillar adhesions. Infect Immun. 1988;56:1987–1993.
- Ghannoum MA. Potential role of phospholipases in virulence and fungal pathogenesis. Clin Microbiol Rev. 2000; 13:122–143.
- Yang YL. Virulence factors of Candida species. J Microbiol Immunol Infect. 2003;36:223–238.
- Hube B. Candida albicans secreted aspartyl-proteinases. Curr Top Med Mycol. 1996;1:55–69.
- Mattei AS, Alves SH, Severo CB, et al. Determination of germ tube, phospholipase, and proteinase production by bloodstream isolates of Candida albicans. Rev Soc Bras Med Trop. 2013;46:340–342.
- Donlan RM. Biofilms and device-associated infections. Emerging Infect Dis. 2001;7:277–281.
- Chandra J, Mukherjee PK, Ghannoum MA. Candida biofilms associated with CVC and medical devices. Mycoses. 2011;55:46–57.
- Saville SP, Lazzell AL, Monteagudo C, et al. Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell. 2003;2:1053–1060.
- Sudbery P, Gow N, Berman J. The distinct morphogenic states of Candida albicans. Trends Microbiol. 2004;12:317–324.
- Nobile CJ, Mitchell AP. Genetics and genomics of Candida albicans biofilm formation. Cell Microbiol. 2006;8:1382–1391.
- Darouiche RO, Mansouri MD, Kojic EM. Antifungal activity of antimicrobial-impregnated devices. Clin Microbiol Infect. 2006; 12:397–399.
- Ghosal S, Singh SK. (Chemical constituents of Amaryllidaceae. Part 24. Crinafoline and crinafolidine, two anti-tumor alkaloids from Crinum latifolium. J Chem Res. 1986;1986 (Suppl):312–313.
- Beckstrom-Sternberg SM, Duke JA, Wain KK. The Ethnobotany Database Genome Informatics Group, National Agricultural Library. Beltsville (MD): U.S. Department of Agriculture; 1994.
- Tram NT, Mitova M, Bankova V, et al. GC-MS of Crinum latifolium L. alkaloids. Z Naturforsch, C J Biosci. 2002;57:239–242.
- Solanki J, Dhiman A, Nanda A, et al. Pharmacognostic and preliminary phytochemical evaluation of the leaves of Crinum latifolium L. Int J Pharma Sci Res. 2011; 2:3219–3223.
- Malik A, Bai S, Teneja R, et al. In vitro screening of some Indian medicinal plants for their activity against extended spectrum beta lactamases. Br Biotechnol J. 2015;8:1–10.
- Rahman MA, Hussain MS, Millat MS, et al. Screenings of in-vitro antimicrobial, cytotoxic and anti-inflammatory activity of crude methanolic extracts of crinum latifolium (Leaves). J Med Plants Res. 2016;10:649–655.
- Khan PA, Malik A, Khan HS. Profile of candidiasis in HIV infected patients. Iran J Microbiol. 2012;4:204–209.
- Clinical and Laboratory Standards Institute. Method for antifungal disk diffusion susceptibility testing of yeasts; Approved guideline. 2nd ed. Wayne: Clinical and Laboratory Standards Institute; 2009.
- Jalal M, Ansari MA, Shukla AK, et al. Green synthesis and antifungal activity of Al2O3 NPs against fluconazole resistant Candida spp. isolated from a tertiary care hospital. RSC Adv. 2016;6:107577–107590.
- Mackenzie DW. Serum tube identification of Candida albicans. J Clin Pathol. 1962;15:563–565.
- Price MF, Wilkinson ID, Gentry LO. Plate method for detection of phospholipase activity in Candida albicans. Med Mycol. 1982;20:7–14.
- Ravichandran S, Muthuraman S. Examining the anti-candidal activity of 10 selected Indian herbs and investigating the effect of Lawsonia inermis extract on germ tube formation, protease, phospholipase, and aspartate dehydrogenase enzyme activity in Candida albicans. Indian J Pharmacol. 2016;48:47–52.
- Rüchel J, Tegeler R, Trost MA. Comparison of secretory proteinases from different strains of Candida albicans. Med Mycol. 1982;20:233–244.
- Ansari MA, Khan HM, Khan AA, et al. Anti-biofilm efficacy of silver nanoparticles against MRSA and MRSE isolated from wounds in a tertiary care hospital. Indian J Med Microbiol. 2015;33:101–109.
- Qian Y, Yao J, Russel M, et al. Characterization of green synthesized nano-formulation (ZnO-A. vera) and their antibacterial activity against pathogens. Environ Toxicol Pharmacol. 2015;39:736–746.
- Nagarajan S, Kuppusamy KA. Extracellular synthesis of zinc oxide nanoparticles using seaweeds of gulf of Mannar, India. J Nanobiotechnol. 2013;11:39.
- Jamdagni P, Khatri P, Rana JS. Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J King Saud UnivSci. 2016. (in press). https://doi.org/https://doi.org/10.1016/j.jksus.2016.10.002.
- Ali K, Dwivedi S, Azam A, et al. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J Colloid Interface Sci. 2016;472:145–156.
- Gawade VV, Gavade NL, Shinde HM, et al. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J Mater Sci Mater Electron. 2017; 28:14033.
- Solanki J, Dhiman A, Nanda A, et al. Pharmacognostic and preliminary phytochemical evaluation of the leaves of Crinum latifolium. IJPSR. 2011;2:3219–3223.
- Barros HR, Cardoso MB, de Oliveira CC, et al. Stability of gum arabic-gold nanoparticles in physiological simulated pHs and their selective effect on cell lines. RSC Adv. 2016;6:9411–9420.
- Anjum S, Abbasi BH. Biomimetic synthesis of antimicrobial silver nanoparticles using in vitro-propagated plantlets of a medicinally important endangered species: Phlomis bracteosa. Int J Nanomedicine. 2016;22:1663–1675.
- Bhuyan T, Mishra K, Khanuja M, et al. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semiconduct Process. 2015;32:55–61.
- Madan HR, Sharma SC, Udayabhanu, et al. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim Acta A Mol Biomol Spectrosc. 2016;5:404–416.
- Maheshwari M, Kaur R, Chadha S. Candida species prevalence profile in HIV seropositive patients from a major tertiary care hospital in New Delhi, India. J Pathog. 2016;2016:6204804.
- Lahkar V, Saikia L, Patgiri SJ, et al. Estimation of biofilm, proteinase & phospholipase production of the Candida species isolated from the oropharyngeal samples in HIV-infected patients. Indian J Med Res. 2017;145:635–640.
- de Paula Menezes R, de Melo Riceto ÉB, Borges AS, et al. Evaluation of virulence factors of Candida albicans isolated from HIV-positive individuals using HAART. Arch Oral Biol. 2016;66:61–65.
- Menezes TOA, Gillet LCS, Menezes SAF, et al. Virulence factors of Candida albicans isolates from the oral cavities of HIV-1-positive patients. Curr HIV Res. 2013;11:304–308.
- Tay ST, Abidin IA, Hassan H, et al. Proteinase, phospholipase, biofilm forming abilities and antifungal susceptibilities of Malaysian Candida isolates from blood cultures. Med Mycol 2011;49:556–560.
- Mane A, Gaikwad S, Bembalkar S, et al. Increased expression of virulence attributes in oral Candida albicans isolates from human immunodeficiency virus-positive individuals. J Med Microbiol. 2012;61:285–290.
- Ansari MA, Khan HM, Khan AA, et al. Synthesis and characterization of the antibacterial potential of ZnO nanoparticles against extended-spectrum β-lactamases-producing Escherichia coli and Klebsiella pneumoniae isolated from a tertiary care hospital of North India. Appl Microbiol Biotechnol. 2012;94:467–477.
- Santhoshkumar J, Kumar SV, Rajeshkumar S. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technol. 2017;3:459–465.
- He L, Liu Y, Mustapha A, et al. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol Res. 2011;166:207–215.
- Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci Mater Int. 2012;22:693–700.
- Rajiv P, Rajeshwari S, Venckatesh R. Bio-Fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim Acta A Mol Biomol Spectrosc. 2013;112:384–387.
- Dhillon GS, Kaur S, Brar SK. Facile fabrication and characterization of chitosan-based zinc oxide nanoparticles and evaluation of their antimicrobial and antibiofilm activity. Int Nano Lett. 2014;4:107.
- Karimiyan A, Najafzadeh H, Ghorbanpour M, et al. Antifungal effect of magnesium oxide, zinc oxide, silicon oxide and copper oxide nanoparticles against Candida albicans. Zahedan J Res Med Sci. 2015;17:e2179.
- Khan MF, Ansari AH, Hameedullah M, et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: potential role as nano-antibiotics. Sci Rep. 2016; 6:27689.
- Lipovsky A, Nitzan Y, Gedanken A, et al. Antifungal activity of ZnO nanoparticles-the role of ROS mediated cell injury. Nanotechnology. 2011;22:105101.
- Bernardes I, Felipe Rodrigues MP, Bacelli GK, et al. Aloe vera extract reduces both growth and germ tube formation by Candida albicans. Mycoses. 2012;55:257–261.
- Mohandas V, Ballal M. Distribution of Candida species in different clinical samples and their virulence: biofilm formation, proteinase and phospholipase production: a study on hospitalized patients in Southern India. J Global Infect Dis. 2011;3:4–8.
- Salyers A, Witt D. Virulence factors that damage the host. In: Salyers A, Witt D, editors. Bacterial pathogenesis: a molecular approach. Washington (DC): ASM Press; 1994. p. 47–62.
- Fotedar R, Al-Hedaithy SSA. Comparison of phospholipase and proteinase activity in Candida albicans and C. dubliniensis. Mycoses. 2005;48:62–67.
- Naglik JR, Albrecht A, Bader O, et al. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 2004;6:915–926.
- Hamid S, Zainab S, Faryal R, et al. Inhibition of secreted aspartyl proteinase activity in biofilms of Candida species by mycogenic silver nanoparticles. Artif Cells Nanomed Biotechnol. 2017;25:1–7.
- Hajjar Esmaeil FHE, Jebali A, Hekmatimoghaddam S. The inhibition of Candida albicans secreted aspartyl proteinase by triangular gold nanoparticles. Nanomed J. 2015;2:54–59.
- Costerton JW. Overview of microbial biofilms. J Ind Microbiol. 1995; 15:137–140.
- Vila T, Fonseca BB, DA Cunha MML, et al. Candida albicans biofilms: comparative analysis of room-temperature and cryofixation for scanning electron microscopy. J Microsc. 2017;267:409–419.
- Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev. 2004;17:255–267.
- Monteiro DR, Silva S, Negri M, et al. Silver nanoparticles: influence of stabilizing agent and diameter on antifungal activity against Candida albicans and Candida glabrata biofilms. Lett Appl Microbiol. 2012;54:383–391.
- Lara HH, Romero-Urbina DG, Pierce C, et al. Effect of silver nanoparticles on Candida albicans biofilms: an ultrastructural study. J Nanobiotechnol. 2015;13:91.
- Yu Q, Li J, Zhang Y, et al. Inhibition of gold nanoparticles (AuNPs) on pathogenic biofilm formation and invasion to host cells. Sci Rep. 2016;6:26667.
- Hamid S, Zainab S, Faryal R, et al. Deterrence in metabolic and biofilms forming activity of Candida species by mycogenic silver nanoparticles. J Appl Biomed. 2017;15:249–255.
- Jothiprakasam V, Sambantham M, Chinnathambi S, et al. Candida tropicalis biofilm inhibition by ZnO nanoparticles and EDTA. Arch Oral Biol. 2017;73:21–24.
- Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol. 2017;45:1751–1761.
- Shoeb M, Singh BR, Khan JA, et al. ROS-dependent anticandidal activity of zinc oxide nanoparticles synthesized by using egg albumen as a biotemplate. Adv Nat Sci Nanosci Nanotechnol. 2013;4:035015.