?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, we explained in detail a targeted nano-photo-thermal therapy (NPTT) method to induce selective apoptosis in cancer cells. Folate-conjugated gold nanoparticles (F-AuNPs) were synthesized by tailoring the surface of AuNPs with folic acid to enhance the specificity of NPTT. KB cancer cells, as a folate receptor over-expressing cell line, and L929 normal cells with low level of folate receptors were incubated with the synthesized F-AuNPs and then irradiated with various laser intensities and exposure durations. Following various regimes of NPTT, we assessed the level of cell viability and the ratio of apoptosis/necrosis. No significant cytotoxicity was observed for both cell lines at concentrations up to 40 μM of F-AuNPs. Moreover, no significant cell lethality occurred for various laser irradiation conditions. The viability of KB and L929 cells incubated with F-AuNPs (40 μM; 6 h) and then irradiated by laser (1 W/cm2; 2 min) was 57 and 83%, respectively. It was also demonstrated that the majority of cancer cell death is related to apoptosis (41% apoptosis of 43% overall cell death). In this process of F-AuNPs based NPTT, it may be concluded that the main factor determining whether a cell dies due to apoptosis or necrosis depends on laser irradiation conditions.
In this study, we explained in detail a targeted nano-photo-thermal therapy (NPTT) method to induce selective apoptosis in cancer cells.
Graphical Abstract
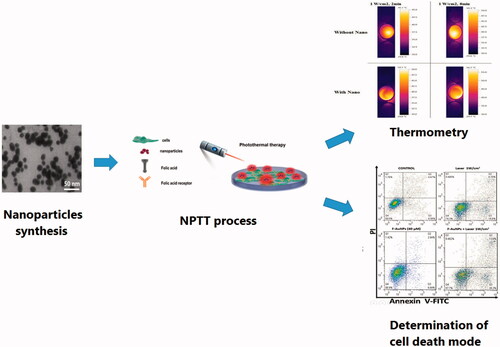
Introduction
Regarding the fact that cancer is one of the main challenges in the field of public health, significant progresses have been made to find some new curative approaches [Citation1]. However, advances in cancer treatment modalities are inadequate and investigations still continue to develop novel therapeutic strategies [Citation2–4]. From the point of view of the need for cancer targeting, nanotechnology seems to be potentially the most important way of employing various nanomaterials to overcome the main limitations of current cancer therapies [Citation5]. Through different targeting ligands, nanomaterials may be specifically targeted towards cancer tissues, to selectively bind to cancer cells and destroy them [Citation6]. Therefore, various smart therapeutic nanomaterials may be synthesized and can be introduced as highly precise, reliable, effective nanoplatforms for cancer treatment. Among various targeted ligands, folic acid provides a promising targeting method to selectively target the cancer cells via receptor-mediated endocytosis [Citation7,Citation8].
Water-soluble folic acid (or folate) molecule plays a pivotal role in the synthesis of thymine by dihydrofolate reductase in DNA synthesis pathway and cell division. This molecule is brought into cytosol by the folate receptor (FR). Some specific cancer cells such as mouth epidermal carcinoma KB cell line tend to overexpress FR due to their extensive requirement for folate. A positive aspect of using folate as a targeting ligand is related to its promising characteristics: non-immunogenic, specificity for many types of cancer cells, and possible conjugation with a number of nanotechnology platforms [Citation9,Citation10].
In the last decade, gold nanoparticles (AuNPs) have been brought to the forefront of cancer research due to their easy synthesis, facile surface modification and functionalization, excellent biocompatibility, intrinsic chemo-physical stability, and enhanced optical tunable characteristics. Synthetic advancement in the last decade engenders a vast array of plasmonic AuNPs with different compositions, structures, shapes and surface coatings properties, boosting their values in photothermal cancer therapy [Citation11–13]. When AuNPs nanoplatforms are deposited into cancer cells, a wide variety of methods can be used to eradicate cells, such as radiation therapy and/or thermal ablation of a tumor.
Plasmonic AuNPs can absorb the external energy of laser, convert the absorbed energy to heat via a series of non-radiative relaxation and dissipate the heat near cancerous cells without harming the surrounding healthy tissues [Citation14]. It seems that plasmonic AuNPs have been mostly identified as a popular area in the field of nano-photo-thermal therapy (NPTT) of cancer because of their intriguing optical properties [Citation15]. The NPTT methods are typically involved in the delivery of nanoparticles to the cancer cells prior to irradiation by laser to improve the spatial selectivity of laser heating [Citation16,Citation17]. Eventually, the consequences of such reactions are irreparable cell damage, protein denaturation, and cell membranes rupture [Citation18–20].
Generally, cell death appears to be typically a two-step process: either physiological cell death, named as apoptosis, or accidental cell death, named as necrosis. Apoptosis has long been regarded to be as an active, regulated, controlled and programmed process of autonomous cellular disassembling. Crucially, apoptotic cells are quickly phagocytosed by macrophages or adjacent normal cells to avoid inflammation and damage to normal cells. In contrast to apoptosis, necrosis has been generally described as a passive, accidental, uncontrolled and toxic mode of cell death resulting from environmental perturbations. Necrotic cells typically undergo the sudden loss of cell membrane integrity, extensive organelle and cell swelling, and uncontrolled release of intracellular constituents into the extracellular milieu. In the clinic, the target must be to mainly induce apoptosis rather than necrosis, which is appealing and very favourable as programing cell death to remove cancer cells without hurting neighboring healthy cells [Citation21].
In this study, we report a possible NPTT approach to selectively induce apoptosis by taking advantages of both the folate targeting method and plasmonic AuNPs properties. We delineate the synthesis of folate-conjugated gold nanoparticles (F-AuNPs) by tailoring the surface of AuNPs with folic acid to enhance the specificity of NPTT of cancer [Citation22–30]. The main hypothesis of this study was that the presence of AuNPs inside the cancer cells and the conditions of laser irradiation are two important factors to induce selective apoptosis in cancer cells. Accordingly, we tested our hypothesis on two different cell lines with various FR expression levels (KB as an over-expressing FR cell line and L929 normal cell line as the control). Also, we examined different laser irradiation conditions and then determined the ratio of necrotic and apoptotic cells after NPTT.
Materials and methods
Material
F-AuNP was prepared in R&D Department of Nanobon Company (Tehran, Iran). Trypan blue, Roswell Park Memorial Institute (RPMI)-1640 cell culture medium, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), streptomycin, penicillin, and trypsin–ethylene diamine tetra acetic acid (EDTA) were purchased from Sigma-Aldrich company (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Gibco® (Carlsbad, CA).
Synthesis of F-AuNPs
F-AuNPs nanoconjugate was prepared according to the method recently reported by Li et al. and Neshastehriz et al. [Citation31,Citation32]. Briefly, to obtain 100 ml of F-AuNPs, an aqueous solution of HAuCl4 (Au; 150 ppm) and trinatrium citrate (150 mg) was heated to 80 °C while vigorously stirred for ∼2 h until the color of the solution turned to deep red. After cooling the solution to room temperature, a solution of FA (150 mg in 5 ml ethanol) was added under ultrasonic probe and sonicated for 10 min. The formation of FA-conjugated AuNPs was confirmed by spectrophotometer, transmission electron microscope (TEM), and Fourier transform infrared spectroscopy (FTIR).
Characterization of nanoparticles
Particles size and morphology determination
The morphological characteristics of nanoparticles were analyzed by a Zeiss LEO 906 TEM (Oberkochen, Germany). For this purpose, nanoparticles were suspended in distilled water and sonicated for 10 min. A small drop of suspension was deposited onto a carbon-coated copper grid and allowed to be evaporated. Then, the perfectly dried grid was observed under TEM at 120 kV. Furthermore, size distribution and effective hydrodynamic size of nanoparticles were determined by dynamic laser light scattering (DLS) using a Malvern Zetasizer, NANO ZS (Malvern Instruments Limited, Malvern, UK).
Surface charge
Zeta potential is an indicator of surface charge, which determines particle stability in dispersion. We determined zeta potential of nanoparticles using a Malvern Zetasizer, NANO ZS (Malvern Instruments Limited).
UV–Visible absorption studies
UV–visible (UV–vis) absorption spectroscopic measurements were recorded on a single-beam UV–vis spectrometer, Agilent 8453 (Palo Alto, CA), using quartz cells of 1 cm path length and DI water as the reference at room temperature.
FTIR studies
FTIR spectra were recorded by using a Shimadzu FT-IR 4300 (Kyoto, Japan) instrument equipped with pressed KBr pellets in the wavenumber range 500–3600 cm−1 at room temperature.
Cell culture
Our experiments were conducted on two different cell lines: human mouth epidermal carcinoma KB cell line and murine fibroblast L929 cell line. The cells were grown as a monolayer using the RPMI-1640 cell growth medium with l-glutamine and NaHCO3 supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) in T-25 tissue culture flasks. The cells were maintained in a humidified atmosphere that contained 5% CO2 and 95% air at 37 °C, and the culture medium was changed every 2 days. The cells were harvested by trypsinizing cultures with 1 mM EDTA/0.25% Trypsin (w/v) in phosphate-buffered saline (PBS).
Light source
Both KB and L929 cells were irradiated with a continuous laser at wavelength of 532 nm (Changchun New Industries Optoelectronics Technology Co. Ltd, Changchun, China). The cells were plated in 24-well cell culture plates (well bottom area =1.9 cm2), incubated overnight to adhere, and then irradiated by laser with various intensities and different exposure time. MTT assay were finally performed as described in “MTT assay” section, to evaluate cell after laser exposure.
Cytotoxicity effects of F-AuNPs
After three passages, KB and L929 cells were counted using a regular hemocytometer and nearly 100,000 cells per well were seeded in 24-well plates and incubated overnight to adhere. Then, cells were treated with various concentrations of F-AuNPs for 6 h (six wells for each concentration were considered). Cell culture medium alone, without nanoparticles, was used as a control. After completion of incubation period, MTT assay was performed to evaluate the level of cell survival.
Cytotoxicity effects of F-AuNPs in the presence of laser
After incubation with F-AuNPs, the cells were washed three times with PBS for removing excess free nanoparticles from culture medium and then fresh medium was added to the cells. For photothermal therapy, each plate was exposed to laser beam (using variable laser intensities and irradiation duration). After photothermal therapy, MTT assay and flow cytometry were performed to evaluate the effects of various treatments.
MTT assay
In all experiments, the viability of KB and L929 cells was evaluated using MTT tetrazolium assay, which measures the ability of metabolically active mitochondria in live cells to reduce a colorless tetrazolium compound to a blue formazan product. To perform this assay, the culture medium was removed. After adding 900 μl of FBS-free culture medium, 100 μl MTT dye (at a concentration of 5 mg/mL) was also added to each well, and the plate was incubated for 4 h. Then, MTT-containing medium was removed and replaced with DMSO (1 ml per well) to dissolve the formazan crystals and kept in the dark at room temperature for 15 min. Finally, the absorbance of the dissolved formazan was read at wavelength of 570 nm by an ELISA reader (Dynex MRX; Dynex Technologies, Carnegie, PA) using a reference wavelength of 630 nm. The relative survival of KB and L929 cells was represented as the absorbance of the treated sample/absorbance of the control group. The optical density (OD) of dissolved formazan is directly proportional to living cells. In our experiments, we designed control groups beside the treated groups, and therefore, the cell viability percentage was calculated by the following formula:
All experiments were repeated at least three times.
Flow cytometry
The extent of apoptosis was measured through annexinV–fluorescein isothiocyanate (FITC) apoptosis detection kit as described in the manufacturer’s instructions (eBioscience, Inc., San Diego, CA). After performing our experiments on cells, the cells were plated at a density of 200,000 cells/well in flat bottom 24-well plates. In brief, cells were harvested and centrifuged at 300g for 5 min. Then, the cells were washed once with PBS and once with 1× binding buffer. After that, cells were suspended in 1× binding buffer and 5 μl of flurochrome-conjugated AnnexinV was added to 100 μl of cell suspension and incubated for 15 min in dark at room temperature. Cells then washed with 2 ml of binding buffer and suspended in 200 μl of 1× binding buffer. Finally, 5 μl of propidum iodide (PI) staining solution was added to 200 μl cell suspensions and assessed by flow cytometer (BD FACSCantoII; BD Biosciences, San Jose, CA).
Thermometry
Sensitive probing of temperature variations in each well of microplate was done to monitor and evaluate heat dissipation in integrated ambience during NPTT. KB cells were seeded in a 24-well cell culture plate at a density of 105 cells/well and incubated overnight at 37 °C in a humidified 5% CO2 atmosphere. The medium solution containing F-AuNPs (40 μM) were prepared and added to the cells. After 6 h of incubation, the culture medium was removed. The cells were washed twice with PBS to remove unloaded nanoparticles and then fresh medium was added. The cells were positioned in front of the laser beam (1 W/cm2 for 1, 2, 3, and 4 min) and irradiation began at a baseline of room temperature. The distance between the plate and laser source was set to cover the entire surface of a well. The temperature variations of the cells were monitored using digital infrared thermal camera (Testo 875-1i, Lenzkirch, Germany) during laser irradiation.
Statistical analysis
All experiments were performed in triplicate. All data are expressed as mean ± SD (standard deviation). For statistical analysis, one-way analysis of variance (ANOVA) followed by Tukey’s test as the post hoc analysis were performed using SPSS version 16 (Chicago, IL). The value of p < .05 was considered to be statistically significant.
Results
Characterization of the F-AuNPs
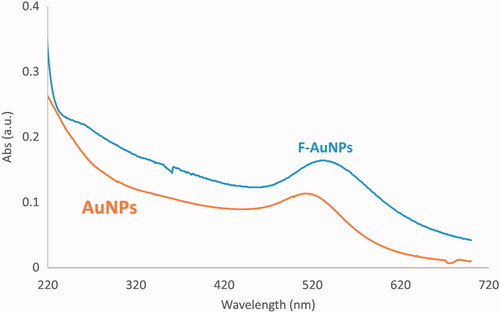
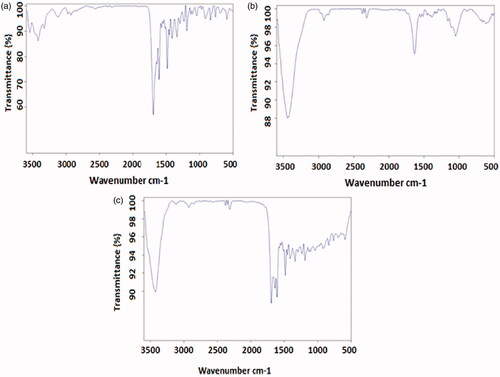
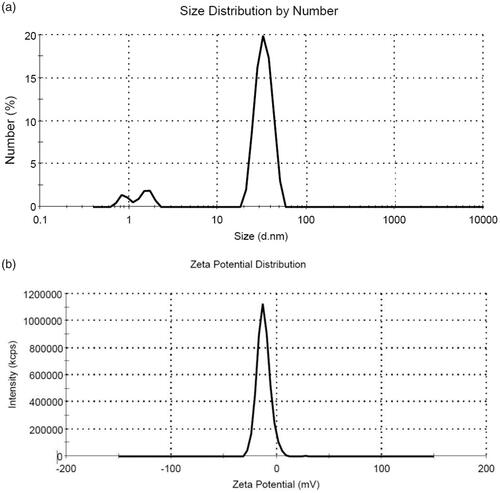
TEM was performed to assess the morphological characteristics of F-AuNPs. shows a typical TEM micrograph of the synthesized F-AuNPs. The results suggest that the nanoparticles are well-dispersed and appear to be spherical in shape with a size distribution of 5–20 nm. The hydrodynamic size and zeta potential of nanoparticles were measured using DLS and zeta potential analyzer, respectively. The obtained results are shown in . It was determined that the hydrodynamic size of nanoparticles is 33.8 nm. Moreover, the zeta potential of the synthesized nanoparticles was −12.5 mV, as shown in . shows the UV–Vis absorption spectrum of F-AuNPs. Accordingly, it may be found that F-AuNPs has three absorption peaks in the range of UV–Vis wavelengths. By looking at the UV–Vis absorption spectrum of F-AuNPs, we identified that F-AuNPs absorption peaks are ∼280 and 360 nm pertaining to folate conjugation and ∼530 nm pertaining to AuNPs. shows FTIR spectrum of folic acid in which wavenumbers of 3418 and 3544 cm−1 correspond to the stretching vibrations of the amide and amine groups. Characteristic bands of C = O group are ∼1694 and 1607 cm−1. FTIR spectrum of AuNPs stabilized by trisodium citrate is presented in . In this figure, the peaks at 2923, 1635, and 3442 cm−1 correspond to the stretching vibration of CH, C = O, and OH groups, respectively. A strong peak must be seen in in the range of 1690–1760 cm−1 for the stretching vibration of the C = O group while this peak is absent. The reason of this phenomenon may pertain to some events that occurred for the carboxyl groups of citrate and the coverage of AuNPs with citrate is an option. Finally, shows FTIR spectrum of F-AuNPs in which peaks at 1693, 1638, and 1607 cm−1 correspond to the stretches of the C = O groups and the peak at 3422 cm−1 correspond to the OH group. By comparing ), it may be inferred that the absence of amine group peaks in is due to the conjugation of amine groups of folic acid to AuNPs and this document shows that folic acid has been conjugated to AuNPs.
Figure 1. TEM image of the synthesized F-AuNPs, suggesting that the nanoparticles are well-dispersed and appear to be spherical in shape with a size distribution of 5–20 nm.
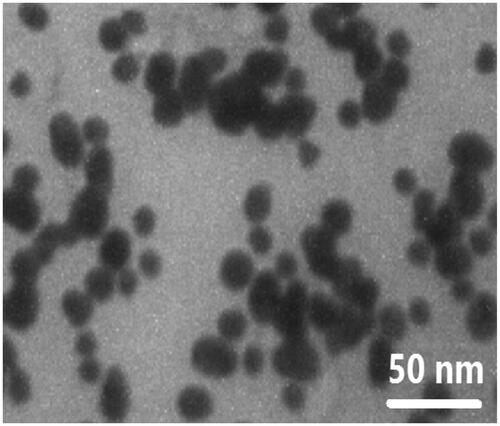
Figure 2. (a) Size distribution of the synthesized F-AuNPs (peak: 33.8 nm). (b) Zeta potential distribution of the synthesized F-AuNPs (peak: −12.5 mV).
MTT assay results
Cytotoxicity of nanoparticles
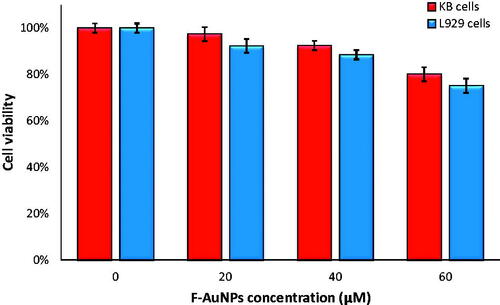
We investigated the cytotoxicity of the F-AuNPs on both KB and L929 cell lines at concentrations of 20, 40 and 60 μM for 6 h incubation period using MTT assay. As seen in , significant cell death occurred at nanoparticles concentration higher than 40 μM. As a result, we selected 40 μM for further studies so that nanoparticles alone would not induce significant cell death.
Cytotoxicity of laser
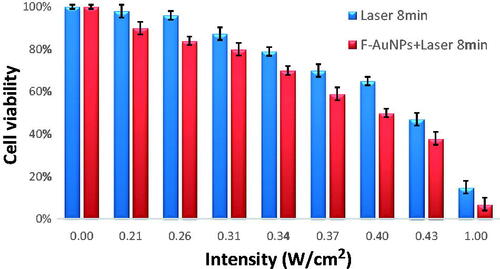
To examine the effect of various laser intensities on KB cells, different intensities (0.21, 0.26, 0.31, 0.34, 0.37, 0.40, 0.43, and 1 W/cm2) were applied for 8 min. These examinations were conducted to observe how the intensity of laser affects cell viability. As seen in , no significant lethality for KB cells occurred when intensity of laser was increased up to 0.26 W/cm2. The KB cell viability dropped from 96% for 0.26 W/cm2 to 87% for 0.31 W/cm2, 79% for 0.34 W/cm2, 70% for 0.37 W/cm2, 65% for 0.40 W/cm2, 47% for 0.43 W/cm2, and to 10% for 1 W/cm2.
Figure 6. The viability of KB cells irradiated by different laser intensities (532 nm for 8 min) in the absence and the presence of F-AuNPs (40 μM for 6 h).
To study the effect of various exposure duration of laser (at the same intensity of 1 W/cm2) on cell viability, different exposure times (1, 2, 3, 4, and 8 min) were applied to the KB cells. As seen in , no significant cell lethality occurred when duration of laser exposure was increased up to 2 min. The KB cell viability dropped from 95% for 2 min to 65% for 3 min, 34% for 4 min. Also, we found a cell viability of 10% for KB cells when irradiated by 1 W/cm2 for 8 min (see ). As a result, we selected 2-min laser exposure time at an intensity of 1 W/cm2 for further studies so that laser irradiation alone would not induce significant cell death.
Cytotoxicity of F-AuNPs in combination with laser irradiation
To evaluate the effect of various laser intensities on cell viability following NPTT of KB cells, different laser intensities were tested for the same exposure duration (8 min). The cells were incubated with 40 μM of F-AuNPs (for 6 h) prior to laser exposure, and then the KB cells were exposed by laser. As seen in , significant cell lethality occurred at all intensities of laser. Moreover, to examine the effect of various laser exposure durations on cell viability, different durations (from 1 to 8 min) at the same laser intensity were tested on KB cells incubated with
F-AuNPs, as seen in both and , significant cell lethality occurred for all durations of laser irradiation.
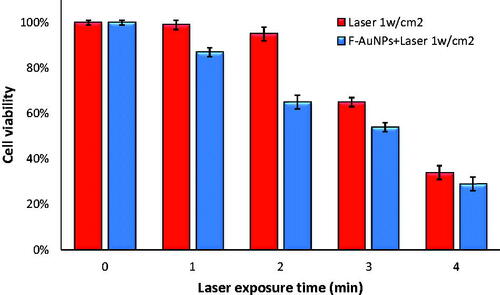
Figure 8. The viability of KB and L929 cells incubated with 40 μM of F-AuNPs for 6 h and then irradiated by laser (532 nm; 1 W/cm2; 2 min).
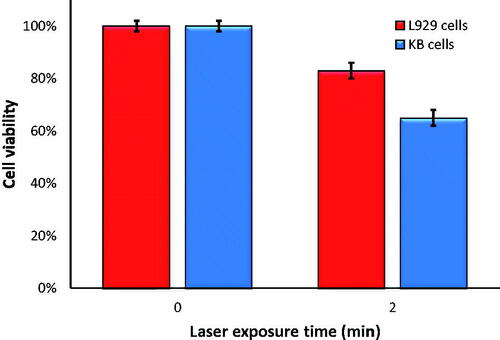
To compare the amount of cell lethality for both KB and L929 at the same conditions of NPTT, additional experiments were designed to investigate whether or not F-AuNPs would operate selectively for cancer cells with high level of FRs. All conditions of laser irradiation for experiments on L929 cells were identical to the conditions for experiments on KB cells (1 W/cm2 for 2 min.). As seen in , cell viability for KB and L929 cells was 65 and 83%, respectively.
Determination of cell death mode following NPTT
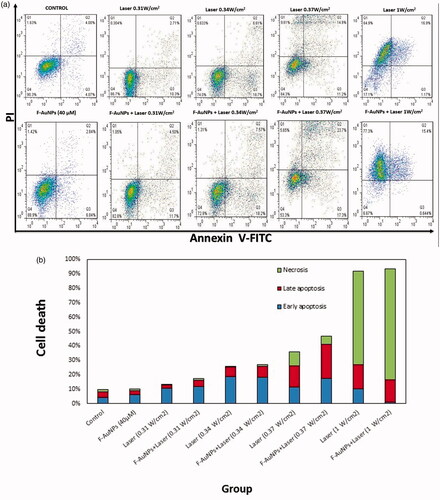
To investigate the effect of various laser intensities on the kinetics of cell death, different laser intensities (0.31, 0.34, 0.37, and 1 W/cm2) were applied to the cells for 8 min. Laser irradiation was applied to the cells in the absence and the presence of F-AuNPs (40 μM). Then, we evaluated phosphatidylserine (PS) the translocation and membrane integrity of the cells. As seen in and , F-AuNPs-treated KB and L929 cells displayed no significant increase in apoptosis as compared to the control group. Laser intensities of 0.31 and 0.34 W/cm2 induced similar percentages of apoptosis in the presence or absence of nanoparticles. However, significant difference in apoptosis was observed between the KB cells receiving laser irradiation alone and the KB cells treated by NPTT at intensity of 0.37 W/cm2. Finally, laser irradiation with intensity of 1 W/cm2 caused a higher percentage of necrosis in both laser-treated and NPTT-treated KB cells (65 and 77%, respectively).
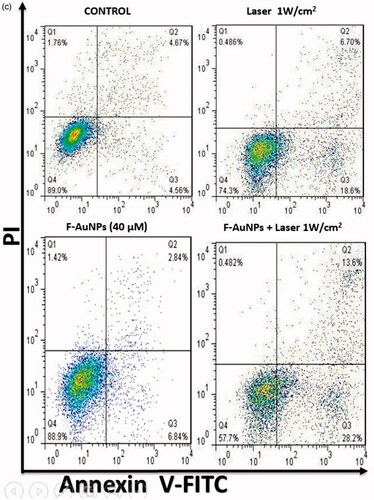
Figure 9. (a) Flow cytometric analysis to determine death modes of KB cells after receiving various treatments (laser irradiation time was 8 min). (b) Proportion of apoptotic and necrotic cell death for KB cells after receiving various treatments (laser irradiation time was 8 min). (c) Flow cytometric analysis to determine the death modes of KB cells after receiving NPTT (laser irradiation time was 2 min).
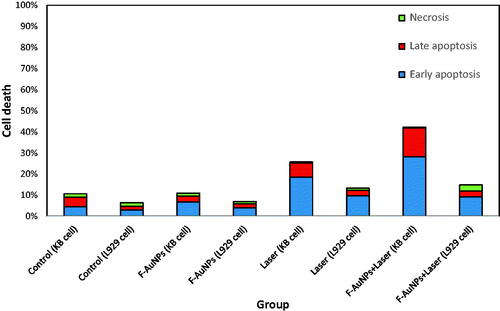
Figure 10. Flow cytometric analysis to determine the death modes of L929 cells after receiving various treatments.
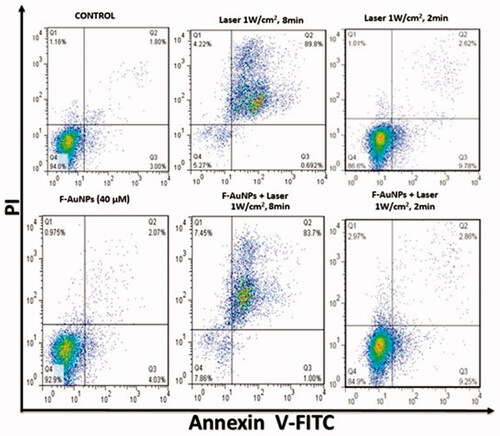
In another experiment on KB cells, we tested the effects of F-AuNPs with and without laser irradiation at an intensity of 1 W/cm2 for 2 min. As seen in , the proportion of apoptosis was 25 and 41% for KB cells treated by laser alone and NPTT, respectively.
The same experiments were conducted on L929 cells to study the various NPTT effects on this normal cell line. As seen in , a significant increase in apoptosis for both groups of L929 cells receiving laser and NPTT were observed (1 W/cm2; 8 min). Also, it was observed that lower level of apoptosis occurred for normal cells when exposed by laser for shorter duration (1 W/cm2; 2 min).
helps the readers make easier comparison between the obtained results for KB and L929 cell lines, following the same NPPT experiments. As seen in this figure, there is a significant difference between cancer and normal cells after NPTT, using the synthesized F-AuNPs.
Temperature distribution during NPTT
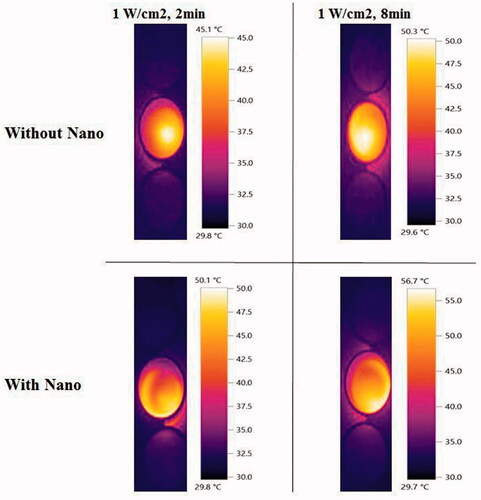
Extra experiments were conducted to do thermometry and compare the localized elevation of temperature of KB cells upon irradiation by laser in the presence or absence of the F-AuNPs. The results are shown in . The temperature of the well containing only KB cells and culture medium was 25 °C. After 8 min laser irradiation with different intensities from 0.21 to 1 W/cm2, temperature of each well was increased from 31.2 to 50.3 °C. In the presence of nanoparticles, this increase was from 34 to 56.7 °C. We also tested the effects of changing exposure time if the intensity of laser is constant. After 1 min laser irradiation at an intensity of 1 W/cm2, a significant increase in temperature was observed (from 25 to 41.6 °C). It was observed that 2, 3, and 4 min irradiation of laser at the same intensity increased the temperature from 25 to 45.1 °C, 47.9 °C, and 49.8 °C. On the other hand, to examine the effect of the presence of F-AuNPs on temperature elevation, we conducted the same experiments on the KB cells incubated with nanoparticles. Increase in temperature was observed from 25 to 44.7 °C, 50.1 °C, 52.2 °C, and 53.8 °C for 1, 2, 3, and 4 min of laser irradiation, respectively. shows some images taken by infrared camera demonstrating that temperature was distributed homogeneously in each well receiving NPTT.
Discussion
Here, we reported the synthesis and characterization of F-AuNPs and then we investigated the cytotoxic effects of the synthesized F-AuNPs alone or in combination with laser irradiation. The in vitro effects were studied on FR-overexpressed KB cancer cells, as the model, and L929 normal fibroblast cells with lower level of FRs, as the blank [Citation22–24]. In fact, AuNPs were modified with folic acid to produce a highly efficient and selective nano-heater for the ablation of cancer cells [Citation9]. UV–Vis and FTIR spectroscopy confirmed the attachment of folic acid to the AuNPs. TEM and DLS determined the shape and size of the synthesized nanoparticles. The main objectives of this study was to determine the roles of (i) the quantity of AuNPs internalized into the cells, as the energy converters, and (ii) the amount of irradiated laser energy in the cellular responses observed following the NPTT [Citation33].
Both the MTT and flow cytometry studies confirmed that the synthesized F-AuNP has low level of cytotoxicity on both KB and L929 cell lines when the nanoparticle concentration is ≤40 μM. Such a low cytotoxicity may be even more reduced if the nanoparticles are coated by poly (ethylene glycol) (PEG). It is well-known that surface coating of the nanoparticles with PEG is one of the best solutions to increase the stability, solubility, and biocompatibility of the nanoparticles [Citation34–36].
As mentioned earlier, the amount of heat generated in the NPTT process may be controlled by laser intensity and exposure time. To determine such effects, we studied cell viability and apoptosis following various NPTTs. In all experiments, the concentration of F-AuNPs was kept constant at 40 μM. shows the effect of various laser intensities on KB cells irradiated for 8 min in the presence and absence of F-AuNPs. As seen in , the cell viability is decreased when higher intensities are applied to the cancer cells and this result is in agreement with other publications [Citation37,Citation38]. It was also observed that F-AuNPs increase the amount of cell death caused by higher intensities of laser. Jin et al. [Citation39] also reported the same results using near-infrared laser-induced hyperthermia of folate-conjugated gold nanorods. According to the method reported by Jin et al. [Citation39], NPTT could break the hepatocellular carcinoma HepG2 cell membrane integrity and homeostasis.
We also determined the effect of various exposure duration of laser (at constant intensity of 1 W/cm2) on cancer cells in the presence and absence of F-AuNPs. In , it is observed that cell death is increased when laser exposure time is elongated. According to , it is also worthy to be noted that a faster decrease in cell viability is observed by changing laser duration from 1 (87%) to 2 min (65%), but a mild decrease is seen when longer irradiation times are applied. In this context, similar observations have been also reported recently [Citation39].
According to the data presented in both and , it is found that laser intensity and exposure time are two important factors to control the level of cell death. Also, it is obvious that lower intensities and longer irradiation times resulted in the same cell death obtained by higher intensities and shorter times. For example, it seems that the conditions below are equivalent from the point of view of cell viability following NPTT suggested in this study:
(0.21 W/cm2; 8 min) ≈ (1 W/cm2; 1 min)
(0.40 W/cm2; 8 min) ≈ (1 W/cm2; 3 min)
It is important to have selectivity in treating cancer and normal cells through NPTT. To compare the amount of cell lethality following NPTT for KB and L929 cells, additional experiments were designed to investigate whether or not F-AuNPs have selective operation. As seen in , L929 cell viability following NPTT is ∼83%, while the viability of KB cells after NPTT is ∼65%. This result shows that F-AuNP may induce a selective response when it encounters cancer or normal cells.
The most important question in the area of NPTT is related to the determination of cell death mode. To date, both necrosis and apoptosis are reported to be responsible for cell death triggered by NPTT [Citation40,Citation41]. More specifically, one of the main challenges in the field of NPTT is the control of the intensity or energy irradiated to the cells. High levels of intensities may lead to cell overheating and easily cause necrosis. On the other hand, there are some reports that NPTT-induced apoptosis occurs through low-energy irradiation which does not cause inflammation [Citation42]. Therefore, given the diverse biological consequences of inducing necrosis versus apoptosis, it would be of great importance to develop a new strategy to selectively induce cancer cell apoptosis and avoid necrosis [Citation43]. The main target must be to know how the kinetics of cell death can be controlled by tailoring treatment parameters such as laser power and exposure time.
In flow cytometry studies on both KB and L929 cells, it was determined that F-AuNPs-treated cells displayed no significant increase in apoptosis as compared to control cells. According to , a significant apoptosis is observed in KB cells when they are irradiated by 0.37 W/cm2. Irradiation (with 1 W/cm2; 8 min) caused a higher percentage of cells to undergo necrosis in both laser-treated (65%) and NPTT-treated (77%) KB cells. With the same condition for L929 cells, a sharp increase (>80%) in late apoptosis is seen for both laser-treated and NPTT-treated cells (). According to these results, it may be concluded that the packages of high intensities and long exposure times are not an appropriate choice to induce apoptosis in cancer cells. Pelaz et al. [Citation44] also demonstrated that NPTT immediately induces necrosis in Vero cells (kidney epithelial cells of an African green monkey). In their experiments, Vero cells were loaded with gold nanoprisms and then exposed to laser irradiation (30 W/cm2 for 2 min).
In another section of current study, the effects of shorter exposure time (1 W/cm2; 2 min) was further investigated (). In KB cells, a significant increase in apoptosis after NPTT was observed (>40%). However, the percentage of apoptosis in L929 cells is lower than 10% after receiving NPTT. As a result, shorter exposure time, for example (1 W/cm2; 2 min), may be a good choice to induce selective apoptosis in cancer cells. There are some other reports revealing the appropriate conditions of laser irradiation and the important role it plays in apoptosis induction. Perez et al. demonstrated that NPTT using gold nanoprisms primarily induce apoptotic cell death [Citation41]. Their data indicate that photothermal treatment induces apoptosis in treated cells, when suitable irradiation conditions are selected. Also, they analyzed the molecular mechanism of apoptosis involved in the process of NPTT using these conditions (5 W/cm2 for 2 or 4 min). However, they did not report any data to determine the effects of various laser intensities in the process of NPTT.
Conclusion
In this study, we delineated the synthesis of F-AuNPs by tailoring the surface of AuNPs with folic acid to enhance the selectivity of NPTT. Moreover, we investigated the in vitro effects of the synthesized nanoparticles on both cancer and healthy cells when treated by the NPTT process. Our findings confirmed that NPTT can either induces cell death via apoptosis or necrosis. The ability that determines whether a cell will die by apoptosis or necrosis on-demand, with NPTT, will probably depend on several factors. The amount of nano-heaters inside the cells and the deposited energy of the laser seem to be the most important factors causing over-heating of the cells and consequently induce necrosis, which results in inflammation and additionally, could damage the surrounding healthy cells. As a result, the main target is better to be a highly triggered apoptosis during NPTT.
In the clinics, it is favorable to use an optimal amount of energy in NPTT so as not to hurt the neighboring healthy cells. In this study, we appointed the energy or intensity threshold requisite for cancer cell apoptosis. There still exists a need to perform detailed studies to know the major players that contribute to the mechanisms of NPTT in vivo. Also, further modifications and characterizations of F-AuNP are needed to determine if such a nanoconjugate can be attached to the target cancer tissue in vivo. In this area, in vivo biodistribution studies [using elemental analysis methods (like ICP-MS) and imaging examinations (like PET scan)] are being accomplished in our laboratory.
| Abbreviations | ||
| NPTT | = | nano-photo-thermal therapy |
| AuNPs | = | gold nanoparticles |
| F-AuNPs | = | folate-conjugated gold nanoparticles |
| FR | = | folate receptor |
| KB cells | = | cancer cells as a folate receptor (FR) over-expressing cell line |
| L929 cells | = | normal cells with low level of FRs |
| RPMI | = | Roswell Park Memorial Institute |
| MTT | = | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| EDTA | = | ethylene diamine tetra acetic acid |
| FBS | = | fetal bovine serum |
| TEM | = | transmission electron microscope |
| FTIR | = | Fourier-transform infrared spectroscopy |
| DLS | = | dynamic light scattering |
| PBS | = | phosphate-buffered saline |
| OD | = | optical density |
| FITC | = | fluorescein isothiocyanate |
| PI | = | propidum iodide. |
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- del Burgo LS, Pedraz J, Orive G. Advanced nanovehicles for cancer management. Drug Discov Today. 2014;19:1659–1670.
- Beik J, Abed Z, Shakeri-Zadeh A, et al. Evaluation of the sonosensitizing properties of nano-graphene oxide in comparison with iron oxide and gold nanoparticles. Physica E Low Dimens Syst Nanostruct. 2016;81:308–314.
- Beik J, Abed Z, Ghoreishi FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221.
- Beik J, Abed Z, Ghadimi-Daresajini A, et al. Measurements of nanoparticle-enhanced heating from 1MHz ultrasound in solution and in mice bearing CT26 colon tumors. J Therm Biol. 2016;62:84–89.
- Bharali DJ, Mousa SA. Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther. 2010;128:324–335.
- Bazak R, Houri M, El Achy S, et al. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769–784.
- Mansoori GA, Mohazzabi P, McCormack P, et al. Nanotechnology in cancer prevention, detection and treatment: bright future lies ahead. World Rev Sci Technol Sustain Dev. 2007;4:226–257.
- Brandenburg K, Shakeri-Zadeh A, Mansoori G. Folate-conjugated gold nanoparticles for cancer nanotechnology applications. Nanotech. 2011;3:404–407. Proceedings.
- Mansoori GA, Brandenburg KS, Shakeri-Zadeh A. A comparative study of two folate-conjugated gold nanoparticles for cancer nanotechnology applications. Cancers. 2010;2:1911–1928.
- Shakeri-Zadeh A, Mansoori GA. Cancer nanotechnology treatment through folate conjugated gold, nanoparticles. Proceedings of WCC 2010; July 13–15, 2010; Italy, Rome. The 2nd World Congress on Cancer. 2010.
- Xia Y, Halas NJ. Shape-controlled synthesis and surface plasmonic properties of metallic nanostructures. MRS Bull. 2005;30:338–348.
- Murphy CJ, Sau TK, Gole AM, et al. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B. 2005;109:13857–13870.
- Huang X, El-Sayed IH, Qian W, et al. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120.
- Bohren CF, Huffman DR. Absorption and scattering of light by small particles. New York: John Wiley & Sons; 2008.
- Dreaden EC, Mackey MA, Huang X, et al. Beating cancer in multiple ways using nanogold. Chem Soc Rev. 2011;40:3391–3404.
- Terentyuk GS, Maslyakova GN, Suleymanova LV, et al. Laser-induced tissue hyperthermia mediated by gold nanoparticles: toward cancer phototherapy. J Biomed Opt. 2009;14:021016–021019.
- Pitsillides CM, Joe EK, Wei X, et al. Selective cell targeting with light-absorbing microparticles and nanoparticles. Biophys J. 2003;84:4023–4032.
- Zharov VP, Mercer KE, Galitovskaya EN, et al. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J. 2006;90:619–627.
- Tong L, Wei Q, Wei A, et al. Gold nanorods as contrast agents for biological imaging: optical properties, surface conjugation and photothermal effects. Photochem Photobiol. 2009;85:21–32.
- Day ES, Thompson PA, Zhang L, et al. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J Neurooncol. 2011;104:55–63.
- Melamed JR, Edelstein RS, Day ES. Elucidating the fundamental mechanisms of cell death triggered by photothermal therapy. ACS Nano. 2015;9:6–11.
- Setua S, Menon D, Asok A, et al. Folate receptor targeted, rare-earth oxide nanocrystals for bi-modal fluorescence and magnetic imaging of cancer cells. Biomaterials. 2010;31:714–729.
- Sahoo B, Devi KSP, Banerjee R, et al. Thermal and pH responsive polymer-tethered multifunctional magnetic nanoparticles for targeted delivery of anticancer drug. ACS Appl Mater Interfaces. 2013;5:3884–3893.
- Bhattacharya D, Das M, Mishra D, et al. Folate receptor targeted, carboxymethyl chitosan functionalized iron oxide nanoparticles: a novel ultradispersed nanoconjugates for bimodal imaging. Nanoscale. 2011;3:1653–1662.
- Chiani M, Norouzian D, Shokrgozar MA, et al. Folic acid conjugated nanoliposomes as promising carriers for targeted delivery of bleomycin. Artif Cells Nanomed Biotechnol. 2017 [Jun 23];1–7. DOI:https://doi.org/10.1080/21691401.2017.1337029.
- Daraee H, Eatemadi A, Abbasi E, et al. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:410–422.
- Ghaznavi H, Hosseini-Nami S, Kamrava SK, et al. Folic acid conjugated PEG coated gold–iron oxide core–shell nanocomplex as a potential agent for targeted photothermal therapy of cancer. Artif Cells Nanomed Biotechnol. 2017 [Oct 10];1–11. DOI:https://doi.org/10.1080/21691401.2017.1384384.
- Tripathi R, Shrivastav A, Shrivastav B. Biogenic gold nanoparticles: as a potential candidate for brain tumor directed drug delivery. Artif Cells Nanomed Biotechnol. 2015;43:311–317.
- Mirrahimi M, Hosseini V, Kamrava SK, et al. Selective heat generation in cancer cells using a combination of 808 nm laser irradiation and the folate-conjugated Fe2O3@ Au nanocomplex. Artif Cells Nanomed Biotechnol. 2017 [Jan 1];1–13. DOI:https://doi.org/10.1080/21691401.2017.1420072
- Beik J, Jafariyan M, Montazerabadi A, et al. The benefits of folic acid-modified gold nanoparticles in CT-based molecular imaging: radiation dose reduction and image contrast enhancement. Artif Cells Nanomed Biotechnol. 2017 [Dec 12];1–9. DOI:https://doi.org/10.1080/21691401.2017.1408019
- Li G, Li D, Zhang L, et al. One‐step synthesis of folic acid protected gold nanoparticles and their receptor‐mediated intracellular uptake. Chem Eur J. 2009;15:9868–9873.
- Neshastehriz A, Tabei M, Maleki S, et al. Photothermal therapy using folate conjugated gold nanoparticles enhances the effects of 6MV X-ray on mouth epidermal carcinoma cells. J Photochem Photobiol B Biol. 2017;172:52–60.
- Qin Z, Bischof JC. Thermophysical and biological responses of gold nanoparticle laser heating. Chem Soc Rev. 2012;41:1191–1217.
- Connor EE, Mwamuka J, Gole A, et al. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327.
- Samadian H, Hosseini-Nami S, Kamrava SK, et al. Folate-conjugated gold nanoparticle as a new nanoplatform for targeted cancer therapy. J Cancer Res Clin Oncol. 2016;142:2217–2229.
- Eyvazzadeh N, Shakeri-Zadeh A, Fekrazad R, et al. Gold-coated magnetic nanoparticle as a nanotheranostic agent for magnetic resonance imaging and photothermal therapy of cancer. Lasers in Medical Science 2017;32:1469–1477.
- El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239:129–135.
- Zhang A-w, Guo W-h, Qi Y-f, et al. Synergistic effects of gold nanocages in hyperthermia and radiotherapy treatment. Nanoscale Res Lett. 2016;11:279.
- Jin H, Yang P, Cai J, et al. Photothermal effects of folate-conjugated Au nanorods on HepG2 cells. Appl Microbiol Biotechnol. 2012;94:1199–1208.
- Kannadorai RK, Chiew GGY, Luo KQ, et al. Dual functions of gold nanorods as photothermal agent and autofluorescence enhancer to track cell death during plasmonic photothermal therapy. Cancer Lett. 2015;357:152–159.
- Pérez-Hernández M, del Pino P, Mitchell SG, et al. Dissecting the molecular mechanism of apoptosis during photothermal therapy using gold nanoprisms. ACS Nano. 2014;9:52–61.
- Davidovich P, Kearney CJ, Martin SJ. Inflammatory outcomes of apoptosis, necrosis and necroptosis. Biol Chem. 2014;395:1163–1171.
- Mehdizadeh A, Pandesh S, Shakeri-Zadeh A, et al. The effects of folate-conjugated gold nanorods in combination with plasmonic photothermal therapy on mouth epidermal carcinoma cells. Lasers Med Sci. 2014;29:939–948.
- Pelaz B, Grazu V, Ibarra A, et al. Tailoring the synthesis and heating ability of gold nanoprisms for bioapplications. Langmuir. 2012;28:8965–8970.