Abstract
Micro/nanostructured TiO2/ZnO coating has been shown to possess multiple functions, including antibacterial activity and bioactivity. Osteoblast-like SaOS-2 cells were employed for evaluating the in vitro osteogenic capacity of this coating and positive results were obtained. However, traditional principles of osseointegration focus only on the osteogenic differentiation alone. The effects of immunomodulation on the osteogenic activity have been largely ignored. In this study, the inflammatory responses of macrophages on the micro/nanostructured TiO2/ZnO coating were investigated. The extract media of macrophage cell line RAW264.7 cultured on the TiO2/ZnO coating were collected as indirect co-culture conditioned media. The osteogenic activity of SaOS-2 cells in the conditioned media was investigated. Adhesion, ALP activity and extracellular mineralization of cells grown in the conditioned media extracted from the micro/nanostructured TiO2/ZnO coating were found to be enhanced, compared to those grown in the conditioned media extracted from the macroporous TiO2 coating. The immune microenvironment produced by the micro/nanostructured TiO2/ZnO coating showed excellent capacity to promote osteogenesis, indicating that this coating could be a promising candidate for implant surface modification in orthopaedic and dental applications. Furthermore, this work could help us understand the interplay between the host immune system and the osteoimmunomodulatory properties of the biomaterials, and optimize the design for coating biomaterials.
Introduction
The principle of osseointegration has been known for several decades. It requires the direct contact between the bone and biomaterials without any fibrous capsule, as observed through an optical microscope [Citation1]. This criterion is feasible for clinical application and has been successfully applied in many cases [Citation2–4]. However, there are some discrepancies between the in vitro and in vivo biological responses to biomaterials. These discrepancies are believed to be due to the complexity of the condition inside the body and the differences between in vitro and in vivo environments [Citation5]. One such cause for the discrepancies is the in vivo immunomodulation. Immune cells act as the first line of host defence to recognize and attack foreign objects [Citation6]. The immune system is also known to participate in the process of bone modulation, which comprises osteogenesis and osteoclastogenesis. Osteoblasts and osteoclasts are involved in these processes. The abnormal functioning of immune cells could lead to an imbalance between osteoclasts and osteoblasts, resulting in pathological bone destruction. Several cytokines, receptors, signalling molecules and transcription factors in the immune system also play a role in the skeletal system [Citation7]. Thus, researchers today have realized that the immune system may play a pivotal role in bone regeneration [Citation8]. Immune cells such as macrophages and T-cells are known to actively participate in osteoclastogenesis and osteogenesis by regulating the release of conjunct molecules for both immune cells and bone cells. T-cells are known to secrete a molecule that inhibits the development and activation of the osteoclasts [Citation9]. Macrophages are the precursors of osteoclasts [Citation10]. Inadequate activation of macrophages could cause undesired inflammatory fibrosis and failure of bone regeneration. It is therefore necessary that immune cells, especially macrophages, should be considered while evaluating the osteogenesis-induction abilities of biomaterials.
When both bone cells and immune cells are involved, in order to mimic the in vivo environment, surface modification of the implant should be performed in such a way that the balance between osteoclastogenesis and osteogenesis is maintained. Surface coating on the implant should favour the development of osteoimmunomodulation to maximize the integration of implants into the bone tissues. Surface topography and chemical composition could affect both the bone cells and immune cells [Citation11]. Although interactions between biomaterials and bone cells have been investigated for many years, the role of immune cells in this mechanism and process has not been fully understood [Citation12]. Thus, the coating materials for implants should be reviewed based on not only the traditional principle, which only considers the skeletal system, but also the role of immune cells in the reaction. Additionally, investigation into osteoimmunomodulation of the coating materials also provides the possible therapy for bone destruction diseases such as rheumatoid arthritis (RA) since these diseases are found to be related to the interplay between immune and skeletal systems [Citation13].
In a previous work, we had fabricated micro/nanostructured TiO2/ZnO coating by combining micro-arc oxidation (MAO) and hydrothermal treatment, followed by calcination to improve the biocompatibility of the coating. The osteogenic activity of this micro/nanostructured TiO2/ZnO has been investigated in our previous study. The secretion of alkaline phosphatase (ALP), collagen, osteopontin (OPN) and osteocalcin (OCN) of cells grown on this coating was increased. However, we have not considered the effects of immunomodulation on the osteogenic activity.
Inflammatory responses of immune cells to ZnO have been examined. ZnO nanoparticles at ultralow concentration are reported to stimulate pro-inflammatory responses of RAW264.7 macrophages and increase the gene expression of pro-inflammatory cytokines IL-6 and IL-1b [Citation14]. ZnO coated discs are found to cause a discontinuous cellular fibrous capsule indicative of unresolved inflammation [Citation15]. Zinc-containing tricalcium phosphate could influence the activity of RAW264.7 macrophages and the formation of multinuclear giant cells [Citation16]. Zinc sulphate is found to suppress osteoclast differentiation and promote osteoblast mineralization [Citation17]. Controversial results on the responses of immune cells to Zn address the necessity of investigation on immunomodulatory effects of the TiO2/ZnO coating in inducing osteogenesis.
In this study, macrophages (RAW264.7 cells) were used to stimulate the immune microenvironment in in vivo. The osteogenesis of osteoblast-like cells SaOS-2 in the conditioned media was reexamined to estimate the performance of the micro/nanostructured TiO2/ZnO coating in the in vivo environment. These results would also provide suggestions for the clinical applications of this coating.
Materials and methods
Preparation
The micro/nanostructured TiO2/ZnO coating was prepared by the combination of MAO and hydrothermal treatment. Briefly, MAO process was performed at positive and negative alternating current (AC) fields of 250 V and 5 V, respectively, for 5 min by a MAO device (WHD-20, Haerbin, China) to create the macroporous TiO2 coating on pure Ti (grade 2, with a diameter of 14.5 mm and thickness of 1 mm). The aqueous electrolyte used in the MAO process contained 0.1 M calcium acetate monohydrate (Ca(CH3COO)2·H2O), 0.1 M disodium edetate (Na2(EDTA)), 0.25 M sodium hydroxide (NaOH) and 0.02 M sodium silicate (Na2SiO3·9H2O). After the MAO process, hydrothermal treatment was performed in autoclaves with 0.02 M zinc acetate (Zn(CH3COO)2) in 8 mL ammonium hydroxide. The pH of the ammonium hydroxide was adjusted to 12.6. The reaction was carried out for 4 h at 200 °C and the obtained sample was referred to as MZn. The samples were then subjected to heat treatment at 450 °C for 3 h, followed by another 3 h at 700 °C. Subsequently, samples underwent an ultrasonic bath with deionized water for 5 min to remove the loosely adhered ZnO. The obtained sample was referred to as MHTZn. Morphologies of MAO and MHTZn samples were observed under scanning electron microscopy (SEM, Zeiss, Oberkochen, Germany). The contents of Zn of the MAO, MZn and MHTZn samples were listed in the supporting information (Table S1). And the release rates of Zn were given in the supporting information (Figure S1).
Inflammatory response of RAW264.7 cells
RAW264.7 cells were seeded on the samples in a 24-well plate at density of 3 × 104 cells per well. Cells were incubated in High-Dulbecco’s Modified Eagle Medium (DMEM, Gibco, Carlsbad, CA) supplemented with 10% foetal bovine serum (FBS, Gibco, Carlsbad, CA), and 1% (v/v) penicillin/streptomycin (Gibco, Carlsbad, CA) at 37 °C. At day 1 and day 3, RAW264.7 cells grown on the samples were observed by SEM. Cells cultured on the two surfaces for 1 and 3 days were fixed with 2.5% glutaraldehyde solution in phosphate buffer (DPBS, WELGENE, Daegu, Korea). Dehydration was performed in serial ethanol solutions (30%, 50%, 75%, 80%, 90%, 95% and 100% [v/v]), followed by tertiary butanol solutions (25%, 50%, 75% and 100% [v/v]). Subsequently, the samples were freeze-dried for 1 h before observation with SEM.
RAW264.7 cells on the MAO and MHTZn samples cultured for 1, 3 and 5 days were fixed with 4% paraformaldehyde, washed thrice with DPBS, stained with 5 µg/mL phalloidin-TRITC (Sigma, St. Louis, MO) for the cell skeleton, and incubated for 40 min at 37 °C. Subsequently, the cells were stained with 2 µg/mL 4′,6-diamidino-2-phenylindole (DAPI, Sigma, St. Louis, MO) for the cell nuclei for 15 min at room temperature. Throughout the experiments, the cells were protected from exposure to light. Finally, the cells were photographed with a laser scanning confocal microscope (Zeiss 710, Zeiss, Germany).
Cell viability was tested using cell counting kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan) at days 1, 3 and 5. The CCK-8 reagent was mixed with a complete medium at 10% (v/v) concentration. The mixture (200 µL) was added to each well. After incubation for 1 h, 100 μL of the liquid was carefully extracted from each well and evaluated in the microplate reader (EnSpire, PerkinElmer, Waltham, MA).
After incubation for 24 h, the DMEM medium was changed to a mixture of DMEM (10% FBS and 1% PS) and McCoy’s 5A media (Gibco, Carlsbad, CA; 15% FBS and 1% PS) in 1:1 (v/v) ratio. The mixed media were collected at day 2 used as conditioned media for growing SaOS-2 cells. The inflammatory cytokines, tumour necrosis factor alpha (TNF-α), interleukin-(IL)-4, IL-6 and IL-10, in the conditioned media were determined using ELISA kits (Quantikine, R&D Systems, Minneapolis, MN).
Osteoimmunomodulation of SaOS-2 cells
SaOS-2 cells were seeded in a 48-well plate at a density of 1 × 104 cell/well. The conditioned media were diluted in the mixture media at a ratio of 1:5. For investigating cell adhesion, cells were cultured on the three surfaces for 1 and 3 days. Cell skeleton and nuclei were stained and observed using the method in section “Inflammatory response of RAW264.7 cells”.
The viability of SaOS-2 cells in the conditioned media was estimated using CCK-8 at days 1, 4 and 7.
The total protein in the cells cultured on the three surfaces for seven days was evaluated using the BCA Protein Assay Kit, according to the manufacturer’s instructions (Beyotime, Shanghai, China). The ALP activity in the three groups was also tested and normalized to the total protein using the ALP kit (Jiancheng Bio-engineering Research Institute of Nanjing, Nanjing, China). ALP activity expression was also evaluated by staining with cALP stain kit (Jiancheng Bio-engineering Research Institute of Nanjing, Nanjing, China). Images were acquired via a fluorescence microscopy (Leica, Wetzlar, Germany).
Mineralization of the cells on the two surfaces was estimated after culturing for 14 days. For the first six days, cells were cultured in complete medium, and then in osteogenic medium (with 10−7 M dexamethasone, 10 mM β-glycerophosphate disodium and 50 μg/mL ascorbic acid). At day 14, cells were fixed with 4% paraformaldehyde in DPBS solution for 30 min, washed thrice with ultra-pure water, and stained with Alizarin Red (Solarbio, Beijing, China) for 2 h. Images were taken under a fluorescence microscopy. After another wash with ultra-pure water, the samples were treated with 10% hexadecylpyridinium chloride for 30 min. The dissolved solution was then evaluated on a microplate reader.
Statistical analysis
The experiments were conducted in triplicate and the data were expressed as means ± standard deviations (SD). Student’s t-test was used to determine the level of significance. Differences with p values < .05 and .01 were considered statistically significant and highly significant, respectively.
Results
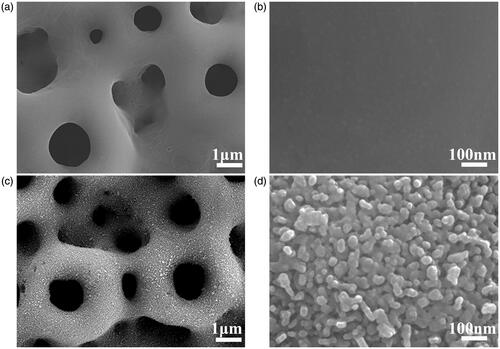
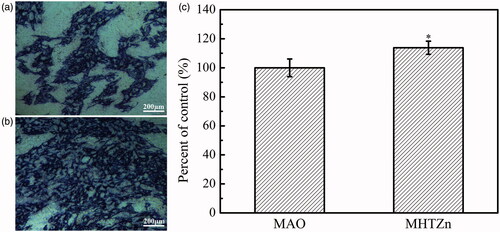
Micro-arc oxidation produced macroporous structures on the Ti substrate (). The hydrothermal treatment and heat treatment created granular nanostructures following the counter of the macropores (). The two coatings were found to have similar microstructures. In our previous study, we found that the MAO coating comprised of TiO2, CaO, SiO2 and CaSiO3. The MHTZn coating contained all these compounds, as well as ZnO. The RAW264.7 cells displayed dramatically different shapes when grown on the MAO and MHTZn coatings. At day 1, macrophages adhering to the MAO coating showed a more elongated morphology with many extended filopodia (), while the majority of macrophages on the MHTZn coating exhibited a round morphology (). At day 3, the RAW264.7 cells on both MAO and MHTZn coatings were found to have aggregated and appeared similar in number and morphology (). Cell skeleton and nuclei staining of RAW264.7 cells cultured on the MAO and MHTZn samples within five days are shown in . Cells on the MAO coating at one day () showed more membrane protrusion and larger spreading area than on the MHTZn coating (). At day 3 (and 5 (), cells on the MAO and MHTZn coatings aggregated into clusters and exhibited spherical morphology. The results were consistent with the observation under SEM and also confirmed in the proliferation test (), which revealed that the number of RAW264.7 cells on the MHTZn coating was lower than that on the MHTZn coating at day 1, but there was no significant difference at day 3 and day 5. The inflammatory response of macrophages was evaluated via the released cytokines. The result of ELISA showed an increase in the expression of TNF-α, IL-6, IL-4 and IL-10 in the MHTZn group compared to the MAO group ().
Figure 2. Morphologies of attached RAW264.7 cells cultured on MAO coating for one day (a), and three days (b), and on MHTZn coating for one day (c) and three days (d).
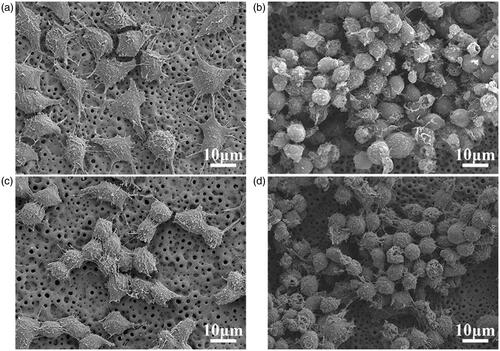
Figure 3. Skeleton and nuclei staining for MAO group at day 1 (a), day 3 (b), and day 5 (c); MHTZn group at day 1 (d), day 3 (e), and day 5 (f). Cell viability of RAW264.7 cells on MAO and MHTZn coatings tested by CCK-8 (g). Values are mean ± SD (n=3); * depicts statistical differences; * p < .05 vs. MAO.
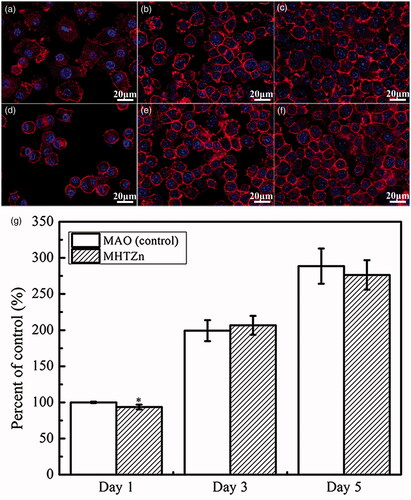
Figure 4. Inflammatory secretions of RAW264.7 cells cultured on MAO and MHTZn coatings for two days. Values are mean ± SD (n = 3); *statistical differences; *p < .05, **p < .01 vs. MAO.
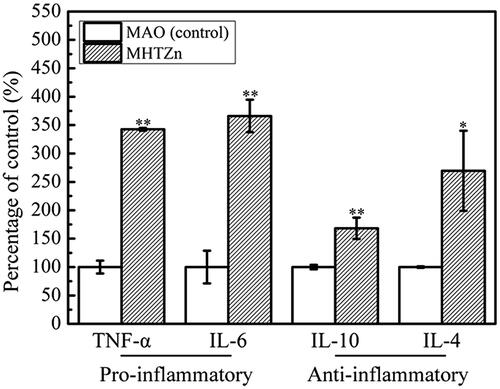
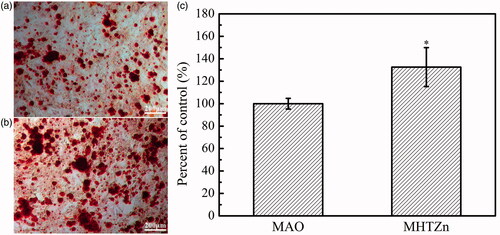
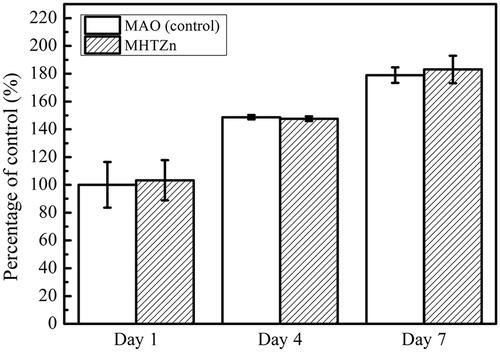
The behaviour of SaOS-2 cells in the conditioned media was also reexamined. The cell skeleton and nucleus are shown in . After one day of culture, more stress fibres crossed through the whole cells in the MHTZn group than in the MAO group, indicating better cell attachment in the MHTZn medium conditioned media. During the incubation from 1 to 3 days, both group showed larger spreading areas for SaOS-2 cells. However, cells in the MHTZn groups displayed more stress fibres. The quality of cell adhesion could affect the following cell behaviour. As shown in , cell proliferation was similar in all surfaces at days 1, 4 and 7. The number of cells growing in the MAO and MHTZn conditioned media increased with the time. However, the difference between the two groups was not statistically significant. ALP activity of the SaOS-2 cells cultured in the two conditioned media for seven days is shown in including ALP staining and quantitative evaluation of ALP activity. As shown in , the ALP-positive areas of the MHTZn group were significantly higher than that of the MAO group. This was in accordance with the further verification by the ALP quantitative analysis (), which showed higher ALP activity in cells grown in the MHTZn conditioned medium. Regarding the mineralization, more calcium nodules were found in the MHTZn group (). The OD values also confirmed that the MHTZn conditioned medium had a higher capacity for promoting osteogenic activity of SaOS-2 cells than the MAO conditioned medium ().
Figure 5. SaOS-2 cells after culturing in the MAO conditioned media for one day (a) and three days (c), in the MHTZn conditioned media for one day (b) and three days (d), showing the nuclei stained by DAPI and the cytoskeleton stained by phalloidin with TRITC.
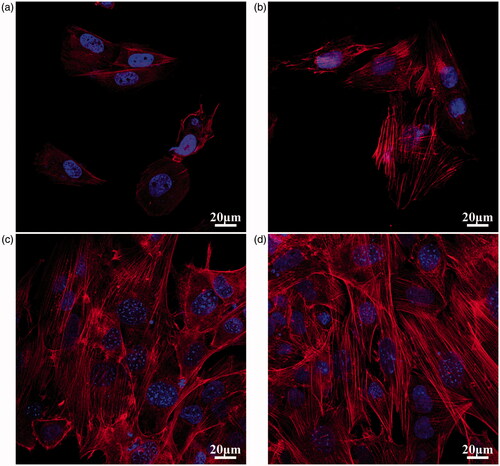
Figure 6. Cell viability of SaOS-2 cells cultured in the MAO and MHTZn conditioned media tested by CCK-8. Values are mean ± SD (n = 3).
Discussion
Macrophages are known to be abundant in the blood and bone marrow [Citation18]. After implantation, macrophages are one of the first cells to interact with the foreign objects. They play critical roles in host defence. As the precursors of osteoclasts, macrophages can release several proteins, factors and cytokines that are involved in both the immune modulation and bone modulation. These factors can influence extracellular matrix deposition and new bone formation. Based on the functions of macrophages, a spectrum of macrophage populations has been proposed. Among them, three populations are defined: classically activated M1 macrophages, alternatively activated M2 macrophages and regulatory macrophages [Citation19]. The secretion from macrophages differs based on different phenotypes. When polarized into the M1 phenotype, macrophages mainly secrete pro-inflammatory cytokines such as TNF-α, IL-6 and IL-12; while macrophages polarize to the M2 phenotype, the major secretion cytokines are anti-inflammatory cytokines such as TGF-β, IL-4 and IL-10 [Citation20]. Excessive inflammation is known to be beneficial for osteoclastogenesis, but not for osteogenesis, and can even cause fibrous encapsulation. Prolonged polarization to the M1 phenotype will cause the recruitment of more macrophages to the tissue/biomaterial interface and lead to excessive inflammation. An excess of M2 phenotype macrophages could cause result in scar tissue or a delay in wound healing. Therefore, the ratio of M1 and M2 phenotype macrophages requires careful control [Citation21]. The polarization of macrophages can be modulated by environmental cues. The properties of the implant surface are crucial for the macrophage phenotype. Microstructures in microscale [Citation22] and nanoscale [Citation23], wettability [Citation24] and chemical composition [Citation25] of the coating materials could all affect adhesion, secretion, proliferation and differentiation of macrophages.
Hydrophilic surfaces have been found to inhibit macrophage adhesion [Citation24], which is in line with our observation that the MHTZn group with higher hydrophilicity had fewer cells when grown in conditioned medium for one day. Besides, nanorods ZnO is reported to reduce the number of adherent macrophages compared to ZnO flat substrate and glass, although the mechanism has not been fully understood [Citation15]. The MHTZn group also showed higher expression of both pro-inflammatory cytokines TNF-α and IL-6, and anti-inflammatory cytokines of IL-4 and IL-10 than the MAO group. High levels of pro-inflammatory cytokines are characteristic of the M1 phenotype. In general, when macrophages polarize into M1 phenotype, the pro-inflammatory cytokines will increase. Whereas for the M2 phenotype, the anti-inflammatory cytokines are highly expressed. The increase of both pro-inflammatory and anti-inflammatory cytokines in this study indicated the polarization of macrophages toward both M1 and M2 extremes. Thus, the micro/nanostructured TiO2/ZnO coating could facilitate the polarization of macrophages better than the MAO coating. Microstructures, wettability and released ions of biomaterials have been shown to effect on the cytokine production by macrophages. MAO coating contained Ca and Si, while MHTZn coating contained Ca, Si and Zn. High concentration of Ca2+ can reduce the expression of TNF-α, thus reducing inflammation [Citation26]. Low concentration of Si ions may enhance the development of osteoclasts and high dose will inhibit the bone resorption activity [Citation27]. Zn2+ decreases the production of TNF-α, increases the production of IL-10 but has no effect on the expression of IL-6 [Citation28]. Hydrophilicity stimulates the release of pro-inflammatory cytokines and chemokines [Citation24]. The microenvironment including microstructures and released ions developed by the MHTZn coating thus facilitates the polarization of macrophages and increases the secretion of both pro-inflammatory cytokines and anti-inflammatory cytokines.
The cell behaviour of osteoblast-like SaOS-2 cells in the conditioned media was examined to investigate the influence of the immune system. Cell proliferation in the MAO and MHTZn conditioned media showed no significant difference. However, cells cultured in the MHTZn conditioned media display enhanced osteogenic activity, including increase in ALP activity and mineralization. These behaviours are likely supposed to be regulated by inflammatory cytokines in the conditioned media secreted by the macrophages. TNF-α is found to ALP activity and mineralization in a dose-dependent manner by activating the NF-kB signalling pathway [Citation29]. IL-6, which is produced by osteoblasts, not only by macrophages, is known to slightly inhibit ALP and collagen synthesis in MC3T3-El cells [Citation30]. Furthermore, IL-6 can regulate the differentiation of both osteoclasts and osteoblasts and promote angiogenesis by stimulating the secretion of vascular endothelial growth factor (VEGF) [Citation29]. TNF-α enhances the growth of human osteoblasts while IL-6 has no effects on osteoblast proliferation [Citation29,Citation30]. Several studies have shown that IL-10 could inhibit the formation of osteoclast like multinucleated cells and suppress the synthesis of ALP, collagen type 1 and OCN without affecting the proliferation of osteoblasts. However, it has been shown that IL-10 does not inhibit ALP activity and collagen synthesis in pre-osteoblastic cell lines like MC3T3 cells in other studies [Citation31]. IL-4 has also been shown to inhibit the formation of osteoclasts and stimulate the proliferation, ALP expression and collagen type 1 secretion of osteoblast-like cells in a concentration-dependent manner [Citation32,Citation33].
Taken together, the micro/nanostructured TiO2/ZnO coating would activate the macrophages, mediating the macrophages to alter their phenotype and play different roles in different bone healing stages. Properly activated macrophages in the early stage of implantation would help to clear debris and stimulate the regeneration process. Balanced activation of M1 and M2 macrophages could prevent excess inflammation and accelerate the healing process. In the adsorption of old bones, cytokines secreted by macrophages, such as TNF-α and IL-6, could facilitate the osteoclasts in the timely removal of the debris. During new bone formation, cytokines like TNF-α, IL-6, IL-4 and IL-10 would promote the osteogenic activity to accelerate bone healing. Furthermore, several anti-inflammatory cytokines such as IL-4 and IL-10 could induce the expression of TGF-β, VEGF and EGF factors, reducing inflammatory reactions in the late stage. Thus, the micro/nanostructured TiO2/ZnO coating material could potentially increase the osteogenesis through in vivo interactions with not only the bone cells but also the immune cells.
Conclusions
A macro/nanostructured TiO2/ZnO coating was developed on pure Ti and its osteogenic activity was investigated based on traditional osseointegration principles in our previous study. However, to understand osteoimmunomodulation, it is essential and critical to study the effects of immune modulation on bone modulation in an in vitro study with conditions similar to the in vivo environment. The osteogenesis capacity of the macro/nanostructured TiO2/ZnO coating was evaluated by the indirect co-culture method in conditioned media. The enhanced expression of differentiation markers of osteoblast-like cells in the conditioned medium indicated that this coating could be promising for dental clinical application, when the immune system was involved. The success of implant integration depends on the balance between M1 macrophages and M2 macrophages to promote wound healing and regeneration. The TiO2/ZnO coating was shown to regulate the polarization of macrophages toward both M1 and M2 phenotypes and enhance osteogenesis, compared to macroporous TiO2 coating. These findings could provide new insights into the design of novel improved dental implant coating materials.
R._ZHANG_ET_AL_supplementary_material.zip
Download Zip (60.9 KB)Disclosure statement
The authors declare no competing financial interest.
Additional information
Funding
References
- Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;3:399–410.
- Adell R, Lekholm U, Rockler B, et al. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;6:387–416.
- Branemark PI, Adell R, Albrektsson T, et al. Osseointegrated titanium fixtures in the treatment of edentulousness. Biomaterials. 1983;1:25–28.
- Le Guehennec L, Soueidan A, Layrolle P, et al. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;7:844–854.
- Chen Z, Yuen J, Crawford R, et al. The effect of osteoimmunomodulation on the osteogenic effects of cobalt incorporated β-tricalcium phosphate. Biomaterials. 2015;61:126–138.
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86–100.
- Schutte RJ, Xie L, Klitzman B, et al. In vivo cytokine-associated responses to biomaterials. Biomaterials. 2009;2:160–168.
- Gruber R. Cell biology of osteoimmunology. Wiener Med Wochenschrift. 2010;17–18:438–445.
- Arron JR, Choi Y. Osteoimmunology – bone versus immune system. Nature. 2000;6812:535–536.
- Simonet WS, Lacey DL, Boyle WJ. Osteoclast differentiation and activation. Nature. 2003;6937:337–342.
- Chen Z, Bachhuka A, Han S, et al. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano. 2017;5:4494–4506.
- Xia Z, Triffitt JT. A review on macrophage responses to biomaterials. Biomed Mater. 2006;1:R1–R9.
- Venkatesha S, Dudics S, Acharya B, et al. Cytokine-modulating strategies and newer cytokine targets for arthritis therapy. Int J Mol Sci. 2015;1:887–906.
- Giovanni M, Yue J, Zhang L, et al. Pro-inflammatory responses of RAW264.7 macrophages when treated with ultralow concentrations of silver, titanium dioxide, and zinc oxide nanoparticles. J Hazard Mater. 2015;297:146–152.
- Zaveri TD, Dolgova NV, Chu BH, et al. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials. 2010;11:2999–3007.
- Luo X, Barbieri D, Davison N, et al. Zinc in calcium phosphate mediates bone induction: in vitro and in vivo model. Acta Biomater. 2014;1:477–485.
- Yamaguchi M, Weitzmann MN. Zinc stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-ΚB activation. Mol Cell Biochem. 2011;1–2:179–186.
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964.
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969.
- Hotchkiss KM, Ayad NB, Hyzy SL, et al. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin Oral Implants Res. 2017;4:414–423.
- Hotchkiss KM, Reddy GB, Hyzy SL, et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016;31:425–434.
- Paul NE, Skazik C, Harwardt M, et al. Topographical control of human macrophages by a regularly microstructured polyvinylidene fluoride surface. Biomaterials. 2008;30:4056–4064.
- Smith BS, Capellato P, Kelley S, et al. Reduced in vitro immune response on titania nanotube arrays compared to titanium surface. Biomater Sci. 2013;3:322–332.
- Dai X, Wei Y, Zhang X, et al. Attenuating immune response of macrophage by enhancing hydrophilicity of Ti surface. J Nanomater. 2015;2015:1–8.
- Chen Z, Klein T, Murray RZ, et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;6:304–321.
- Macleod RJ, Hayes M, Pacheco I. Wnt5a secretion stimulated by BHE extracellular calcium-sensing receptor inhibits defective wnt signaling in colon cancer cells AJP. Gastrointest Liver Physiol. 2007;1:G403–G411.
- Pietak AM, Reid JW, Stott MJ, et al. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28:4023–4032.
- Scuderi P. Differential effects of copper and zinc on human peripheral blood monocyte cytokine secretion. Cell Immunol. 1990;2:391–405.
- Mountziaris PM, Mikos AG. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng B: Rev. 2008;2:179–186.
- Ishimi Y, Miyaura C, Jin C, et al. Il-6 is produced by osteoblasts and induces bone-resorption. J Immunol. 1990;10:3297–3303.
- Vanvlasselaer P, Borremans B, Vandenheuvel R, et al. Interleukin-10 inhibits the osteogenic activity of mouse bone-marrow. Blood. 1993;8:2361–2370.
- Ueno K, Katayama T, Miyamoto T, et al. Interleukin-4 enhances in vitro mineralization in human osteoblast-like cells. Biochem Biophys Res Commun. 1992;3:1521–1526.
- Nohtomi K, Sato K, Shizume K, et al. Stimulation of interleukin-4 of cell-proliferation and messenger-RNA expression of alkaline-phosphatase and collagen type-I in human osteoblast-like cells of trabecular bone. Bone Miner. 1994;1:69–79.