Abstract
The present study reports the biosynthesis of silver nanoparticles (IH-AgNPs) using aqueous leaf extract of Indigofera hisruta L. The biosynthesized IH-AgNPs were found to be FCC crystals, 5–10 nm in size, spherical in shape and stable. The biosynthesized IH-AgNPs showed dose-dependant cytotoxicity against prostate cancer (PC3) (IC50 = 68.5 μg/mL), colon cancer (COLO205) (IC50 = 85.2 μg/mL), and mouse melanoma (B16F10) (IC50 = 80.9 μg/mL). IH-AgNPs were found to be nontoxic towards normal CHO (Chinese hamster ovary) cells. The biosynthesized IH-AgNPs showed effective in vitro antioxidant activity against DPPH (IC50 = 63.43 μg/mL) and H2O2 (IC50 = 89.93 μg/mL) radicals. IH-AgNPs exhibited effective antibacterial activity against both Gram+ve and Gram-ve bacteria. MIC values of IH-AgNPs against S. aureus, B. subtilis, P. aeruginosa and E. coli were found to be 7.8 μg/mL, 3.9 μg/mL, 15.6 μg/mL and 15.6 μg/mL respectively. IH-AgNPs also showed inhibitory activity against fungal pathogens including C. albicans, C. nonalbicans and C. tropicalis. Considering the results together, we demonstrate that IH-AgNPs exhibits three different bioactivities (3-in-1 system) and they could be employed as future antimicrobial, antioxidant and anticancer agents/drug delivery vehicles in the field of biomedicine.
Introduction
Metal nanoparticles (NPs) have attracted significant attention due to their unique physicochemical properties such as optical, electronic, thermal and magnetic properties [Citation1]. The unique physicochemical properties of metal NPs mainly depend on the size, shape and surface chemistry [Citation2]. In the last decade, metal NPs such as silver, gold, copper, zinc oxide and titanium have been reported to play a key role in the drug delivery, cancer therapy and bio-imaging [Citation3–5]. Metal NPs have been employed for the diagnosis and treatment of neurodegenerative (Parkinson’s and Alzheimer’s) diseases [Citation6,Citation7]. Metal NPs based nanotherapeutic solutions for cardiovascular diseases have been developed [Citation8]. Chemical and biologically synthesized Metal NPs have been employed as antimycobacterial agents against tuberculosis [Citation9]. Gold nanobioconjugates (hyaluronate-AuNPs/tocilizumab) and gold/iron/gold plasmonic NPs have been successfully developed for the treatment of rheumatoid arthritis [Citation10,Citation11]. Metal NPs based biotherapeutics have been developed for the treatment of diabetes and inflammation [Citation12,Citation13]. Metal NPs possess antimicrobial activity against different bacteria and fungi including human pathogens, crop pathogens and oral pathogens [Citation14–16]. Antimicrobial activity of the metal NPs is an important application in the medical and diagnostic fields to prepare medicines, implants, injectables, diagnostic kits, devices, polymers and dressing materials to prevent infections. Metal NPs are also emerged as promising antiparasitic agents. Metal NPs particularly silver and gold possess antileishmanial activity and emerged as promising leishmanicidal agents [Citation17]. Metal NPs have been reported to exhibit antihelminthic activity against platy-helminths (flat worms). Biogenic AgNPs and AuNPs have been found to possess scolicidal activity against Echinococcus granulosus protoscolices [Citation18].
Among the metal NPs, silver races upfront due to several advantages including tunable photo-physical attributes, simple methods of production, ease of characterization and disinfectant activity of silver. AgNPs have been found to possess variety of biological applications such as catalysis [Citation19], biosensors [Citation20,Citation21], gene delivery [Citation22], biomarkers [Citation23], bioimaging [Citation5,Citation21], antimicrobial, antioxidant and anticancer agents [Citation24–30]. Different physical and chemical methods have been reported for the synthesis of AgNPs. But most of these methods possess limitations including application of various toxic materials that are not eco-friendly and also hazardous to animalia. Chemically synthesized AgNPs are non-biocompatible as they contain toxic chemicals on their surface. Hence, biological approaches have become prime choice for the production of clean, non-toxic and biocompatible AgNPs. AgNPs have been successfully produced using different plant extracts including Alysicarpus monilifer [Citation15], Justicia glauca [Citation24], Mimusops elengi [Citation25], Glycyrrhiza uralensis [Citation26], Cibotium barometz [Citation27], Rhynchosia suaveolens [Citation30], Borago officinalis [Citation31], Catharanthus roseus [Citation32], Chaenomeles sinensis [Citation33], Chrysanthemum indicum [Citation34], Elettaria cardamomum [Citation35], Centella asiatica [Citation36], Euphrasia officinalis [Citation37], Gymnema sylvestre [Citation38], Indigofera tinctoria [Citation39], Ipomoea batatus [Citation40], Melia dubia [Citation41], Panax ginseng [Citation42] and Rheum palmatum [Citation43].
Indigofera hirsuta is an important medicinal plant belongs to the family Fabaceae. Leaf extracts of I. hirsuta contain alkaloids, saponins, glycosides, flavonoids, tannins and steroids. They possess antimicrobial, anthelmintic, anti-inflammatory and antidiabetic properties [Citation44]. In the present study, we demonstrate the synthesis of IH-AgNPs using the leaf extract of I. hirsuta. In this study, leaf extract acts as both reducing and capping agent. Characterization of biosynthesized IH-AgNPs was carried out using different techniques like ultraviolet-visible (UV-Vis) spectroscopy, transmission electron microscopy (TEM), X-ray diffraction (XRD), dynamic light scattering (DLS) and Fourier transform infrared spectroscopy (FTIR) analysis. In this study, we demonstrate the antimicrobial activity of IH-AgNPs against various dreadful bacterial and fungal pathogens. IH-AgNPs exhibit antioxidant activity against DPPH and H2O2 free radicals. We also demonstrate that IH-AgNPs exhibit anticancer activity against mouse melanoma (B16F10), human colon carcinoma (COLO205) and human prostate carcinoma (PC3).
Materials and methods
Collection of plant material
Indigofera hirsuta plants were collected from Seshachalam hills, Eastern ghats, Andhra Pradesh, India. The taxonomic authentication was done and the voucher specimen was deposited in the Herbarium (SVUTYIH-0205), Department of Botany, Sri Venkateswara University, Andhra Pradesh, India.
Preparation of aqueous leaf extract of I. Hirsuta
Young and healthy leaves of I. hirsuta were collected and washed under running tap water to remove the dust. Then, the leaves were surface sterilized using Milli-Q water for three times and then blotted on the tissue paper to remove the moisture. Thereafter, the leaves were dried completely in the shade and ground into fine powder. Twenty grams of dried leaf powder was added into 100 mL of Milli-Q water, boiled for 15 min at 60 °C and incubated at room temperature for 2 h. After incubation, the extract was filtered and stored in an airtight container.
Biosynthesis of IH-AgNPs
Silver nitrate (AgNO3) was purchased from Molychem, India. Fifty millilitres of the aqueous leaf extract of I. hirsuta was added to 100 mL of 1 mM AgNO3 and heated for 30 min at 50 °C. The reaction mixture was incubated in the dark for 4 h at room temperature. Initially, bioreduction of silver ions (Ag+) into IH-AgNPs was detected visually by the colour change of the reaction solution.
Characterization of IH-AgNPs
Bioreduction of Ag + into IH-AgNPs was confirmed by UV-Vis double-beam spectrophotometer (Model: Spectro UV 2060 Plus; Analytical Technologies Ltd, Baroda, India). UV-Vis spectrum was recorded between 200 and 700 nm. Colloidal solution of IH-AgNPs was centrifuged at 2000 rpm for 15–20 min. Supernatant was discarded and the pellet of IH-AgNPs was collected. The pellet was redispersed in Milli-Q water and centrifuged at 16,000 rpm for 20 min. Repeated the centrifugation process for three times and the final pellet was collected. The pellet was air dried to get the pure powder of IH-AgNPs. This pure powder of IH-AgNPs was used for further characterization. FTIR spectrum of IH-AgNPs was recorded between 500–4000 cm−1 range by using Alpha interferometer (Bruker, Zurich, Switzerland). The crystalline structure of IH-AgNPs was determined by XRD using Cu Kα radiation source (Ultima IV X-ray diffractometer Rigaku Ltd, Tokyo, Japan). TEM (Philips Optics Ltd, Eindhoven, Netherlands) analysis of IH-AgNPs was carried out to determine the size and shape. Selected area electron diffraction (SAED) pattern of IH-AgNPs was also recorded using TEM. Particle size distribution, average particle size and zeta potential value (surface charge) were determined by DLS technique (SZ-100; Horiba Scientific, Stanmore, UK).
Antimicrobial activity of IH-AgNPs
Antibacterial activity of the IH-AgNPs was evaluated against dreadful human pathogenic bacteria including S. aureus and B. subtilis (Gram-positive), P. aeruginosa and E. coli (Gram-negative) by employing disc diffusion method [Citation36]. Two hundred microlitres of bacterial inoculum was spread on the surface of nutrient agar (NA) plates. Each NA plate contains three sterile paper discs. First disc was impregnated with aqueous leaf extract of I. hirsuta. Second disc was impregnated with 50 μL of IH-AgNPs (1 mg/1 mL) and third disc was impregnated with standard drug streptomycin. Then, the NA plates were incubated in BOD chamber at 37 °C for 24 h. After incubation, observed the NA plates for growth inhibition and measured the diameter of zone of inhibition.
Hundred microlitres (1 × 105 cells/mL) of actively growing bacterial suspension was poured into a set of test tubes. Different concentrations of IH-AgNPs (0–250 µg/mL) were added into each test tube. The final volume was adjusted to 1 mL by addition of nutrient broth and the samples were incubated at 37 °C for 24 h. Manifestation of turbidity reveals the active growth of bacteria, while disappearance of turbidity reveals the inhibition of growth of bacteria. The turbidity was measured using UV–Vis spectrophotometer at 600 nm wavelength.
Antifungal activity of the IH-AgNPs was evaluated against pathogenic fungi C. albicans, C. nonalbicans and C. tropicalis by employing disc diffusion method [Citation38]. Two hundred microlitres of fungal inoculum was spread on the surface of potato dextrose agar (PDA) plates. Each PDA plate contains three sterile paper discs. First disc was impregnated with aqueous leaf extract of I. hirsuta. Second disc was impregnated with 50 μL of IH-AgNPs (1 mg/1 mL) and third disc was impregnated with standard drug voriconazole. Then, the PDA plates were incubated at 25 °C for 72 h. After incubation, observe for the inhibition zones and recorded the diameter of zone of inhibition.
In vitro antioxidant activity of IH-AgNPs
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay
In vitro antioxidant activity of the IH-AgNPs was determined by DPPH radical scavenging assay [Citation45]. 0.1 mM of DPPH stock solution was prepared by dissolving 4 mg of DPPH in 100 mL of methanol. DPPH stock solution was stored at 4 °C. 20 µL of various concentrations (50, 100, 150 and 200 μg/mL) of IH-AgNPs was added to 180 µL of DPPH solution and then incubated for 45 min at room temperature in the dark. After incubation, the absorbance was measured at 517 nm using UV-Vis spectrometer. The free radical scavenging activity (RSA) was calculated by the following equation. RSA (%) = [(AControl – ATest)/AControl] × 100. The concentration of test sample required to scavenge/inhibit 50% of the radicals (IC50) was calculated using linear regression graphs.
Hydrogen peroxide (H2O2) radical scavenging assay
In vitro antioxidant activity of the IH-AgNPs was further confirmed by H2O2 radical scavenging assay [Citation46]. Fifty microlitres of different concentrations of IH-AgNPs (50, 100, 150 and 200 μg/mL) was added to 100 µL of H2O2 solution and kept in the dark at room temperature for 30 min. Then measured the absorbance at 230 nm. The percentage of RSA was calculated using the following equation. RSA (%) = [(AControl – ATest)/AControl] × 100. IC50 value was calculated using linear regression curve.
Anticancer activity of IH-AgNPs
B16F10, COLO205, PC3 and CHO cell lines were obtained from National Centre for Cellular Sciences, Pune, India. Cells were cultured either in RPMI (Roswell Park Memorial Institute) medium (COLO205) or DME (Dulbecco’s modified Eagle’s) medium (B16F10, PC3 and CHO) supplemented with 10% foetal bovine serum (heat inactivated), 1% penicillin, 1% streptomycin, 2 mM glutamine and 1 mM NaHCO3 at 37 °C in 5% CO2 atmosphere.
Anticancer activity of IH-AgNPs was measured by using MTT [3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide] assay [Citation47]. The cells were seeded in 96-well plates at a density of 5 × 103 per each well and incubated for overnight. After incubation, exactly 100 μL of IH-AgNPs at different concentrations (0, 10, 50, 100, 150 and 200 μg/mL) were added to the cell suspension and incubated for 24 h. After 24 h of incubation, 10 μL of MTT solution (at a concentration of 5 mg/mL PBS) was added into each well and incubated further for 3–4 h at 37 °C. Then discarded the media and MTT solution. The formazan crystals formed were dissolved using 100 μL of dimethyl sulfoxide. The absorbance of the each well was recorded at 570 nm wavelength. Percentage of cell viability was calculated and the IC50 values were also determined.
Results and discussion
Biosynthesis of IH-AgNPs
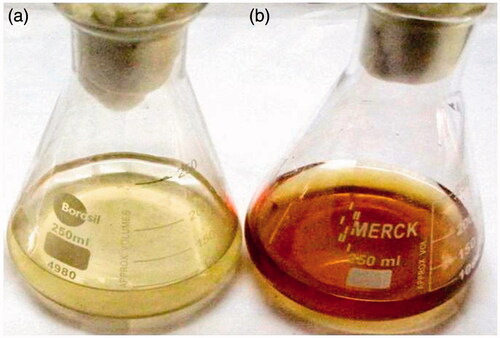
In the present study, we demonstrated the synthesis of IH-AgNPs using aqueous leaf extract of I. hirsuta. The leaf extract of I. hirsuta acts as both reducing and capping agent. Initially, the biosynthesis of IH-AgNPs was confirmed by observing the colour change of the colloidal solution from light yellow to dark brown (). Further the biosynthesis was authenticated by UV-Vis analysis of colloidal solution of IH-AgNPs.
UV-vis analysis of IH-AgNPs
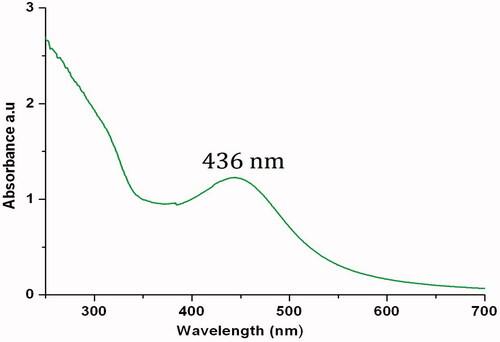
UV-Vis spectrum of the colloidal solution of IH-AgNPs showed an absorbance peak at 436 nm () which is the characteristic peak of AgNPs due to surface plasmon resonance (SPR), an unique interaction of light where the free electrons of the AgNPs undergo a oscillation with respect to the metal lattice in the presence of the oscillating electromagnetic field of the light [Citation1,Citation2,Citation48]. A band shift in the UV-Vis spectra signifies changes in the particle size, chemical surrounding, adsorbed species in the surface and dielectric constant. The SPR peak observed in this study indicates the formation of small sized, monodispersed and spherical shaped AgNPs without much agglomeration. The results are in consistence with previous reports. Ultra sized (10–20 nm) AgNPs synthesized by Justicia glauca exhibited SPR peak at 445 nm [Citation24]. Small size and spherical shaped AgNPs synthesized by Rheum palmatum, Chrysanthemum indicum and Mimusops elengi showed SPR peaks respectively at 440, 430 and 434 nm [Citation25,Citation34,Citation43].
TEM analysis of IH-AgNPs
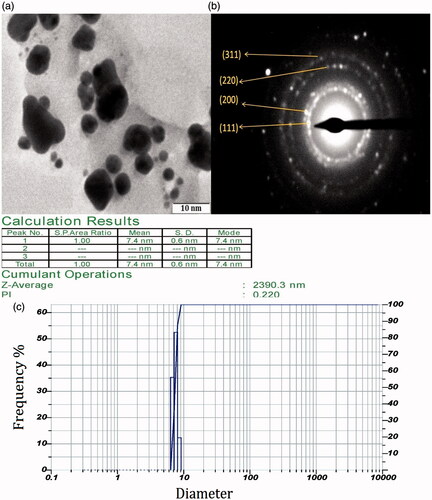
TEM analysis of IH-AgNPs was carried out to determine the morphology and size. The TEM micrograph () depicted that the biosynthesized IH-AgNPs are monodispersed in nature without agglomeration. TEM micrograph revealed that IH-AgNPs are 5–10 nm in size (ultra sized) and spherical in shape. SAED pattern () showed Debye-Scherrer rings correspond to (111), (200), (220) and (311) planes of the FCC crystal structure. SAED pattern was further supported by XRD spectra of IH-AgNPs. The size of the IH-AgNPs was further confirmed by particle size distribution using DLS (). DLS analysis showed that the IH-AgNPs were found to be 5–10 nm in size with average hydrodynamic radius of 7.4 ± 0.6 nm. The poly dispersity index (PDI) value of IH-AgNPs was found to be 0.220. TEM analysis, particle size distribution, average hydrodynamic radius and PDI value strongly support that the biosynthesized IH-AgNPs are ultra sized.
XRD analysis of IH-AgNPs
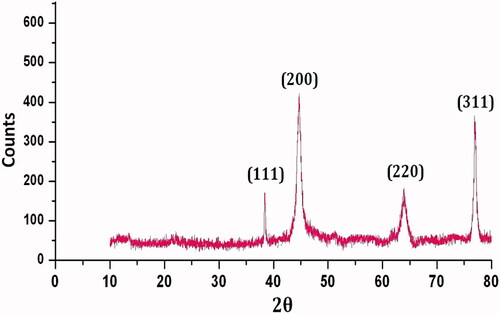
The crystalline nature of IH-AgNPs was determined by XRD analysis (). XRD pattern showed the Bragg’s diffraction peaks at 38.12°, 44.31°, 64.45° and 77.4°, respectively corresponding to (111) (200) (220) and (300) planes of the face centred cubic (FCC) lattice. The results are in consistent with standard JCPDS data (JCPDS card No.89- 3722). The XRD results are in line with previous reports [Citation31–43].
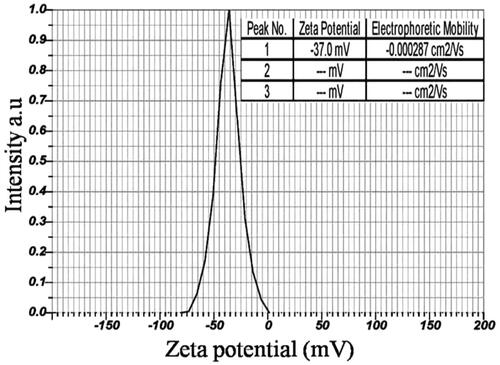
Zeta potential measurement of IH-AgNPs
DLS analysis of IH-AgNPs was carried out to determine the zeta potential value. Zeta potential is an essential measurement to determine the stability of the IH-AgNPs coated with plant proteins. In this study, Zeta potential of the IH-AgNPs was found to be −37.0 mV (). The high negative zeta potential revealed that IH-AgNPs are long-term stable without agglomeration. The high negative zeta potential value supports good colloidal nature, high dispersity and long-term stability of biosynthesized IH-AgNPs due to negative-negative repulsion. The high stability is very crucial for the application of AgNPs in biomedical and diagnostic fields. Further, we have carried out the stability studies for the biosynthesized IH-AgNPs using different buffer solutions as dispersion media including 5% NaCl, pH-7 PBS (phosphate-buffered saline), pH- 8 PBS, FBS (buffered solution containing fetal bovine serum) and DPBS (Dulbecco's phosphate-buffered saline). UV-Vis spectra were recorded at 24 h and 15 days. UV-Vis spectra showed similar SPR peaks for the IH-AgNPs dispersed in different buffer solutions after 24 h and 15 d. Thus, the biosynthesized IH-AgNPs were proved to be high stable.
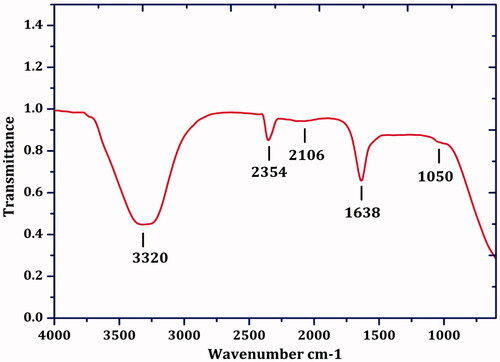
FTIR analysis of IH-AgNPs
FTIR spectrum showed the peaks at 3320, 2354, 2106, 1638 and 1050 cm-1 (). The broad peak at 3320 cm−1 is due to O-H stretching vibrations of phenolic compounds [Citation46]. The peak at 2354 cm − 1 is corresponding to C-O vibrational stretching of carbonyl compounds [Citation38]. The peak at 2106 cm−1 could be raised due to C=O stretching of the aromatic groups. The peak at 1638 cm − 1 is corresponding to amide linkage of proteins. The peak at 1051 cm−1 could be assigned to C-OH of carboxylic acids [Citation46]. The peaks clearly revealed the participation of proteins and polyphenols in the bioreduction of silver ions into IH-AgNPs. The proteins plausibly involved in the coating/capping around the particles. Biofunctionalization of AgNPs with proteins leads to long-term stability and prevent the agglomeration of the IH-AgNPs. The stability of AgNPs was already confirmed by zeta potential measurement.
We state that IH-AgNPs are ultra sized (5–10 nm in size) and spherical in shape (based on the TEM and particle size distribution analysis), pure crystalline in nature (based on XRD and SAED pattern analysis), and highly stable due to high negative surface charge and capping by proteins (based on zeta potential measurement and FTIR analysis).
Antimicrobial activity of IH-AgNPs
IH-AgNPs effectively inhibited the growth of bacteria by producing the clear inhibition zones around the discs. The inhibition zones produced by IH-AgNPs, aqueous leaf extract of I. hirsuta and standard antibiotic (streptomycin) were showed in the . IH-AgNPs produced the inhibition zones of 14.8, 16.5, 13.8 and 12.4 mm, respectively, against S. aureus, B. subtilis, P. aeruginosa and E. coli. MIC values of AgNPs were found to be 7.8 μg/mL, 3.9 μg/mL, 15.6 μg/mL and 15.6 μg/mL respectively against S. aureus, B. subtilis, P. aeruginosa, E. coli. Based on the inhibition zones and MIC values, it is determined that IH-AgNPs showed maximum inhibition against Gram + ve bacteria compared to Gram-ve bacteria. The lowest MIC value for B. subtilis revealed its more susceptibility to IH-AgNPs. The difference in the sensitivity of the Gram-positive and Gram-negative strains could be due to difference in their membrane composition, cell structure, metabolism and the interaction with the IH-AgNPs.
Figure 7. Antimicrobial activity of the IH-AgNPs against bacterial species (a) S. aureus, (b) B. subtilis, (c) P. aeruginosa (d) E. coli and fungal species (e) C. albicans, (f) C. nonalbicans and (g) C. tropicalis; (i) Inhibition zones observed by aqueous leaf extract of I. hirsuta, (ii) Inhibition zones observed by biosynthesized IH-AgNPs and (iii) Inhibition zones by standard drug.
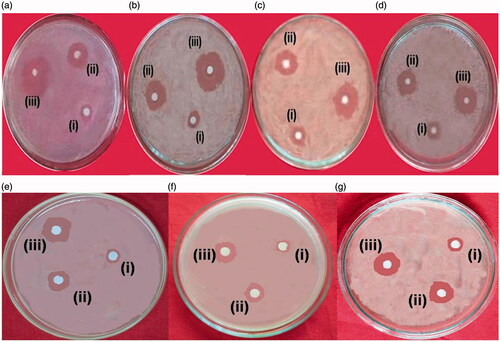
Antifungal activity of the IH-AgNPs was also studied against different Candida species such as C. albicans, C. nonalbicans and C. tropicalis. IH-AgNPs exhibited effective inhibition against all the Candida species used in this study. The inhibition zones produced by aqueous leaf extract, IH-AgNPs and voriconazole were represented in . IH-AgNPs formed highest growth inhibition zone against C. tropicalis (15.6 mm) followed by C. albicans (14.3 mm) and C. nonalbicans (12.1 mm).
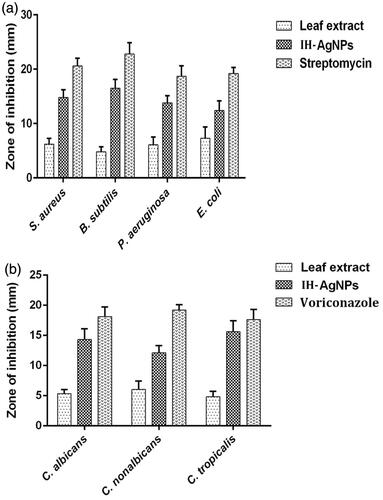
The diameter of inhibition zones (mm) produced by aqueous leaf extract of I. hirsuta, IH-AgNPs and streptomycin were compared (). IH-AgNPs produced the inhibition zones of 12-17 mm against different bacteria and fungi used in this study, whereas aqueous leaf extract produced the inhibition zones of 4–7 mm only and the standard antibiotic formed the inhibition zones of 18-23 mm. IH-AgNPs exhibited about 2- to 4-fold effective antibacterial and antifungal activities compared with that of pristine aqueous leaf extract of I. hirsuta. The effective inhibitory activity of IH-AgNPs might be due to synergistic effect of silver, polyphenolic compounds (involved in the synthesis of NPs) and proteins (coated around the NPs).
Figure 8. Comparison of (a) antibacterial and (b) antifungal activities of pristine aqueous leaf extract of I. hisuta, IH-AgNPs and standard drug (streptomycin/voriconazole).
The results of antimicrobial activity of IH-AgNPs are in line with many previous reports. Justicia glauca leaf synthesized AgNPs exhibited effective antimicrobial activity with the inhibition zones of 25, 29, 23, 28 and 26 mm, respectively, against S. aureus, B. subtilis, P. aeruginosa, E. coli and C. albicans [Citation24]. Mimusops elengi leaf synthesized AgNPs showed antimicrobial activity with inhibition zones of 10 and 18 mm against S. aureus and K. pneumonia [Citation25]. Rheum palmatum root synthesized AgNPs showed inhibition against S. aureus (15 mm) and P. aeruginosa (13 mm) [Citation43]. Saravanan et al. (2011) reported that biosynthesized AgNPs exhibited effective inhibition against drug resistant pathogens Salmonella typhi and Streptococcus pneumoniae [Citation49]. Biosynthesized AgNPs also showed inhibition against methicillin resistant Staphylococcus aureus and methicillin-resistant Staphylococcus epidermidis [Citation50,Citation51]. Biosynthesized AgNPs using Bacillus subtilis showed broad spectrum antibacterial activity against different bacteria including Staphylococcus aureus, Streptococcus pyogens, Salmonella typhi and Klebsiella pneumonia [Citation52]. In this study, IH-AgNPs exhibited maximum inhibition against Gram-positive bacteria compared to Gram-negative bacteria. Our results are supported by many previous reports. Biosynthesized AgNPs using different plants and bacteria including Justicia glauca, Rheum palmatum, Bacillus megaterium, Aspergillus clavatus, Bacillus subtilis showed more inhibition against Gram-positive bacteria compared to Gram-positive bacteria [Citation24,Citation25,Citation49–52].
The antimicrobial activity of the IH-AgNPs mainly depends on the size, shape and negative surface charge. The difference in inhibitory activity of the IH-AgNPs against different microbes could also due to the variations in membrane composition of bacteria and fungi. Ultra sized IH-AgNPs possess larger surface area to volume ratio which enhances the contact surface of IH-AgNPs to microorganism which in turn enhances the antimicrobial activity. The effective activity of the IH-AgNPs synthesized in this study might be due to their smaller size (5–10 nm) and high negative surface charge (−37.0 mV). The plausible mechanism behind the inhibitory activity of IH-AgNPs against different bacteria and fungi was also explained. IH-AgNPs caused the bacterial cell death by membrane disruption and bacterial DNA fragmentation [Citation53,Citation54]. IH-AgNPs bind with active site of regulatory enzymes that are important for bacterial cell division. As a result bacterial cell death occurs. Proteomic analysis revealed that IH-AgNPs can disrupt the cell membranes by altering the expression of membrane proteins which leads to leakage of intracellular ions and reducing sugars and eventually leads to death [Citation55].
In vitro antioxidant activity of IH-AgNPs
Antioxidant activity of the IH-AgNPs was measured by DPPH and H2O2 radical scavenging assays. IH-AgNPs exhibited dose-dependant radical scavenging activity against DPPH free radicals (). IH-AgNPs showed the maximum scavenging activity of 70.81% at the highest concentration (200 µg/mL) used in this assay. IC50 value of IH-AgNPs against DPPH radicals was found to be 63.43 µg/mL. IH-AgNPs also showed concentration-dependant scavenging activity against H2O2 radicals (). IH-AgNPs showed maximum inhibition of 65.75% at the highest concentration (200 µg/mL) used in this assay. IC50 value of IH-AgNPs against H2O2 radicals was found to be 89.93 µg/mL.
Figure 9. Free radical scavenging activity of IH-AgNPs. (a) DPPH radical scavenging activity and (b) H2O2 radical scavenging activity.
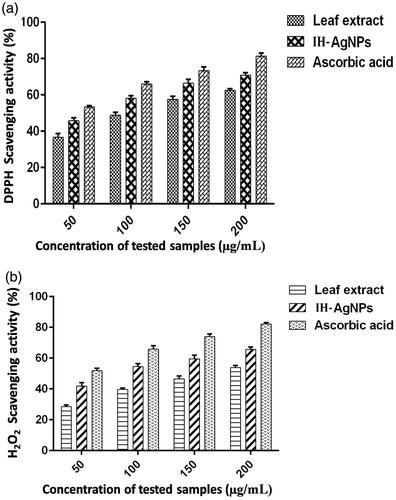
The effective scavenging activity of the IH-AgNPs could be due to antioxidant compounds like polyphenols and flavonoids present in the leaf extract. Polyphenols and flavonoids mainly participated in the biosynthesis of IH-AgNPs. Our results are in consistence with many earlier reports. Mohamed et al (2014) showed antioxidant activity of green synthesized AgNPs against DPPH radicals [Citation56]. AgNPs prepared by using Cibotium barometz root showed effective scavenging activity against DPPH radicals [Citation27]. Chaenomeles sinensis leaf synthesized AgNPs showed effective DPPH quenching activity [Citation33]. Reactive oxygen species (ROS) such as hydroxyl, epoxyl, superoxide, peroxylnitrile and singlet oxygen causes oxidative stress linked to various human diseases such as inflammation, atherosclerosis, ageing, cancer and neurodegenerative disorders. Therefore, more attention has been focused on the application of natural antioxidants to protect the cells against the damage by ROS. Hence the present study provides the evidence for the application of IH-AgNPs as natural antioxidants in future.
Anticancer activity of IH-AgNPs
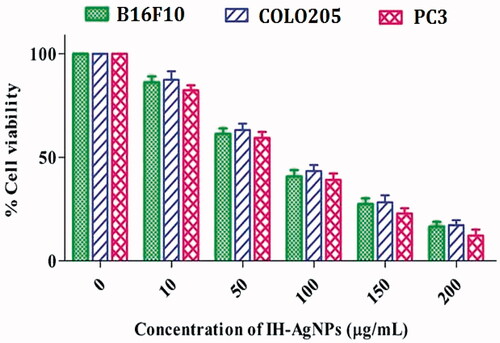
The cytotoxic effects of IH-AgNPs on cancer cells B16F10, COLO205 and PC3 were checked. The percentage of cell viability of different cell lines used in this study was depicted in . It was observed that percentage of cell viability decreases with increase in the concentration of IH-AgNPs. Increase in the concentration of IH-AgNPs decreases the cell viability and increases the cytotoxicity against B16F10, COLO205 and PC3 cells. IH-AgNPs showed maximum inhibition at the highest concentration (200 µg/mL) used in this assay. IH-AgNPs exhibited 85.4% inhibition of B16F10 cells, 82.2% inhibition of COLO205 cells, and 88.1% inhibition of PC3 cells. IC50 values of IH-AgNPs against B16F10, COLO205 and PC3 cells were found to be 80.9, 85.2 and 68.5 μg/mL respectively.
Figure 10. Effect of the IH-AgNPs on the cell viability of different cancer cells including B16F10, COLO205 and PC3.
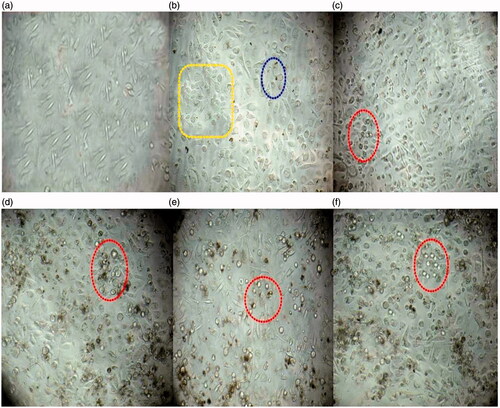
Among the three cell lines used in this study, PC3 cells were found to be more susceptible to IH-AgNPs. showed the light microscopic observations of the morphology of PC3 cells treated with IH-AgNPs. Increase in the concentration of IH-AgNPs decreases the cell viability and increases the number of dead cells or non-viable cells. The non-viable cells are clearly observed in the red round circles indicated in the figure. As the concentration of IH-AgNPs increases, the cells become clustered and exhibited morphological alterations which eventually leads to cell death or apoptosis. The exact mechanism of anticancer activity of AgNPs is not yet elucidated. But, the plausible reasons for the anticancer activity of IH-AgNPs were explained. AgNPs causes the apoptosis of cancer cells through caspase-dependent and mitochondrial-dependent pathways. IH-AgNPs entered into mammalian cells through various mechanisms such as endocytosis, pinocytosis and phagocytosis. AgNPs causes reactive oxygen species (ROS) generation which leads to up-regulation of caspase-3 and p53 protein expressions. Caspase-3 plays a major role in cell apoptosis by arresting G2/M phase of cell cycle [Citation3,Citation29]. Biocompatibility is crucial step for the successful application of NPs in biomedical field. Hence, the IH-AgNPs were checked for biocompatibility on normal CHO cell lines. IH-AgNPs did not show any dose dependant increase in cytotoxicity towards normal CHO cells. 90% cells are viable even at the highest concentration of IH-AgNPs used in this assay. This proved that IH-AgNPs synthesized in this study are biocompatible towards normal cells. Ovais et al. (2016) reported that phytochemical coating around biosynthesized AgNPs make them high bioactive and biocompatible compared with chemically synthesized AgNPs [Citation29].
Figure 11. Effect of the IH-AgNPs on the cell viability of PC3 cells (a) Normal control (Without any treatment), (b) PC3 cells treated with 10 µg/mL of IH-AgNPs, (c) PC3 cells treated with 50 µg/mL of IH-AgNPs, (d) PC3 cells treated with 100 µg/mL of IH-AgNPs, (e) PC3 cells treated with 150 µg/mL of IH-AgNPs and (f) PC3 cells treated with 200 µg/mL of IH-AgNPs. Yellow box indicates cells undergo morphological changes, Green round circle indicates the initial apoptotic cells, Red round circles indicates dead cells or nonviable cells. Increase in the concentration of IH-AgNPs increases the cytotoxic activity (or increase in the number of non-viable cells).
It was reported that the inhibitory effects of IH-AgNPs on different cell lines mainly depends on the size, morphology and plant compounds coated around AgNPs (proteins and flavonoids etc). IH-AgNPs biosynthesized in this study are 5–10 nm in size with the mean size of 7.4 nm. The ultra sizes of NPs possess the advantages of more permeability, high accumulation and high retention properties. Ultra-sized NPs can be up taken easily by the cells through different endocytic processes which involve complex intrinsic pathways. Therapeutic efficiency of ultra-sized NPs can be enhanced due to their significant diffusion, deep penetration and high accumulation in the tumour matrix. Whereas the NPs ranged between 20–100 nm could not be penetrated deeply in the tumour matrix and accumulate at the walls of blood vessels and can reach only few top layers of tumour matrix. Consequently, the tumour cells which are far from the blood vessels cannot be eradicated completely [Citation46]. Elimination of the tumour cells at/near the walls of blood vessels may check the tumour’s growth but the tumorigenic/oncogenic cells cannot be eradicated utterly. Based on this results, it is evident that the IH-AgNPs synthesized in this study are biocompatible to normal cells and cytotoxic to cancer cells and hence they can be employed as future anticancer agents or drug delivery systems.
This study clearly demonstrated that the synthesized IH-AgNPs are non-toxic and biocompatible towards normal CHO cells and are potent cytotoxic against cancer cell lines. Our results are in line with the many previous reports portrayed the anticancer activity of AgNPs synthesized using different plant extracts. AgNPs synthesized using Olax scandens showed effective cytotoxicity against different cancer cells including B16F10, A549 (human lung carcinoma) and MCF-7 (human breast cancer) and they showed biocompatibility towards normal cells including CHO, H9C2 (rat cardiomyoblast), and HUVEC (human umbilical vein endothelial cells) [Citation5]. AgNPs from Panax ginseng showed broad spectrum cytotoxicity against different cell lines including A549, HepG2 (Hepatic carcinoma) and MCF-7 cells [Citation42]. AgNPs from Clerodendrum phlomidis showed significant cytotoxicity against HT29 (human colorectal adenocarcinoma) and EAC (Ehrlich ascites carcinoma) cells [Citation57]. Biosynthesized AgNPs using Gymnema sylvestre and Rubus glaucus leaves showed effective cytotoxicity against HepG2 cell lines [Citation58,Citation59]. Biosynthesized AgNPs using Hydrastis canadensis, Gelsemium sempervirens, Phytolacca decandra, and Thuja occidentalis showed anticancer activity against A375 (skin melanoma) by arresting G2/M phase of cell cycle [Citation60].
Characteristic features and biological activities of biosynthesized IH-AgNPs were represented clearly in .
Table 1. Represents the characteristic features and biological activities of biosynthesized IH-AgNPs using leaf extract of I. hirsuta.
Conclusions
In the present study, we report a simple, rapid, efficient and eco-friendly approach for the successful synthesis of clean, non-toxic and biocompatible IH-AgNPs using leaf extract of I. hirsuta. The synthesized IH-AgNPs were ultra-sized, spherical in shape and highly stable. Biosynthesized IH-AgNPs were proved their biomedical importance by showing three different biological activities (3-in-1 system) including antimicrobial activity against bacterial and fungal pathogens, antioxidant activity against different free radicals, and anticancer activity against cancer cells, as well as biocompatible nature towards normal cells. Hence, we believe that the synthesized IH-AgNPs can be used as antimicrobial ingredients in topical ointments to prevent bacterial and fungal infections. Further we strongly believe that the IH-AgNPs could be bound with many drugs and proteins for targeting against cancer cells. Considering the results, IH-AgNPs could be employed as a source for the exploration of novel therapeutic agents in biomedical field.
Acknowledgements
We are highly grateful to Dr. J. Venkateswara rao, Scientist-G and Dr. Bojja Sreedhar, Scientist-F, IICT, Hyderabad, for technical assistance
Disclosure statement
All the authors declare that there is no conflict of interest.
References
- El-Sayed MA. Some interesting properties of metals confined in time and nanometer space of different shapes. Acc Chem Res. 2001;34:257–264.
- Kelly KL, Coronando E, Zhao LL, et al. The Optical properties of metal nanoparticles: the influence of size, shape and dielectric environment. J Phys Chem B. 2002;107:668–677.
- Barabadi H, Ovais M, Khan Z, et al. Anti-cancer green bionanomaterials: present status and future prospects. Green Chem Lett Rev. 2017;10:285–314.
- Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782.
- Mukherjee S, Chowdhury D, Rajesh K, et al. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics. 2014;4:316–335.
- Mustafa N, Salina M, Laszlo P. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: focus on selenium nanoparticles. Expert Rev Clin Pharmacol. 2017; doi: https://doi.org/10.1080/17512433.2017.1324781
- Vio V, Marchant MJ, Araya E, et al. Metal nanoparticles for the treatment and diagnosis of neurodegenerative brain diseases. CPD. 2017;23:1916–1926.
- Mulder WJM, Fayad ZA. Nanomedicine captures cardiovascular disease. Arterioscler Thromb Vasc Biol. 2008;28:801–802.
- Richa S, Laxman UN, Manisha A, et al. Chemical and biological metal nanoparticles as antimycobacterial agents: a comparative study. Int J Antimicrob Agents. 2015;46:183–188.
- Lee H, Lee MY, Bhang SH, et al. Hyaluronate-gold nanoparticle/tocilizumab complex for the treatment of rheumatoid arthritis. ACS Nano. 2014;8:4790–4798.
- Kim HJ, Lee SM, Park KH, et al. Drug-loaded gold/iron/gold plasmonic nanoparticles for magnetic targeted chemo-photothermal treatment of rheumatoid arthritis. Biomaterials. 2015;61:95–102.
- Sung HW, Kiran S, Feng SS. Nanomedicine for diabetes treatment. Nanomedicine. 2011;6:1297–1300.
- Luminita D, Bianca M, Adriana V, et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Col Surf B Biointerfaces. 2014;122:767–777.
- Saravananan M, Gopinath V, Chaurasia MK, et al. Green synthesis of anisotropic zinc oxide nanoparticles with antibacterial and cytofriendly properties. Microb Pathogen. 2018;115:57–63.
- Kasithevar M, Saravanan M, Prakash P, et al. Green synthesis of silver nanoparticles using Alysicarpus monilifer leaf extract and its antibacterial activity against MRSA and CoNS isolates in HIV patients. J Interdisciplin Nanomed. 2017;2:131–141.
- Emmanuel R, Saravanan M, Ovais M, et al. Antimicrobial efficacy of drug blended biosynthesized colloidal gold nanoparticles from Justicia glauca against oral pathogens: a nanoantibiotic approach. Microb Pathogen. 2017;113:295–302.
- Ovais M, Akhtar N, Khalil AT, et al. Biosynthesized colloidal silver and gold nanoparticles as emerging leishmanicidal agents: an insight. Nanomedicine (Lond). 2017;12:2807–2819.
- Barabadi H, Soheila H, Milad AM, et al. Green chemical synthesis of gold nanoparticles by using Penicillium aculeatum and their scolicidal activity against hydatid cyst protoscolices of Echinococcus granulosus. Environ Sci Pollut Res. 2017;24:5800–5810.
- Campelo JM, Luna D, Luque R, et al. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chem Sus Chem. 2009; 2:18–45.
- Cobley CM, Skrabalak SE, Campbel DJ, et al. Shape controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics. 2009;4:171–179.
- Georgios AS, Pratsinis SE. Engineering nanosilver as an antibacterial, biosensor and bioimaging material. Curr Opin Chem Eng. 2011;1:3–10.
- Chen XJ, Sanchez GBL, Qian ZX, et al. Noble metal nanoparticles in DNA detection and delivery. Wiley Interdisci Rev Nanomed Nanobiotech. 2012;4:273–290.
- Liang J, Liu H, Huang C, et al. Aggregated silver nanoparticles based surface-enhanced Raman scattering enzyme-linked immunosorbent assay for ultrasensitive detection of protein biomarkers and small molecules. Anal Chem. 2015;87:5790–5796.
- Emmanuel R, Palanisamy S, Chen SM, et al. Antimicrobial efficacy of green synthesized drug blended silver nanoparticles against dental caries and periodontal disease causing microorganisms. Mater Sci Eng C Mater Biol Appl. 2015;56:374–379.
- Prakash Gnanaprakasam P, Emmanuel R, et al. Green synthesis of silver nanoparticles from leaf extract of Mimusops elengi, Linn. for enhanced antibacterial activity against multi drug resistant clinical isolates. Col Surf B Biointerfaces. 2013;108:255–259.
- Huo Y, Singh P, Kim YJ, et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotechnol. 2018;46:303–312.
- Wang D, Markus J, Wang C, et al. Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root. Artif Cells Nanomed Biotechnol. 2017;45:1548–1555.
- Subbaiya R, Saravanan M, Priya AR, et al. Biomimetic synthesis of silver nanoparticles from Streptomyces atrovirens and their potential anticancer activity against human breast cancer cells. IET Nanobiotechnol. 2017;11:965–972.
- Ovais M, Khalil AT, Raza A, et al. Green synthesis of silver nanoparticles via plant extracts: beginning a new era in cancer theranostics. Nanomedicine (Lond). 2016; 11:3157–3177.
- Bethu MS, Netala VR, Domdi L, et al. Potential anticancer activity of biogenic silver nanoparticles using leaf extract of Rhynchosia suaveolens: an insight into the mechanism. Artif Cells Nanomed Biotechnol. 2018;1–11. doi: https://doi.org/10.1080/21691401.2017.1414824.
- Singh H, Du J, Yi TH. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomed Biotechnolo. 2017;45:1310–1316.
- Kotakadi VS, Rao YS, Gaddam SA, et al. Simple and rapid biosynthesis of stable silver nanoparticles using dried leaves of Catharanthus roseus.Linn. G. Donn and its anti microbial activity. Colloids Surf B Biointerfaces. 2013;105:194–198.
- Oh KH, Soshnikova V, Markus J, et al. Biosynthesized gold and silver nanoparticles by aqueous fruit extract of Chaenomeles sinensis and screening of their biomedical activities. Artif Cells Nanomed Biotechnol. 2017; doi: https://doi.org/10.1080/21691401.2017.1332636.
- Arokiyaraj S, Dinesh KV, Elakya V, et al. Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.–potential for malaria vector control. Environ Sci Pollut Res. 2015;22:9759–9765.
- Soshnikova V, Kim YJ, Singh P, et al. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif Cells Nanomed Biotechnol. 2018;46:108–117.
- Netala VR, Kotakadi VS, Nagam V, et al. First report of biomimetic synthesis of silver nanoparticles using aqueous callus extract of Centella asiatica and their antimicrobial activity. Appl Nanosci. 2015;5:801–807.
- Singh H, Du J, Singh P, et al. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells Nanomed Biotechnol. 2017; doi: https://doi.org/10.1080/21691401.2017.1362417.
- Netala VR, Kotakadi VS, Domdi L, et al. Biogenic silver nanoparticles: efficient and effective antifungal agents. Appl Nanosci. 2016;6:475–484.
- Vijayan R, Joseph S, Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif Cells Nanomed Biotechnol. 2017; doi: https://doi.org/10.1080/21691401.2017.1345930.
- Pavithra BV, Ragavendran C, Murugan N, et al. Ipomoea batatas (Convolvulaceae)-mediated synthesis of silver nanoparticles for controlling mosquito vectors of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus (Diptera:Culicidae). Artif Cells Nanomed Biotechnol. 2017;45:1568–1580.
- Netala VR, Kotakadi VS, Ghosh SB, et al. Biofabrication of silver nanoparticles using aqueous leaf extract of Melia dubia, characterization and antifungal activity. Int J Pharm Pharm Sci. 2014;6:298–300.
- Singh P, Kim YJ, Wang C, et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 2016;44:811–816.
- Arokiyaraj S, Vincent S, Saravanan M, et al. Green synthesis of silver nanoparticles using Rheum palmatum root extract and their antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosa. Artif Cells Nanomed Biotechnol. 2017;45:372–379.
- Suvarnalatha A, Yasodamma N. Quantitative phytochemical evaluation of Indigofera hirsuta L. Plant parts. Int J Pharma Res Rev. 2015;4:1–5.
- Netala VR, Kotakadi VS, Bobbu PL, et al. Endophytic fungal isolate mediated biosynthesis of silver nanoparticles and their free radical scavenging activity and anti microbial studies. 3 Biotech. 2016;6:132.
- Netala VR, Murali BS, Bobbu PL, et al. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. IJN. 2016;11:5683–5696.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Link S, El-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54:331–366.
- Saravanan M, Vemu AK, Barik SK. Rapid biosynthesis of silver nanoparticles from Bacillus megaterium (NCIM 2326) and their antibacterial activity on multi drug resistant clinical pathogens. Col Surf B Biointerfaces. 2011;88:325–331.
- Saravanan M, Nanda A. Extracellular synthesis of silver bionanoparticles from Aspergillus clavatus and its antimicrobial activity against MRSA and MRSE. Colloids Surf B Biointerfaces. 2010;77:214–218.
- Nanda A, Saravanan M. Biosynthesis of silver nanoparticles from Staphylococcus aureus and its antimicrobial activity against MRSA and MRSE. Nanomedicine. 2009;5:452–456.
- Saravanan M, Jacob V, Arockiaraj J, et al. Extracellular biosynthesis, characterization and antibacterial activity of silver nanoparticles synthesized by Bacillus subtilis (NCIM—2266)). J Bionanosci. 2014;8:21–27.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology 2005;16:2346–2353.
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotech Biol Med. 2007;3:168–171.
- Lok CN, Ho CM, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924.
- Mohamed SA, Mohamed SS, Aziza AE, et al. Antioxidant and antibacterial activity of silver nanoparticles biosynthesized using Chenopodium murale leaf extract. J Saudi Chem Soc. 2014;18:356–363.
- Sriranjani R, Srinithya B, Vellingiri V, et al. Silver nanoparticle synthesis using Clerodendrum phlomidis leaf extract and preliminary investigation of its antioxidant and anticancer activities. J Mol Liquids. 2016;220:926–930.
- Nakkala JR, Rani M, Bhaga E, et al. Green synthesis of silver and gold nanoparticles from Gymnema sylvestre leaf extract: study of antioxidant and anticancer activities. J Nanopart Res. 2015;17:151.
- Kumar B, Kumari S, Seqqat R, et al. In vitro evaluation of silver nanoparticles cytotoxicity on Hepatic cancer (Hep-G2) cell line and their antioxidant activity: green approach for fabrication and application. J Photochem Photobiol B: Biol. 2016;159:8–13.
- Sreemanti D, Jayeeta D, Asmita S, et al. Biosynthesized silver nanoparticles by ethanolic extracts of Phytolacca decandra, Gelsemium sempervirens, Hydrastis canadensis and Thuja occidentalis induce differential cytotoxicity through G2/M arrest in A375 cells. Coll Surfaces B: Biointerfaces. 2013;101:325–336.