Abstract
A green synthesis route was developed for preparation of the ZnO nanoparticles and ZnO/CuO nanocomposites by using Vaccinium arctostaphylos L. fruit extract, and their anti-bacterial activities against two pathogenic bacteria, Escherichia coli (Gram negative) and Staphylococcus aureus (Gram positive), were investigated. The fabricated nanomaterials were characterized by XRD, EDX, FESEM, TEM, TGA, FT-IR, UV–vis DRS and BET instruments. The results showed that the sizes of ZnO and CuO nanoparticles in the ZnO/CuO (10%) nanocomposite were 12 and 8 nm, respectively. In addition, specific surface areas of the ZnO (W), ZnO (ext) and ZnO/CuO samples were 29.3, 27.5 and 18.0 m2/g−1, respectively. The results obtained from EDX, TGA, FT-IR and UV–Vis DRS analyses displayed that some of the organic compounds from the fruit extract have bonded to the surface of the samples fabricated in the presence of the fruit extract. The results revealed that the anti-bacterial activity of the fabricated samples is as ZnO/CuO (10%) > ZnO/CuO (5%) > ZnO (ext) > ZnO (W). Viability percentages for E. coli and S. aureus bacteria were 12.1 ± 0.39 and 14.9 ± 0.65 in the presence of the ZnO/CuO (10%) nanocomposite, respectively. The SEM images clearly demonstrated the disruption of the bacterial membranes during the inactivation process.
Graphical Abstract
Introduction
Infectious diseases are among the most popular challenges in the medical field. In the last decade, we were witnessing the emergence of more than 300 new infectious diseases and the revitalization of multiple past infectious diseases [Citation1]. Recently, the utilization of conventional anti-bacterial drugs has led to the microbial resistance to these drugs. This situation is reaching a perilous level and going to be the major cause of mass population deaths worldwide [Citation1–3]. Moreover, the lack of financial resources, existing scientific barriers and regulatory challenges have slowed down the design and development of new antibiotics [Citation4].
In this regard, metal oxide nanoparticles have attracted much more attention from the research community to address these issues [Citation5]. These nanoparticles possess unique properties such as super-durability, lower toxicity and high surface-to-volume ratio [Citation6]. There are different physical/chemical techniques to prepare these nanoparticles, including microwave-assisted synthesis, sol–gel, chemical vapour deposition, co-precipitation, thermal decomposition, hydrothermal and combustion methods [Citation7]. Among these methods, microwave irradiation route is a facile, fast and controllable method to synthesize these nanoparticles [Citation8,Citation9]. On the other hand, a green synthesis method, using environmentally friendly compounds/microorganisms have been accepted as a promising technique to prevail the limitations accompanied with the above-mentioned methods. In recent years, extensive efforts have been done for the application of plant extracts to fabricate different metal oxides and metallic nanoparticles, due to their availability, low cost and biocompatibility [Citation10,Citation11]. Vaccinium L. belongs to the Ericaceae family that is morphologically diverse. This plant includes almost 450 species in the cold areas of the northern Hemisphere, and some tropical areas such as Madagascar and Hawaii. The chemical constituents of the fruit extract such as flavonoids, anthocyanins, coumarins, lignans, iridoids, benzoic acids and triterpenoids have been well-documented. In this plant, more than 116 anthocyanins and flavonoids compounds have identified in its fruits and leaves. Vaccinium arctostaphylos L. is an only member of Vaccinium genus in Iran, which has anti-microbial, anti-diabetic and anti-hypertension activities [Citation12–14]. In addition, it has been reported that anti-oxidant activity for the bioactivities compounds of this plant [Citation15,Citation16].
Up to now, various metal oxides such as zinc oxide (ZnO) nanoparticles have been synthesized using plants extract [Citation17–25]. The ZnO nanoparticles have unique properties, including optical and electrical conductivity, UV-filtering, photochemical, photocatalytic, anti-viral, anti-bacterial, anti-fungal and anti-cancer activities [Citation26–30]. This metal oxide, which listed in the generally recognized as safe materials by the U.S. Food and Drug Administration, is biocompatible and non-toxic for human beings and animals [Citation31–33]. Another metal oxide that has been used as a biocide for many years is copper oxide (CuO). This metal oxide is a p-type semiconductor with optical, thermal, electrical, catalytic, anti-fungal, anti-viral and anti-bacterial activities [Citation3,Citation7,Citation34].
In this paper, ZnO powder in water, denoted as ZnO (W), in the fruit extract of V. arctostaphylos L., denoted as ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) were synthesized in the extract by a microwave-assisted method. The prepared nanomaterials were characterized using X-ray diffraction (XRD), energy-dispersive X-ray spectroscopy (EDX), field emission scanning electron microscopy (FESEM), transmission electron microscopy (TEM), thermo gravimetric analysis (TGA), Fourier transform-infrared spectroscopy (FT-IR), UV–vis diffuse reflectance spectroscopy (UV–vis DRS) and Brunauer–Emmett–Teller (BET) instruments. Furthermore, to examine the anti-bacterial activities, the prepared nanomaterials were used against Escherichia coli (Gram-negative) and Staphylococcus aureus (Gram-positive) bacteria. The experimental results displayed that the fabricated nanomaterials in the presence of the extract had excellent potential as a growth inhibitor for the studied bacteria.
Experimental section
Materials
In this research, zinc (II) nitrate [Zn(NO3)2.6H2O], copper (II) acetate [Cu(CH3 COO)2.H2O], sodium hydroxide (NaOH) and absolute ethanol were purchased from Merck (Darmstadt, Germany). E. coli (PTCC 1047) and S. aureus (PTCC 1189) were provided from Iranian Research Organization for Science and Technology (IROST). Nutrient broth (NB) and nutrient agar (NA) were purchased from Pronadisa (Spain). Double distilled water (DDW) was used in this work.
Preparation of the extract
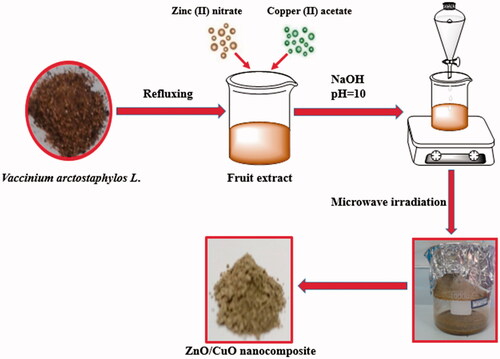
The dried fruit of V. arctostaphylos L. was bought from a local supplier in Ardabil province (Northwestern Iran) and authenticated by the Botanical lab of the University of Mohaghegh Ardabili, Ardabil, Iran. The dried fruit was entirely washed by DDW and then dried at room temperature in a dust free condition. The fruit was crushed into fine powder by a grinding mill. Then, 10 g of the prepared powder was mixed with 100 ml of DDW and refluxed at 95 °C for 5 h. The fruit extract was obtained by filtering the mixture and stored at 4 °C to be used in the green synthesis of the nanomaterials.
Preparation of the nanomaterials
A simple microwave oven (2.45 GHz and 1000 W) was applied to synthesize the nanomaterials. For synthesis of the ZnO (W) sample, 7.31 g of zinc nitrate was dissolved in 100 ml of DDW under stirring at room temperature. In order to adjust pH of the solution to 10, an aqueous solution of NaOH (5 M) was drop-wisely added into the solution under stirring. Afterward, the suspension was irradiated for 10 min in the microwave oven, followed by a centrifugation at 6000 rpm for 5 min. The resulted precipitate was then washed with DDW and ethanol repeatedly and oven-dried at 60 °C for 24 h. The green synthesized ZnO nanoparticles, ZnO (ext), were also prepared with the same procedure, except that the zinc nitrate was dissolved in a solution with a 20:80 v/v ratio of the extract to DDW.
For preparation of the ZnO/CuO (10%) nanocomposite, 1.644 g zinc (II) nitrate and 0.1254 g copper (II) acetate were dissolved in 80 ml of DDW and 20 ml of the plant extract. The pH of the solution was adjusted to 10 by drop-wise adding an aqueous solution of NaOH (5 M), and then irradiated for 10 min at the microwave oven. The obtained precipitate was separated from the system by centrifugation. After washing with DDW and ethanol repeatedly, it was oven-dried at 60 °C for 24 h. In the case of the ZnO/CuO (5%) nanocomposite, the amounts of zinc (II) nitrate and copper (II) acetate were 3.47 and 0.152 g, respectively. The diagram for synthesis of the samples is illustrated in Scheme 1.
Characterization techniques
The XRD patterns were used for determination of the crystallinity, purity and average size of the powders, recorded on a Philips Xpert X-ray diffractometer with Cu K∝ radiation (λ = 0.15406 nm). The morphology and shape analysis of the samples were carried out with FESEM (MIRA3 TESCAN-XMU) equipped with an EDX system to analyse purity of the samples. The TEM images were obtained with a CM30 Philips, using an acceleration voltage of 150 kV. The surface morphology of bacteria was investigated by LEO 1430VP SEM, using an accelerating voltage of 15 kV. The TGA of the samples were obtained using Linseis STA PT 1000 by heating under air atmosphere from room temperature to 700 °C at 10 °C/min. The FT-IR analysis was performed by Perkin Elmer Spectrum RX I apparatus. UV–vis DRS plots were recorded by a Scinco 4100 apparatus. The textural properties were collected by Belsorp apparatus at −196 °C.
Anti-bacterial assessment
All the equipment and tools used in these experiments were autoclaved for 20 min at 121 °C. The S. aureus and E. coli strains were cultured in 50 ml of NB and incubated at 37 °C overnight before using. The concentration of cells was adjusted to 107 ml−1 colony forming units (CFU) in the solution. Anti-bacterial activities of the nanomaterials were studied by the CFU method [Citation35,Citation36]. Different concentrations of the samples were prepared in the sterilized NB medium in a final volume of 3 ml. The growth of bacteria was monitored without and with nanomaterials at an optimized concentration of 128 µg/mL. Subsequently, the cultures were incubated in a shaker incubator for 24 h at 190 rpm/min and at 37 °C. Afterwards, the optical density was measured at 600 nm. The agar plates were then surface inoculated with 100 µL of each bacterial suspension. The plates were incubated at 37 °C for 48 h and the number of viable colonies was counted. The experiments were repeated three times for each isolate.
Results and discussion
Characterization
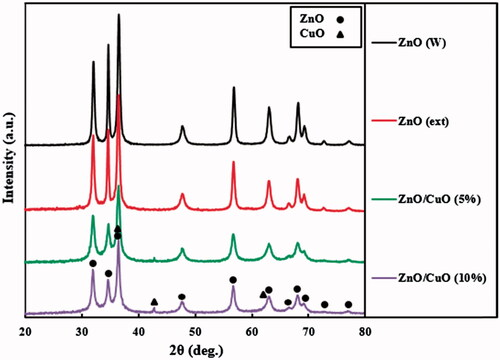
Crystalline structure of the fabricated samples was identified by XRD analysis. The recorded patterns for ZnO (W), ZnO (ext) and ZnO/CuO nanocomposites are displayed in . For the ZnO (W) and ZnO (ext) samples, all of the peaks were ascribed to hexagonal crystalline phase of ZnO (JCPDS card No. 36–1451) [Citation37]. Average crystallite sizes for the ZnO (W) and ZnO (ext) samples were calculated using Scherrer’s equation to be 29 and 23 nm, respectively. Due to the presence of different functional groups in the V. arctostaphylos L. fruit extract, including OH, C = O, O–CH3 and COOH, the size of the ZnO particles synthesized in the aqueous solution of V. arctostaphylos L. fruit extract was decreased in comparison with the ZnO (W) sample. Similar results have been reported for the ZnO particles during fabrication in the presence of different extracts [Citation14,Citation38,Citation39]. In the ZnO/CuO (5%) and ZnO/CuO (10%) nanocomposites, besides the peaks of hexagonal crystalline phase of ZnO, the reflection peaks of CuO with low intensity were observed, due to its low weight percentages [Citation40]. The observed peaks of CuO are attributed to its cubic crystalline phase (JCPDS card No. 78–0428) [Citation41]. In the ZnO/CuO (5%) nanocomposite, the average crystallite sizes for the ZnO and CuO nanoparticles were calculated as 14 and 8 nm, respectively, whereas these sizes in the ZnO/CuO (10%) nanocomposite were 12 and 8 nm, respectively.
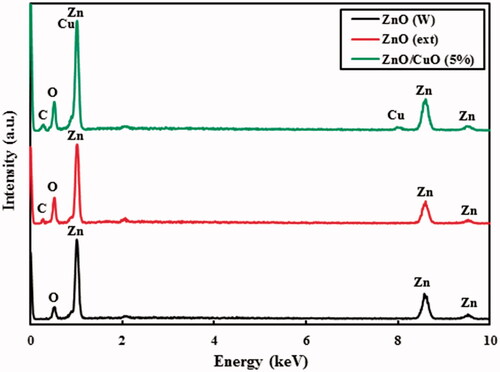
EDX analysis was used to evaluate purity of the ZnO (W), ZnO (ext) and ZnO/CuO (5%) nanocomposite, as the results are shown in . According to these spectra, no characteristic peaks of any other impurities or precursors were observed, indicating the formation of pure materials. The weight percentages of Zn, O and C in the ZnO (ext) were 67.14%, 27.66% and 5.20%, respectively. Additionally, the weight percentages of Zn, O, C and Cu elements in the ZnO/CuO (5%) nanocomposite were found to be 69.74%, 20.36%, 5.55% and 4.35%, respectively. The presence of C element in the spectra of the samples prepared in the presence of the fruit extract is ascribed to binding different organic compounds from the fruit extract to the surface of the fabricated ZnO [Citation13].
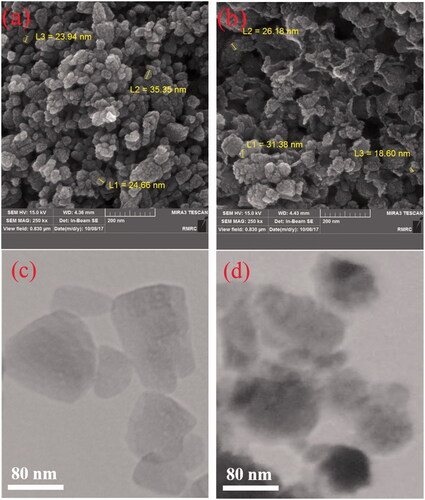
Furthermore, morphology of the ZnO (W) and ZnO (ext) samples was investigated by FESEM and TEM instruments, and the results are displayed in . As can be seen, most of the particles are nearly spherical (). Moreover, for the sample prepared in the presence of the extract, mean particle sizes have been decreased, which are in concordance with the XRD analysis.
Figure 3. FESEM images for the prepared samples: (a) ZnO (W) and (b) ZnO (ext). TEM images for the prepared samples: (c) ZnO (W) and (d) ZnO (ext).
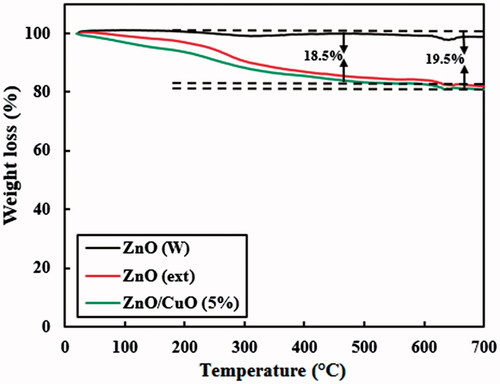
displays the TGA curves for the ZnO (W), ZnO (ext) and ZnO/CuO (5%) nanocomposite. The ZnO (W) powder shows a weight loss of about 1.4% after heating up to 700 °C. This decrease can be ascribed to desorption of water molecules during the heating process. It is clear that the thermal behaviour of the ZnO (ext) and ZnO/CuO (5%) samples is similar to each other. The weight loss of these samples, by heating up to about 200 °C, is attributed to the dehydration step, as observed for the ZnO (W) powder. However, by further heating of these samples, much more decrease in the weight was observed. These decreases are related to decomposition of the organic compounds from the fruit extract attached to the surface of the ZnO (ext) and ZnO/CuO (5%) samples [Citation14]. The weight percentages of organic compounds attached to the surface of ZnO (ext) and ZnO/CuO (5%) samples were calculated after heating the samples up to 650 °C. As observed, the weight percentages of organic compounds over the ZnO (ext) and ZnO/CuO (5%) samples were calculated to be 18.5% and 19.5%, respectively.
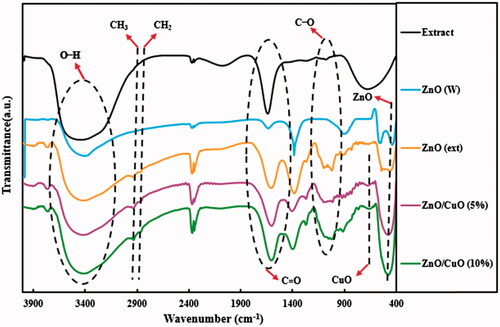
FT-IR analysis was performed to determine the functional groups in the synthesized samples in the range of 400–4000 cm−1, and the spectra are illustrated in . The FT-IR spectra of V. arctostaphylos L. fruit extract and all synthesized samples showed broad absorption peaks at 3100–3648 cm−1, representing the stretching vibration mode of hydroxyl groups [Citation42]. Regarding the prepared samples in the presence of the fruit extract, the absorption peaks for C–H stretching vibrations of CH2 and CH3 groups were observed at 2862 and 2926 cm−1, respectively [Citation14]. Furthermore, the peak around 1638 cm−1 is related to the C = O group of the molecules from fruit extract [Citation43]. The absorption peaks in the region between 1050 and 1200 cm−1 are attributed to C–O stretching vibrations of alcohols and carboxylic acids of the extract [Citation44]. The Zn–O bond stretching vibration appeared at 530 cm−1 [Citation45,Citation46]. Additionally, the FT-IR spectra of ZnO/CuO (5%) and ZnO/CuO (10%) samples exhibited a peak at 670 cm−1, which is attributed to the vibrations of Cu–O bond [Citation43].
Figure 5. FT-IR spectra for the V. arctostaphylos L. fruit extract, ZnO (W), ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples.
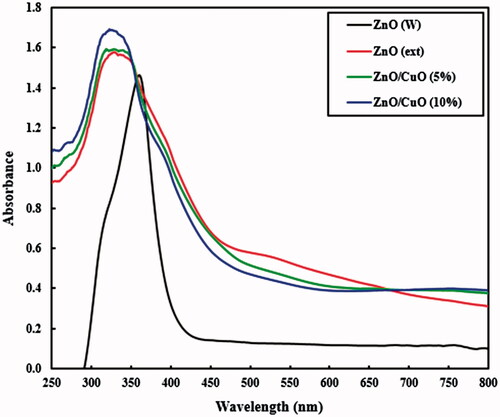
The UV–Vis DRS spectra gave useful information about the electronic absorption characteristics of the fabricated samples. displays the UV–vis spectra in the range of 250–800 nm for the ZnO (W), ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples. The ZnO (W) sample showed an intensive absorption in 370 nm [Citation47]. The absorption spectrum of ZnO (ext) showed a distinct blue shift relative to the ZnO (W), due to a quantum confinement effect [Citation22]. In addition, the ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples showed a remarkable absorption in visible region, caused by the organic compounds from the extract, confirming the results obtained from the EDX, TGA and FT-IR analyses.
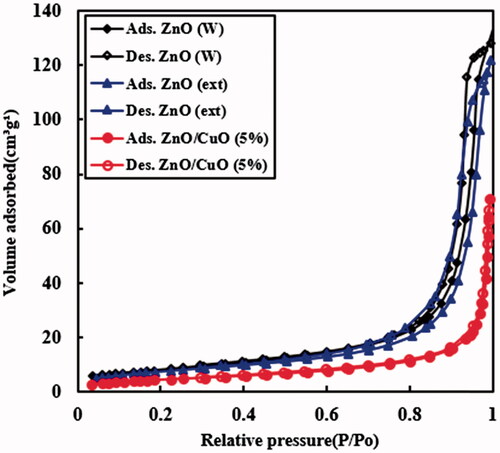
The BET analysis for the ZnO (W), ZnO (ext) and ZnO/CuO (5%) samples were assessed by using nitrogen adsorption–desorption. In , the BET results revealed that the samples show typical IV isotherms, indicating the fact that they almost have mesopores structure [Citation48]. The values of BET specific surface areas for the ZnO (W), ZnO (ext) and ZnO/CuO (5%) samples were 29.3, 27.5 and 18.0 m2g−1, respectively (). The total pore volumes of ZnO (W), ZnO (ext) and ZnO/CuO (5%) samples were 0.1981, 0.1824 and 0.0996 cm3g−1, respectively. The decrease in specific surface area and pore volume could be attributed to the blocking surface of the prepared samples by attached organic compounds from the fruit extract.
Table 1. Specific surface area and pore properties for the ZnO (W), ZnO (ext) and ZnO/CuO (5%) samples.
Anti-bacterial activity of the samples
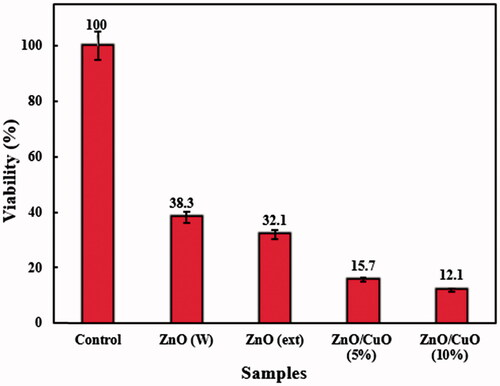
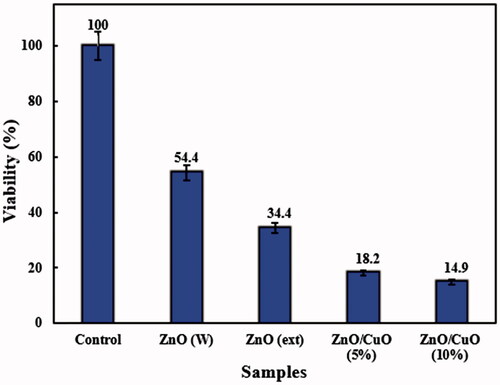
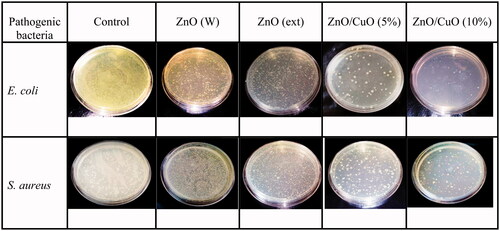
Anti-bacterial activity of the ZnO (W), ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples was investigated against E. coli and S. aureus bacteria, using the CFU method. and display the activities in the presence of 128 µg/mL of the prepared samples. The differences in the viability of E. coli and S. aureus bacteria among the as-prepared samples were statistically significant by one-way ANOVA at p < .05, as indicated. As observed, the ZnO (W) sample exhibited weak anti-bacterial activity against both bacteria. Nonetheless, when ZnO was synthesized in the presence of V. arctostaphylos L. extract, the viability percentages of the bacteria were reduced. Interestingly, the ZnO/CuO nanocomposites revealed high anti-bacterial activities against both E. coli and S. aureus. The anti-bacterial activity of ZnO/CuO nanocomposites was slightly influenced by the concentration of CuO in the nanocomposite and increased by increasing its concentration. In addition, the anti-bacterial activity of the ZnO/CuO nanocomposite (10%) against E. coli (12.1 ± 0.39) was higher than that observed for S. aureus (14.9 ± 0.65).
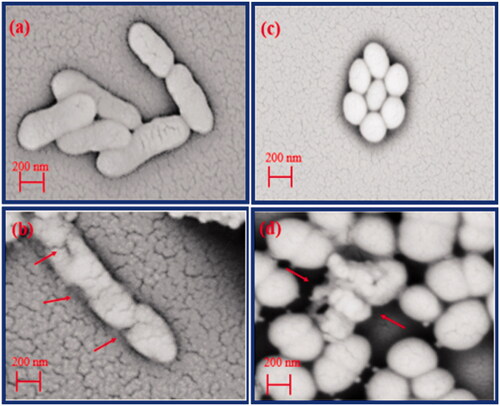
For more insights, E. coli and S. aureus bacteria were inactivated with the ZnO (W), ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples, and their optical images are displayed in . Based on this figure, the anti-bacterial activities were substantially dependent on the type of nanomaterials and bacteria. In addition, for all samples, the number of cell colonies for E. coli was less than those for S. aureus. As clearly observed, the anti-bacterial activity of the as-prepared samples was as ZnO/CuO (10%) > ZnO/CuO (5%) > ZnO (ext) > ZnO (W). Thus, in accordance with the viability analysis, these images confirm an enhanced activity for the samples fabricated in the presence of the fruit extract.
Figure 10. Anti-bacterial activities of the ZnO (W), ZnO (ext), ZnO/CuO (5%) and ZnO/CuO (10%) samples against E. coli and S. aureus.
In order to observe the effect of the ZnO/CuO (10%) nanocomposite on the surface morphology of E. coli and S. aureus bacteria, their SEM images were provided, as depicted in . According to ) for E. coli bacteria, the SEM images clearly revealed obvious cracks in the bacterial membranes, caused by the nanocomposite. Furthermore, as shown in ), the ZnO/CuO (10%) nanocomposite ruptured the membrane of S. aureus, resulting in a disorganization and secretion of the cell wall. This could be attributed to connection of the nanocomposite to thiol (–SH) groups present on the cell membranes, causing the cell lyses [Citation49–51].
Conclusion
In the present study, the ZnO nanoparticles and ZnO/CuO nanocomposites were fabricated by a microwave-assisted method in the presence of V. arctostaphylos L. fruit extract, and their anti-bacterial activities were investigated against E. coli and S. aureus bacteria. The XRD, FESEM and TEM analyses displayed that the ZnO particles synthesized in the presence of the fruit extract have smaller size in comparison with the sample prepared in water. The anti-bacterial activity measurements showed that the ZnO (W) sample exhibited a weak anti-bacterial activity against both bacteria. However, the ZnO sample fabricated in the presence of the extract showed an enhanced anti-bacterial activity compared to the sample prepared in water. Interestingly, the ZnO/CuO nanocomposites revealed high anti-bacterial activities against both E. coli and S. aureus. The activity of the ZnO/CuO (10%) nanocomposite against E. coli (12.1 ± 0.39) was higher than that observed for S. aureus (14.9 ± 0.65). The SEM images clearly revealed the disruption of the bacterial membranes by the nanocomposite. Due to the promising characteristics of the prepared nanocomposites and their favourable anti-bacterial efficacy against tested bacteria, the green synthesized ZnO/CuO nanocomposite can be introduced as a potential proficient disinfectant.
Disclosure statement
The authors have no conflicts of interest regarding the publication of this manuscript.
Additional information
Funding
References
- Jones KE, Patel NG, Levy MA, et al. Global trends in emerging infectious diseases. Nature. 2008;451:990–993.
- Khan ST, Musarrat J, Al-Khedhairy AA. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles, current status. Colloids Surf B Biointerfaces. 2016;146:70–83.
- Kumar R, Umar A, Kumar G, et al. Antimicrobial properties of ZnO nanomaterials: a review. Ceram Int. 2017;43:3940–3961.
- Wright GD. Something old, something new: revisiting natural products in antibiotic drug discovery. Can J Microbiol. 2014;60:147–154.
- Dizaj SM, Lotfipour F, Barzegar-Jalali M, et al. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284.
- Stankic S, Suman S, Haque F, et al. Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnol. 2016;14:20.
- Videa JR, Huang PY, Parsons J, et al. Plant-based green synthesis of metallic nanoparticles: scientific curiosity or a realistic alternative to chemical synthesis? Nanotechnol Environ Eng. 2016;4:1–29.
- Gawande MB, Shelke SN, Zboril R, et al. Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc Chem Res. 2014;47:1338–1348.
- Rathi AK, Gawande MB, Zboril R, et al. Microwave-assisted synthesis-catalytic applications in aqueous media. Coord Chem Rev. 2015;291:68–94.
- Singh P, Kim YJ, Zhang D, et al. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016;34:588–599.
- Ahmed S, Ahmad M, Swami BL, et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7:17–28.
- Côté J, Caillet S, Doyon G, et al. Antimicrobial effect of cranberry juice and extracts. Food Control. 2011;22:1413–1418.
- Su Z. Anthocyanins and flavonoids of Vaccinium L. Pharm Crops. 2012;3:7–37.
- Bayrami A, Parvinroo S, Habibi-Yangjeh A, et al. Bio-extract-mediated ZnO nanoparticles: microwave-assisted synthesis, characterization and antidiabetic activity evaluation. Artif Cells Nanomed Biotechnol. 2017. DOI:https://doi.org/10.1080/21691401.2017.1337025
- Wang H, Guo X, Hu X, et al. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017;217:773–781.
- Spínola V, Pinto J, Castilho PC. Hypoglycemic, anti-glycation and antioxidant in vitro properties of two Vaccinium species from Macaronesia: a relation to their phenolic composition. J Funct Foods. 2018;40:595–605.
- Vanathi P, Rajiv P, Narendhran S, et al. Biosynthesis and characterization of phyto mediated zinc oxide nanoparticles: a green chemistry approach. Mater Lett. 2014;134:13–15.
- Bala N, Saha S, Chakraborty M, et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;5:4993–5003.
- Bhuyan T, Mishra K, Khanuja M, et al. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process. 2015;32:55–61.
- Ramesh M, Anbuvannan M, Viruthagiri G. Green synthesis of ZnO nanoparticles using Solanum nigrum leaf extract and their antibacterial activity. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;136:864–870.
- Fu L, Fu Z. Plectranthus amboinicus leaf extract–assisted biosynthesis of ZnO nanoparticles and their photocatalytic activity. Ceram Int. 2015;41:2492–2496.
- Elumalai K, Velmurugan S, Ravi S, et al. Green synthesis of zinc oxide nanoparticles using Moringa oleifera leaf extract and evaluation of its antimicrobial activity. Elsevier. 2015;143:158–164.
- Jafarirad S, Mehrabi M, Divband B, et al. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: a mechanistic approach. Mater Sci Eng. 2016;59:296–302.
- Singh A, Singh N, Afzal S, et al. Zinc oxide nanoparticles: a review of their biological synthesis, antimicrobial activity, uptake, translocation and biotransformation in plants. J Mater Sci. 2018;53:185–201.
- Jalal M, Ansari MA, Ali SG, et al. Anticandidal activity of bioinspired ZnO NPs: effect on growth, cell morphology and key virulence attributes of Candida species. Artif Cells Nanomed Biotechnol. 2018. DOI:https://doi.org/10.1080/21691401.2018.1439837
- Qi K, Cheng B, Yu J, et al. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J Alloys Compd. 2017;727:792–820.
- Yadavalli T, Shukla D. Role of metal and metal oxide nanoparticles as diagnostic and therapeutic tools for highly prevalent viral infections. Nanomed: Nanotechnol Biol Med. 2017;13:219–230.
- Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol. 2017;45:1751–1761.
- Abdolmohammadi MH, Fallahian F, Fakhroueian Z, et al. Application of new ZnO nanoformulation and Ag/Fe/ZnO nanocomposites as water-based nanofluids to consider in vitro cytotoxic effects against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol. 2017;45:1769–1777.
- Pirhashemi M, Habibi-Yangjeh A, Rahim-Pouran S. Review on the criteria anticipated for the fabrication of highly efficient ZnO-based visible-light-driven photocatalysts. J Ind Eng Chem. 2018. DOI:https://doi.org/10.1016/j.jiee.2018.01.012
- Xiong HM. ZnO nanoparticles applied to bioimaging and drug delivery. Adv Mater Weinheim. 2013;25:5329–5335.
- Prashanth GK, Prashanth PA, Nagabhushana BM, et al. Comparison of anticancer activity of biocompatible ZnO nanoparticles prepared by solution combustion synthesis using aqueous leaf extracts of Abutilon indicum, Melia azedarach and Indigofera tinctoria as biofuels. Artif Cells Nanomed Biotechnol. 2017. DOI:https://doi.org/10.1080/21691401.2017.1351982
- Zhong Q, Tian J, Liu T, et al. Preparation and antibacterial properties of carboxymethyl chitosan/ZnO nanocomposite microspheres with enhanced biocompatibility. Mater Lett. 2018;212:58–61.
- Ingle AP, Duran N, Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: a review. Appl Microbiol Biotechnol. 2014;98:1001–1009.
- Gao B, He S, Guo J, et al. Preparation and antibacterial character of a water-insoluble antibacterial material of grafting polyvinylpyridinium on silica gel. Mater Lett. 2007;61:877–883.
- Fernandes SC, Sadocco P, Alonso-Varona A, et al. Bioinspired antimicrobial and biocompatible bacterial cellulose membranes obtained by surface functionalization with aminoalkyl groups. ACS Appl Mater Interfaces. 2013;5:3290–3297.
- Omidi A, Habibi-Yangjeh A, Pirhashemi M. Application of ultrasonic irradiation method for preparation of ZnO nanostructures doped with Sb +3 ions as a highly efficient photocatalyst. Appl Surf Sci. 2013;276:468–475.
- Anbuvannan M, Ramesh M, Viruthagiri G, et al. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater Sci Semicond Process. 2015;39:621–628.
- Ambika S, Sundrarajan M. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J Photochem Photobiol B. 2015;146:52–57.
- Saravanan R, Karthikeyan S, Gupta V, et al. Enhanced photocatalytic activity of ZnO/CuO nanocomposite for the degradation of textile dye on visible light illumination. Mater Sci Eng C Mater Biol Appl. 2013;33:91–98.
- Zhang Y, Guo WW, Zheng TX, et al. Engineering hierarchical Diatom@ CuO@ MnO2 hybrid for high performance supercapacitor. Appl Surf Sci. 2018;427:1158–1165.
- Oh BT, Jeong SY, Velmurugan P, et al. Probiotic-mediated blueberry (Vaccinium corymbosum L.) fruit fermentation to yield functionalized products for augmented antibacterial and antioxidant activity. J Biosci Bioeng. 2017;124:542–550.
- Padil VV, Černík TM. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomed. 2013;8:889–889.
- Zhou L, Lie Y, Briers H, et al. Natural product recovery from bilberry (Vaccinium myrtillus L.) presscake via microwave hydrolysis. ACS Sustain Chem Eng. 2018;6:3676–3685.
- Pirhashemi M, Habibi-Yangjeh A. Ultrasonic-assisted preparation of novel ternary ZnO/Ag3VO4/Ag2CrO4 nanocomposites and their enhanced visible-light activities in degradation of different pollutants. Solid State Sci. 2016;55:58–68.
- Azam A, Ahmed A, Oves SM, et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed. 2012;7:6003–6009.
- Shankar S, Rhim JW. Facile approach for large-scale production of metal and metal oxide nanoparticles and preparation of antibacterial cotton pads. Carbohydr Polym. 2017;163:137–145.
- Pirhashemi M, Habibi-Yangjeh A. Photosensitization of ZnO by AgBr and Ag2CO3: nanocomposites with tandem n-n heterojunctions and highly enhanced visible-light photocatalytic activity. J Colloid Interface Sci. 2016;474:103–113.
- Zhang H, Chen G. Potent antibacterial activities of Ag/TiO2 nanocomposite powders synthesized by a one-pot sol − gel method. Environ Sci Technol. 2009;43:2905–2910.
- Li H, Chen Q, Zhao J, et al. Enhancing the antimicrobial activity of natural extraction using the synthetic ultrasmall metal nanoparticles. Sci Rep. 2015;5:13.
- Lam SM, Quek AJ, Sin JC. Mechanistic investigation of visible light responsive Ag/ZnO micro/nanoflowers for enhanced photocatalytic performance and antibacterial activity. J Photochem Photobiol A: Chem. 2018;353:171–184.