Abstract
Doxorubicin (DOX) is an effective anticancer drug which is widely used in clinical treatment. However, the severe cardiotoxicity limits its use. Thus, it is an urgent need to attenuate the toxicity of DOX without impairing its efficacy. Many studies show that Se may protect normal tissues from damages of some anticancer drugs. Recently, Se@SiO2 nanocomposites emerges as better substitutes for direct element Se in treatment of cancer cells for their ideal biocompatibility. In the present article, we synthesized Se@SiO2 nanocomposites and confirmed their characterization according to previous studies. We accomplished a conjunctive use of Se@SiO2 nanocomposites with DOX then explored the toxicity and efficacy of this combination. In the in vivo experiments, the survival rate of mice with DOX treatment was significantly increased by Se@SiO2. And Se@SiO2 has few interference to the therapeutic effect of DOX. Particularly, Se@SiO2 significantly attenuated DOX-induced myocardial tissue damage (serum index, apoptosis index, western-blot index) and protected mice from reduction in LVEF induced by DOX in mice model. In summary, we concluded that the protective effect of Se@SiO2 in DOX-induced cardiotoxicity was possibly attributable to the inhibition of ROS production, showing great potential of Se@SiO2 nanocomposite in the clinical use of DOX.
Introduction
Cancer is a severe disease. Among the strategies combating cancer, chemotherapy is an important and highly effective treatment which has been promoted by nanotechnology [Citation1]. Doxorubicin (DOX) is the first line chemotherapeutic agent used in many cancers [Citation2]. Despite of its effectiveness, DOX is often limited in the clinic use due to the severe cardiotoxicity [Citation2]. DOX may reduce left ventricular ejection fraction (LVEF), eventually leading to heart failure [Citation3]. Cardiomyopathy induced by DOX is reported to be involved with poor prognosis and low survival rate [Citation3]. Thus, there is an urgent need to develop new strategies to prevent cardiotoxicity induced by DOX without compromising its antitumor efficacy.
In recent years, many studies have focused on the possible mechanisms underlying DOX-induced cardiotoxicity including oxidative stress, lipid peroxidation, imbalance of Ca2 + and so on [Citation4,Citation5]. Oxidative stress and lipid peroxidation could enhance the production of reactive oxygen species (ROS). It is well-established that increased ROS may result in various subcellular changes in the myocardium, including changes which are typical in DOX-induced cardiomyopathy [Citation6]. Furthermore, previous studies showed that doxorubicin was associated with a decrease in endogenous antioxidants and an increase in free radicals [Citation7–9]. The addition of probucol, an antioxidant which could also promote the activities of endogenous antioxidants, to DOX effectively prevented cardiomyopathy and heart failure [Citation7,Citation10]. ROS generation is thought to be an important mechanism underlying the cardiotoxicity of DOX [Citation6]. Thus, the agents with antioxidant properties may attenuate DOX-induced cardiotoxicity.
ROS inhibition by nanotechnology has already been reported [Citation11,Citation12]. In our previously researches, it has been proved that Se@SiO2 nanocomposites can decrease ROS damage in the disease process of steroid-induced necrosis [Citation13] and acute lung injury [Citation14]. Meanwhile, Se@SiO2 nanocomposites proved their high competence of inhibiting cancer cell and targeting efficacy [Citation15,Citation16]. Selenium (Se) is an essential micronutrient for humans and animals with a range of biological and antioxidative properties. Se can reduce the risk of various cancers, such as breast cancer, prostate cancer and so on [Citation17]. Se was proved to have the effects of immune response and cancer prevention activity. Se also can sensitize cancer cells to antitumor drugs and enhance their effect [Citation17,Citation18]. In some cases, Se even can protect normal tissues from the damage of antitumor drugs [Citation17]. However, Se has a narrow margin between the beneficial and toxic effects [Citation19]. Thus, Se@SiO2 nanocomposites is developed as an ideal formulation in our formal study [Citation16]. The combination with drug and nanoparticles may depress toxic side effects and simultaneously enhance the therapeutic efficiency [Citation20]. And we believe the same results may come when it comes to DOX and Se@SiO2 [Citation21].
In this study, we combined Se@SiO2 and DOX and found that Se@SiO2 has protective effect against DOX-induced cardiotoxicity with the potential ability to strengthen the antitumor efficacy of DOX, both in vivo and in vitro.
Materials and methods
Materials
DOX was purchased from HARVEY (USA). Cell Counting Kit CCK-8/WST-8 and TUNEL assay kit was purchased from DOJINDO (Japan) and Abcam (USA), respectively. The primary and sencondary antibodies were all obtained from Cell Signaling Technology (USA). Water and all buffers were of Millipore grade and pre-treated with Chelex 100 resin to ensure that the aqueous solution was free of heavy metal. DOX and all other reaction buffers and chemicals were acquired from Sinopharm Chemical Reagent (China).
Six-week-old male Kunming mice and six-week-old male BALB/c nude mice obtained from the Shanghai Experimental Animal Center of Chinese Academy of Sciences were used for experiment in vivo This study was performed following the National Institutes of Health guidelines for the use of experimental animals, and all animal protocols were approved by the Institutional Animal Care and Use Committee of The Second Military Medical University.
Synthesis and characterization of Se@SiO2 nanocomposites
Se@SiO2 nanocomposites were synthesized according to the previously reported method [Citation16]. The synthesis of Cu2-xSe nanocrystals were carried out using standard Schlenk-line techniques under nitrogen. 43.5 mg selenium powders and 6 ml oleic acid (OA) were mixed in three-necked flask and 280 °C for 0.5 h under magnetic stirring to form Se-OA precursor. 49.5 mg of CuCl, 5 ml oleylamine (OAm) and 5 ml OA were mixed in another three-necked round-bottom flask and heated to 220 °C and maintained for 10 min under stirring. Subsequently, Se-OA precursor was quickly injected into the CuCl mixture and then aged at 220 °C for 5 min. Ethanol was added to the reaction solution after cooled to 60 °C, followed by centrifuging and washing with ethanol. Then, the Cu2-xSe nanocrystals were obtained and dispersed in 10 ml n-hexane. Subsequently, 1 ml above solution, 10 ml n-hexane, 1 ml n-hexanol, 1 ml Triton X-100, 0.3 ml deionized water and 0.04 ml TEOS. Then, 0.05 ml ammonium hydroxide was added into the mixture and reacted with Cu2-xSe nanocrystals to form [Cu(NH3)4]2 + . Se quantum dots were developed by oxidizing Se2− at same time. The silica coated around the Se quantum dots by orthosilicate hydrolysis in alkaline environment, forming solid Se@SiO2 nanocomposites. Finally, solid Se@SiO2 nanocomposites were coated with PVP and etched in hot water (95 °C) to construct porous structures. Se@SiO2 nanocomposites were characterized by means of a D/max-2550 PC X-ray diffractometer (XRD; Rigaku, Cu-Kα radiation), and a transmission electronic microscopy (TEM; JEM-2100 F). And the Se release was evaluated by the dialysis method reported before [Citation16].
Cell culture studies
The rat myocardial cell line H9C2 and the human cervical cancer cell line Hela were purchased from the American Type Culture Collection. All the cell lines were authenticated twice by morphologic and isoenzyme analyses during the study period. Cell lines were routinely checked for contamination by mycoplasma using Hoechst staining and consistently found to be negative.
Cell proliferation assay
H9C2 or HeLa cells were seeded at a density of 5000 cells per well in a flat-bottomed 96-well plate in a humidified 37 °C and 5% CO2 atmosphere. Next day, cells were incubated with an increasing concentration of Se@SiO2 (ranging from 0 to 180 μg/ml). The medium was replaced every 2 or 3 days. Five days later, the cell proliferation was determined by Cell Counting Kit CCK-8/WST-8 (DOJINDO, Japan).
HeLa cells were seeded at a density of 5000 cells per well in a flat-bottomed 96-well plate in a humidified 37 °C and 5% CO2 atmosphere. Next day, cells were incubated with an increasing concentration of DOX or the corresponding concentration of DOX plus 40 μg/ml Se@SiO2 (the concentration of DOX ranges from 0 to 50 μg/ml). The medium was replaced every 2 or 3 days. Five days later, the cell proliferation was determined by Cell Counting Kit CCK-8/WST-8 (DOJINDO, Japan). Then, IC50 was calculated based on the cell proliferation rates.
ROS measurement
ROS measurement was determined by dichloro-dihydro-fluorescein diacetate (DCFH-DA, Sigma, D6883), according to manufactures’ instruction. Briefly, cells were loaded with DCFH-DA (final concentration of 10 mM for 30 min), washed with ice cold HBSS (Hank’s Balanced Salt Solution, pH 7.2), then observed under a Zeiss LSM710 laser confocal microscope (Carl Zeiss, Germany) equipped with Zen software to process the image. In addition, the intensity of DCFH-DA was determined by microplate reader (Thermo Scientific, USA).
TUNEL assays
Primary myocardial cells were acquired from mice. Myocardial cells in the blank group and H2O2 group received no treatments in the first 12 h, while cells from Se@SiO2 group and H2O2 + Se@SiO2 group were treated with 40 μg/ml Se@SiO2 for 12 h. In the subsequent 12 h, the H2O2 and H2O2 + Se@SiO2 group received 50 mM H2O2 for 12 h. After that, the cells were stained and examined according to the manufacturer’s protocol (Abcam, ab66110). Blue fluorescence shows DAPI and red the expression of a-actinin. In each group, several visual fields were selected randomly and images were acquired randomly by a Zeiss LSM710 laser confocal microscope (Carl Zeiss, Germany). Apoptosis rates were determined by the percentages of dead cells from three independent visual fields in each group.
In vivo toxicity experiment
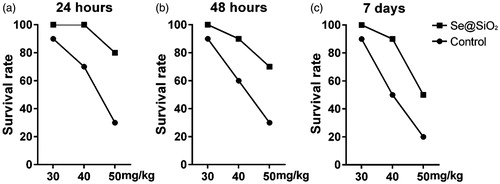
Six-week-old Kunming mice were randomly assigned into 6 groups. Each group included 10 Kunming mice. The experiment group received an intravenous injection of Se@SiO2 (1 mg/kg), and the control group received an intravenous injection of saline with the same volume as Se@SiO2. Twenty hours later, the mice were administrated intravenously with 30 mg/kg, 40 mg/kg or 50 mg/kg DOX. The number of survival mice were recorded 24 h, 48 h and 7 days after treatment. The survival rates were calculated as the survival number/the total number.
In vivo tumour growth studies and cardiotoxicity evaluation
Six-week-old male BALB/c nude mice were subcutaneously implanted with Hela cells (5 × 106 per mouse). When tumours reached an average tumour volume of 100–200 mm3, animals were distributed into treatment cohorts of 10 mice each randomly. The pre-treatment of Se@SiO2 (1 mg/kg) were given 24 h before the administration of DOX (20 mg/kg). Control mice were administered with saline alone.
Tumour size measurement
Tumours were measured with digital calipers twice a week and tumour volumes were calculated by the formula: volume (mm3) = length × (width)2/2. The tumour volumes were recorded for 21 days after tumour implantation. In the 21st day, the mice were subjected to echocardiography test. The blood and heart samples of mice were collected for further studies.
Echocardiography test
Echocardiography was performed using a high-resolution ultrasound imaging system equipped with a 7V3 probe with a frequency of 6.0 MHz (Acuson Sequoia; Siemens, Washington, DC, USA). End diastolic volume and end systolic volume were calculated from bi-dimensional long-axis parasternal views by means of the single-plane area-length method. The ejection fraction (EF) was calculated as follows: EF (%) = (LVEDV − LVESV)/LVEDV ×100%.
Western blot
After myocardium tissues were lysed, cell lysates were subjected to SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes. Then, the membranes were blocked in Tris-buffered saline tween (TBST) containing 5% skim milk for 1 h at room temperature and probed with indicated primary monoclonal antibodies (Cell Signaling Technology, USA) at 4 °C overnight. After that, the membranes were washed and then incubated with horseradish peroxidase-conjugated secondary antibodies (Cell Signaling Technology, USA) for 1 h at room temperature. After washing with TBST for 15 min, bands were detected using an enhanced chemiluminescence detection system and autography.
Histological examination
Myocardial tissues were excised by horizontal intercept from the middle part of the hearts of mice. After a 24-h fixation in 10% buffered formalin, tissues were embedded in paraffin and sliced into 5 µm sections. The sections were stained with haematoxylin and eosin. Then, sections were visualized using light microscopy.
Myocardial enzyme spectrums
After the serum of mice was collected, the CK level and LDH-L level in serum were examined by Hitachi 7170 biochemical analyzer (Japan).
Statistical analysis
All the statistical analyses were performed using the software package SPSS 21.0 (SPSS, Chicago, IL). Data from ROS assay, Western blot assay, TUNEL assay, cell proliferation assay, tumour growth studies, EF% and Se release were presented as the mean ± standard error of the mean. Data among different groups were analysed by one-way analysis of variance. SNL was used to correct for multiple comparisons. Differences were considered statistically significant at a p values < .05.
Results
We designed a novel delivery system which combined ultrasmall Se particles (quantum dots) with anticancer drug (DOX) for synergistic treatment of cancers. To our best knowledge, it is first time to fabricate porous silicananocarriers for release of Se particles and drug, leading to synergistic inhibition of cancer cells without appreciable negative effect on normal cells.
The characterization of Se@SiO2 nanocomposites
XRD pattern was used to determine the phase structure of Se@SiO2 nanocomposites. As shown in , many well-defined characteristic peaks in Se@SiO2 nanocomposites showed the hexagonal phase, referenced by the standard Se phase (JCPDS card no. 65–1876). The XRD pattern of the Se@SiO2 nanocomposites exhibited a steady increase in the low angle region, which may be explained by amorphous silica.
Figure 1. The characterization of the Se@SiO2 nanocomposites. (a) XRD pattern of the solid Se@SiO2 nanocomposites and the standard hexagonal phase of Se (JCPDS card no: 65–1876). (b) Low and (c) High magnification TEM image of as prepared solid Se@SiO2 nanocomposites. (d) TEM image of the porous Se@SiO2 nanocomposites. (e,f) The Se@SiO2 nanocomposites in the water in the first day (e) and the fourteenth day (f) during the test. (g) The cumulative release kinetics of Se from the porous Se@SiO2 nanocomposites in PBS (pH 7.4) at 37 °C.(h,i) The cell viability of H9C2 cells (h) and Hela cells (i) incubated with different concentrations of Se@SiO2 nanocomposites for 24 h.
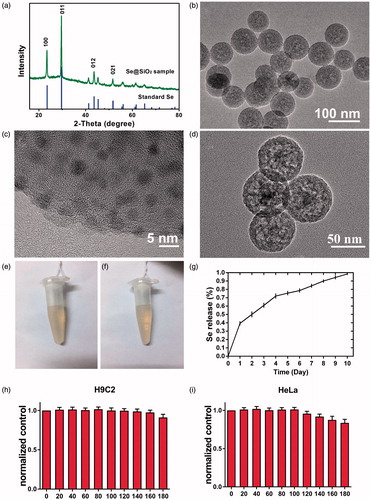
Next, transmission electronic microscope (TEM) was used to evaluate the morphology and size of the Se@SiO2 nanocomposites. The nanocomposites have a diameter of about 55 nm, in which many small quantum dots were interspersed from the centre to the surface (). The quantum dots were irregular and the size was less than 5 nm (). As shown in , the Se@SiO2 nanocomposites were porous.
The stability assay showed that Se@SiO2 was stable, because Se@SiO2 nanocomposites were dispersed evenly in the water during the 2-week test (). No precipitation or aggregation was observed in the Se@SiO2 solution. Se release was measured under conditions close to physiological ones, that is, at pH 7.4 and 37 °C. Se quantum dots released slowly from the porous Se@SiO2 nanocomposites (). As shown in , the release rate of Se is rapid in the first day and gradually slowed down in next 9 days.
Moreover, CCK-8 assay was used to examine the cytotoxicity of Se@SiO2 nanocomposites on H9C2 cells and Hela cells. Both H9C2 and Hela cell lines were incubated with an increasing concentration of Se@SiO2 nanocomposites, ranging from 0 to 180 μg/ml (). All the cell viabilities of H9C2 cells with indicated treatments were above 90.0%, whereas the cell viabilities of Hela cells decreased significantly when the concentration of Se@SiO2 nanocomposites was above 140 μg/ml (). It indicated that Se@SiO2 nanocomposites were relatively safe to H9C2 cells.
The inhibition of DOX-induced ROS production
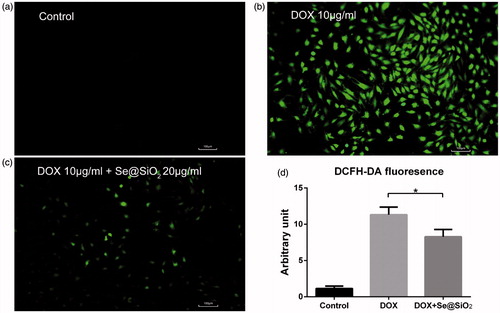
The ROS level in H9C2 cells was assessed by cellular fluorescence intensity using DCFH-DA staining analysis. Compared with the negative control, DOX dramatically increased the ROS level in H9C2 cells (). However, the addition of Se@SiO2 to DOX alleviated DOX-induced ROS production significantly (). Thus, Se@SiO2 may protect cells from the ROS outbreak triggered by DOX.
Figure 2. Comparison of cellular ROS induced by indicated drugs. (a,b,c) Cells were treated with the control (a), 10 μg/ml DOX (b) or 10 μg/ml DOX plus 20 μg/ml Se@SiO2 (c) for 4 h, followed by cellular ROS measurement, as reflected by DCFH-DA signals. (d) The fluorescence intensity of DCFH-DA was quantitated. Experiments were repeated 3 times. *, p < .05.
The reduction in ROS-induced apoptosis by Se@SiO2 in primary myocardium cells
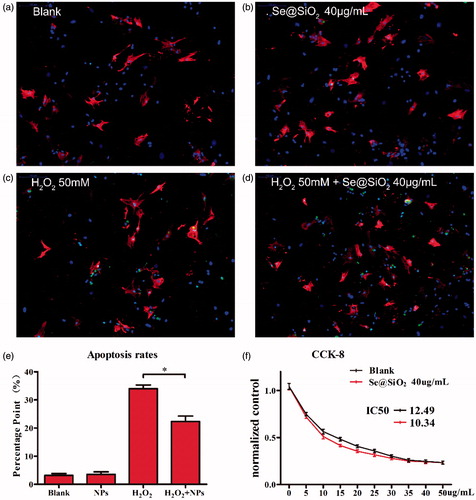
TUNEL assay was used to evaluate the apoptosis-inducing ability of drugs in primary myocardium cells treated with control, Se@SiO2, H2O2, and H2O2 + Se@SiO2. The results showed that no significant statistics difference was observed between the control group and Se@SiO2 group (). Apoptosis was significantly induced by H2O2, whereas Se@SiO2 could dramatically attenuate the apoptosis-inducing ability of H2O2 ().
Figure 3. Identification of myocardium cells and confirmation of biosafety of the porous Se@SiO2 nanocomposites: Immunofluorescence staining of aggrecan and collagenase type I in the myocardium cells treated with blank (a), Se@SiO2 NPs (b), H2O2 (c), and H2O2 + Se@SiO2 NPs (d) (200×). (e) Quantitative analysis of TUNEL positive H9C2 cells for blank group,(a), Se@SiO2 NPs (b), H2O2 (c), and H2O2 + Se@SiO2 NPs (d). Data (means ± SEM) represent the means of independent experiments (n = 5 in each group). *, p < .05. Decreased TUNEL positive cells were observed in H2O2 group versus H2O2 + Se@SiO2 NPs group. (f) Normalized by control group the cell activity decreased along with the increasing DOX concentrations. Significant protection of the porous Se@SiO2 nanocomposites as reflected by the CCK-8 assay. *, p < .05.
The IC50 value of Se@SiO2 plus DOX
The IC50 value of DOX + Se@SiO2 (10.34 μg·mL−1) is much lower than that of DOX (12.49 μg·mL−1) alone (), indicated that the addition of Se@SiO2 to DOX enhanced the efficient inhibition effect of DOX on cancer cells.
In vivo toxicity test of Se@SiO2 plus DOX
As shown in , in 24 h after treatment, the survival rates of mice decreased significantly with the concentrations of DOX increasing. The pre-treatment of Se@SiO2 could increase the survival rates of mice treated with 30 mg/kg, 40 mg/kg or 50 mg/kg DOX (). Similar results were obtained in 48 h and 7 days after treatment (). Thus, Se@SiO2 may protect mice from the fatal damage caused by DOX.
The antitumour efficacy and cardiotoxicity of Se@SiO2 plus DOX in hela-bearing nude mice
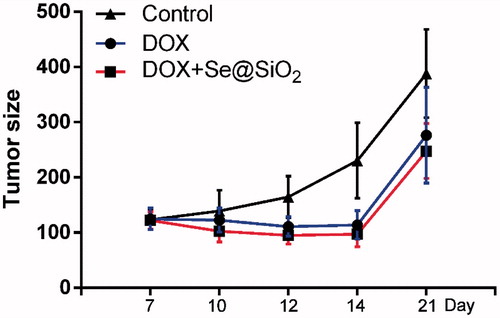
As shown in , the tumour volumes of DOX group and the DOX plus Se@SiO2 group were significantly smaller than that of control group. However, no significant statistic difference was observed between the DOX group and the DOX plus Se@SiO2 group ().
Figure 5. Tumour volume of Hela tumour xenografts after treatment with the control (saline, intravenously), DOX (20 mg/kg, intravenously), and DOX (20 mg/kg, intravenously) plus Se@SiO2 (1 mg/kg, intravenously). Data are shown as means ± SEM.
After the in vivo tumour growth studies, echocardiography was performed in each mice to measure relative parameters of cardiac functions. As shown in , two-dimensional and M-mode short-axis views of the left ventricle were acquired at the level of the papillary muscles in mice. Compared with the control group, treatment with DOX decreased LVEF (). LVEF in the DOX plus Se@SiO2 group were significantly higher than that in the DOX group (), suggesting that Se@SiO2 improved the impaired cardiac functions induced by DOX in mice.
Figure 6. Two-dimensional (upper panel) and M-mode (lower panel) short-axis views of the left ventricle at the level of the papillary muscles in four different animals from the control (a), DOX (b), and DOX + Se@SiO2 (c) groups, respectively. Results of LVEF in the three groups. *, p < .05. (e,f) The levels of LDH-L (e) and CK (f) of mice treated with DOX or DOX plus Se@SiO2.
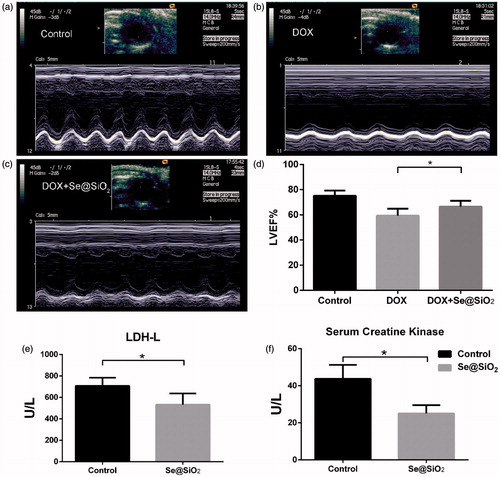
In addition, we determined the serum levels of creatine kinase (CK) and L-lactic dehydrogenase (LDH-L) of mice treated with DOX and DOX plus Se@SiO2. The results showed that Se@SiO2 group significantly reduced the CK and LDH-L levels increased by DOX ().
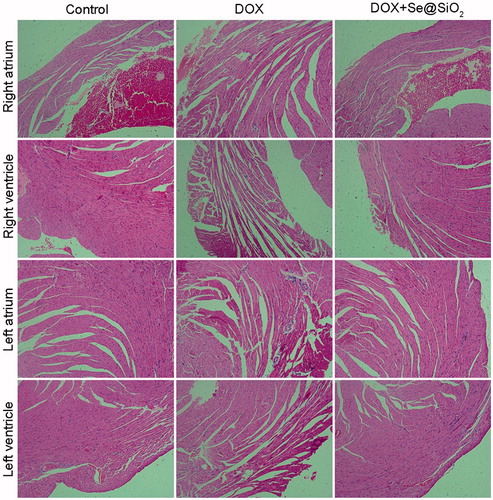
H and E staining was used to evaluate the changes in myocardial tissues from mice with different treatments. The results showed that in right atrium tissue sections, the volume of the myocardium in the DOX group was smaller than that in the control group (). Meanwhile, the spaces between myocardial fibres in the DOX group also become larger (). Compared with the DOX group, the DOX + Se@SiO2 group attenuated the myocardial atrophy (). Similar results were also obtained in the left atrium, left ventricle and right ventricle ().
Figure 7. Histology of heart tissues exposed to control, DOX and DOX + Se@SiO2 treatment. Haematoxylin and eosin (H&E) staining (400×magnification) of various tissue slices from mice with indicated treatments.
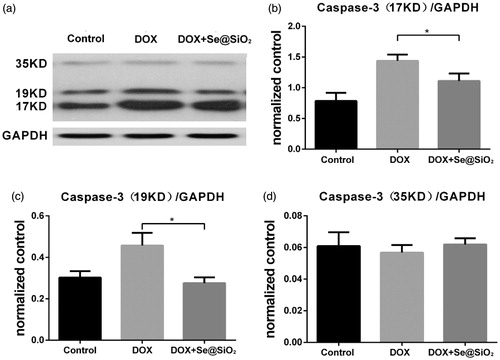
Also, we performed Western blot to evaluate the apoptosis of myocardial cells from mice treated with DOX and DOX + Se@SiO2. There were no difference in the levels of pro-caspase-3 among the control group, DOX group and DOX + Se@SiO2 group (). Compared with the control group, cleaved Caspase-3 proteins showed an increase in the DOX group (). The cleavage of Caspase-3 induced by DOX was reduced significantly by Se@SiO2 (). Therefore, Se@SiO2 could attenuate the apoptosis of myocardial cells caused by DOX.
Figure 8. Western blot evaluating the apoptosis of myocardial cells from mice treated with DOX, and DOX + Se@SiO2. (a) Immunoblots examining the cleavage of Caspase-3 in heart tissue from mice treated with DOX and DOX + Se@SiO2. (b,c,d) The relative density of the 17 kD and 19 kD cleaved caspase-3 proteins (b,c), and 35 kD pro-caspase-3 (d) was normalized to GAPDH. All experiments were performed in triplicate (n = 3). Error bars represent mean ± SEM. *, p < .05; t-test.
Discussion
DOX is a highly effective and common chemotherapeutic agent. Nevertheless, the severe cardiotoxicity has become a tough nut to crack in the clinical use of DOX [Citation2,Citation22]. A great number of previous studies show that the combination of Se and many anticancer drugs could decrease the damages of anticancer drugs to normal tissues and simultaneously enhance the therapeutic efficiency [Citation23–27]. Compared with direct element Se whose margin between beneficial effect and toxic effect is quite narrow [Citation2,Citation19], Se nanoparticles are better choice for their higher biological activity and lower toxicity which are attributable to smaller size and targeting efficacy [Citation19,Citation28–30]. Moreover, compared with the general Se nanoparticles, Se@SiO2 possess advantages including good compatibility, tailorable pore structure and controllable release of Se [Citation31].
In our study, we developed porous Se@SiO2 nanocomposites and explored the side effects and antitumor effects of the anticancer drug DOX in combination with Se@SiO2 nanocomposites. Our data showed that Se quantum dots released sustainably from the porous Se@SiO2 nanocomposites during the test, which reduce the toxicity due to the avoidance from dose fluctuation. As predicted, DOX induced myocardial tissue damage, whereas Se@SiO2 could attenuated the damage caused by DOX. Se@SiO2 also could reduce the DOX-induced apoptosis of myocardial cells. The TUNEL assay confirmed the protective effect of Se@SiO2 on myocardial cells upon DOX-induced apoptosis in vitro. In addition, we also determined cardiac dysfunction of mice treated with the control, DOX, and Se@SiO2 plus DOX. Compared with the negative control, DOX decreased LVEF%, whereas Se@SiO2 reversed the abnormalities in LVEF% induced by DOX. Previous study suggested that the severity and timing of degenerative myocardial lesions and cardiac dysfunction may influence the mortality of rats [Citation22]. Our study showed that the survival rates of mice reduced significantly with the increasing concentrations of DOX. However, the pre-treatment of Se@SiO2 could improve the survival rates of mice treated with DOX at all the indicated concentrations (ranging from 30 mg/kg to 50 mg/kg) for both 2d and 7d. Furthermore, Se@SiO2 exhibited no appreciable negative effect on the viability of H9C2 cells, which could be attributable to PVP coating and released Se below the toxic margin for normal cells. Thus, Se@SiO2 protected myocardial cells from the damage of DOX.
Next, we wondered why Se@SiO2 nanocomposites could decrease DOX-induced cardiotoxicity. Selenium is closely related to the regulation of oxidative stress. It is the main form of selenocysteine and plays a physiological role [Citation32]. The selenoproteins that have been identified so far mainly include: various forms of glutathione peroxidase, selenoprotein P, iodothyronine deiodinase, selenoprotein W, thioredoxin reduction Enzymes and selenophosphate synthase [Citation33], all of them were related to the balance of oxidative stress. Thus, the supporting of selenium can promote anti-oxidative stress capacity. In the present study, we found that DOX increased the ROS level dramatically, whereas Se@SiO2 could significantly inhibit the ROS production induced by DOX. Se@SiO2 alleviated the apoptosis induced by ROS. It is well-known that ROS generation is one key mechanism underlying the cardiotoxicity of DOX [Citation6]. Enhanced ROS production leads to injuries in the myocardium, including changes which are typical in DOX-induced cardiomyopathy [Citation6]. It was also reported that a reduction in endogenous antioxidants and an increase in free radicals were observed in the DOX treatment [Citation7–9]. In the present study, we found that DOX increased the ROS level dramatically, whereas Se@SiO2 could significantly inhibit the ROS production induced by DOX. Se@SiO2 alleviated the apoptosis induced by ROS. Thus, the protective effect of DOX may be mainly attributable to the antioxidant property of Se@SiO2. Therefore, the same function may happen when it comes to any other drugs with same mechanism (noscapine for example) [Citation34]. The side effects of oxidative stress can be reduced too.
ROS plays an complicated role in cancer process, it can not only mediated cancer cytoprotective autophagy [Citation35] but also directly induce apoptosis. Additionally, we also found that the combination of DOX and Se@SiO2 could augment the antitumour efficacy of DOX. In the present study, although little statistic difference was observed between DOX group and DOX + Se@SiO2 group in vivo, Se@SiO2 could significantly decrease the IC50 of DOX upon Hela cells in vitro.
In summary, Se@SiO2 decrease DOX-induced cardiac damage with potential ability to strengthen the antitumor efficacy of DOX, possibly through combatting oxidative damage. Our study suggested that Se@SiO2 may be an ideal and effective drug to attenuate side effects and enhance the therapeutic effects of DOX and other drugs with ROS damage mechanism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cao J, Chen Z, Chi J, et al. Recent progress in synergistic chemotherapy and phototherapy by targeted drug delivery systems for cancer treatment. Artif Cells Nanomed Biotechnol. 2018 [cited 2018 Feb 6]; [14 p.]. DOI:10.1080/21691401.2018.1436553
- Shabalala S, Muller CJF, Louw J, et al. Polyphenols, autophagy and doxorubicin-induced cardiotoxicity. Life Sci. 2017;180:160–170.
- Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084.
- Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642.
- Jones LW, Haykowsky MJ, Swartz JJ, et al. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50: 1435–1441.
- Singal PK, Iliskovic N. Doxorubicin-induced cardiomyopathy. N Engl J Med. 1998;339:900–905.
- Singal PK, Siveski-Iliskovic N, Hill M, et al. Combination therapy with probucol prevents adriamycin-induced cardiomyopathy. J Mol Cell Cardiol. 1995;27:1055–1063.
- Doroshow JH, Locker GY, Myers CE. Enzymatic defenses of the mouse heart against reactive oxygen metabolites: alterations produced by doxorubicin. J Clin Invest. 1980;65:128–135.
- Singal PK, Iliskovic N, Li T, et al. Adriamycin cardiomyopathy: pathophysiology and prevention. FASEB J. 1997;11:931–936.
- Iliskovic N, Singal PK. Lipid lowering: an important factor in preventing adriamycin-induced heart failure. Am J Pathol. 1997;150:727–734.
- Bian Y, Wei G, Chang TM. Lowering of elevated tissue PCO2 in a hemorrhagic shock rat model after reinfusion of a novel nanobiotechnological polyhemoglobin-superoxide dismutase-catalase-carbonic anhydrase that is an oxygen and a carbon dioxide carrier with enhanced antioxidant properties. Artif Cells Nanomed Biotechnol. 2013;41:60–68.
- Rezaei-Sadabady R, Eidi A, Zarghami N, et al. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif Cells Nanomed Biotechnol. 2016;44:128–134.
- Deng G, Niu K, Zhou F, et al. Treatment of steroid-induced osteonecrosis of the femoral head using porous Se@SiO2 nanocomposites to suppress reactive oxygen species. Sci Rep. 2017;7:43914.
- Zhu Y, Deng G, Ji A, et al. Porous Se@SiO2 nanospheres treated paraquat-induced acute lung injury by resisting oxidative stress. IJN. 2017;12:7143–7152.
- Wang Y, Chen P, Zhao G, et al. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem Toxicol. 2015;85:71–77.
- Liu X, Deng G, Wang Y, et al. A novel and facile synthesis of porous SiO2-coated ultrasmall Se particles as a drug delivery nanoplatform for efficient synergistic treatment of cancer cells. Nanoscale. 2016;8:8536–8541.
- Whanger PD. Selenium and its relationship to cancer: an update. Br J Nutr. 2004;91:11–28.
- Tan L, Jia X, Jiang X, et al. In vitro study on the individual and synergistic cytotoxicity of adriamycin and selenium nanoparticles against Bel7402 cells with a quartz crystal microbalance. Biosens Bioelectron. 2009;24:2268–2272.
- Estevez H, Garcia-Lidon JC, Luque-Garcia JL, et al. Effects of chitosan-stabilized selenium nanoparticles on cell proliferation, apoptosis and cell cycle pattern in HepG2 cells: comparison with other selenospecies. Colloids Surf B Biointerfaces. 2014;122:184–193.
- Sulaiman GM, Jabir MS, Hameed AH. Nanoscale modification of chrysin for improved of therapeutic efficiency and cytotoxicity. Artif Cells Nanomed Biotechnol. 2018 [cited 2018 Jan 31]; [13 p.]. DOI:10.1080/21691401.2018.1434661
- Doane TL, Burda C. The unique role of nanoparticles in nanomedicine: imaging, drug delivery and therapy. Chem Soc Rev. 2012;41: 2885–2911.
- Andreadou I, Mikros E, Ioannidis K, et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J Mol Cell Cardiol. 2014;69:4–16.
- Liu T, Zeng L, Jiang W, et al. Rational design of cancer-targeted selenium nanoparticles to antagonize multidrug resistance in cancer cells. Nanomedicine. 2015;11:947–958.
- Zheng W, Cao C, Liu Y, et al. Multifunctional polyamidoamine-modified selenium nanoparticles dual-delivering siRNA and cisplatin to A549/DDP cells for reversal multidrug resistance. Acta Biomater. 2015;11:368–380.
- Nasrolahi Shirazi A, Tiwari RK, Oh D, et al. Cyclic peptide-selenium nanoparticles as drug transporters. Mol Pharm. 2014;11: 3631–3641.
- Gao F, Yuan Q, Gao L, et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials. 2014;35:8854–8866.
- Liu W, Li X, Wong YS, et al. Selenium nanoparticles as a carrier of 5-fluorouracil to achieve anticancer synergism. ACS Nano. 2012;6:6578–6591.
- Wang H, Zhang J, Yu H. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: comparison with selenomethionine in mice. Free Radic Biol Med. 2007;42:1524–1533.
- Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101:22–31.
- He Y, Chen S, Liu Z, et al. Toxicity of selenium nanoparticles in male Sprague-Dawley rats at supranutritional and nonlethal levels. Life Sci. 2014;115:44–51.
- Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24: 1504–1534.
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241.
- Holben DH, Smith AM. The diverse role of selenium within selenoproteins: a review. J Am Diet Assoc. 1999;99:836–843.
- Pannu V, Rida PC, Ogden A, et al. Induction of robust de novo centrosome amplification, high-grade spindle multipolarity and metaphase catastrophe: a novel chemotherapeutic approach. Cell Death Dis. 2012;3:e346.
- Wang H, Yu X, Su C, et al. Chitosan nanoparticles triggered the induction of ROS-mediated cytoprotective autophagy in cancer cells. Artif Cells Nanomed Biotechnol. 2018 [cited 2018 Jan 9]; [9 p.]. DOI:10.1080/21691401.2017.1423494