 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Primary hepatocytes, as the gold standard cell type for in vitro models, lose their characteristic morphology and functions after few days. There is an urgent need to develop physiologically relevant models that recapitulate liver microenvironment to obtain mature hepatocyte from stem cells. We designed and fabricated a micro-bioreactor device mimicking the physiological shear stress and cell–cell interaction in liver sinusoid microenvironment. Induced pluripotent stem cells (iPSCs) were co-cultured with human umbilical vein endothelial cells (HUVECs) in the micro-bioreactor device with continuous perfusion of hepatic differentiation medium (100 μL/h). Simulation results showed that flow field inside our perfusion device was uniform and shear stress was adjusted to physiological condition (<2 dyne/cm2). IPSCs-derived hepatocytes (iPSCs-Heps) that were cultured in micro-bioreactor device showed a higher level of hepatic markers compared to those in static condition. Flow cytometry and immunocytochemistry analysis revealed iPSCs cultured in the device sequentially acquired characteristics of definitive endodermal cells (SOX17 positive), hepatoblasts (AFP positive) and mature hepatocyte (ALB positive). Moreover, the albumin and urea secretion were significantly higher in micro-bioreactor device than those cultured in culture dishes during experiment. Thus, based on our results, we propose our micro-bioreactor as a beneficial device to generate mature hepatocytes for drug screening and basic research.
Introduction
Liver is a highly metabolic organ with complex tissue architecture that is responsible for many vital functions in body [Citation1]. Currently, there are satisfactory in vitro models for studying liver biology, liver related disease and drug screening [Citation2,Citation3]. However, there is an urgent need to develop physiologically relevant models to obtain mature hepatocyte for cell therapy and drug toxicity screening. Primary hepatocytes can be isolated from human liver and serve as golden standard cell type for such in vitro models. However, primary hepatocytes cultured on conventional culture plates retain their characteristic morphology, polarity and functions for no more than 1 week, eventually converting to fibroblast-like cells [Citation4–6]. To overcome this limitation, some investigations have used human pluripotent stem cells (hPSCs) as an alternative source for obtaining functional hepatocytes [Citation7–9]. Human induced pluripotent stem cells (hiPSCs) have the ability to form any cell type of the human body and thus, they are broadly used to generate hepatocyte-like cells for liver biology and pathology studies [Citation10–12], as well as various biomedical applications such as drug testing [Citation13,Citation14].
During embryonic development, endothelial cells envelope liver bud-derived hepatoblasts and stimulate bud’s expansion and invasion into surrounding septum transversum mesenchyme [Citation15]. Several studies have demonstrated that co-culturing primary hepatocytes or stem cells-derived hepatocyte-like cells with endothelial cells maintain hepatocyte maturation via paracrine signalling and cell–cell contacts [Citation6,Citation16]. On the other hand, in hepatic tissue, hepatocytes are permanently exposed to portal pressure in the form of fluid shear stress [Citation17]. Despite the evidence indicating the importance of fluid shear stress on phenotype maintenance and metabolic activity of hepatocytes [Citation18–20], few studies evaluated this factor in the hepatic differentiation.
Since static and two-dimensional (2D) culture plates do not accurately model the cell–cell interactions, and physical/chemical stimuli provided by hepatic microenvironment, micro-fabrication and microfluidics technologies have been used to overcome these problems [Citation3,Citation21–23]. In recent years, using these technologies, several studies have tried to simulate the various parameters of hepatocytes microenvironment to obtain viable and functional hepatocytes. Domansky et al. (2010) showed that primary rat hepatocytes, co-cultured with sinusoid endothelial cells, were viable for 1 week under perfusion medium flow [Citation24]. Moreover, Kang et al. [Citation3] reported that primary rat hepatocytes, co-cultured with an endothelial cell line, maintained their hepatic morphology and function for more than 30 days in dual micro-channel under continuous perfusion. In another study, efficient differentiation of mesnchymal stem cells (MSCs) into hepatocyte-like cells were facilitated using a microfluidic device [Citation21]. It has also been shown that iPSCs-derived hepatocytes (iPSCs-Heps) generated in microfluidic systems had functional phenotypes and responded to drug treatment [Citation20]. Despite these valuable results, none of these studies has been able to produce highly pure, viable, and functional hepatocytes suitable for pharmaceutical applications and/or fundamental research.
The aim of this study is to design and fabricate a perfusion micro-bioreactor device for co-culturing iPSCs and human umbilical vein endothelial cells (HUVECs) to enhance the hepatic differentiation capacity of iPSCs. Our results showed that the fabricated micro-bioreactor device is a highly promising platform compared to the conventional culture dishes, and could efficiently improve hepatic differentiation and maturation of iPSCs.
Material and methods
Design and fabrication of the micro-bioreactor device and cell culture
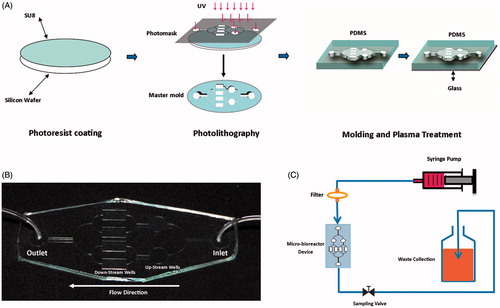
Photomask designed by Corel Draw software (CorelDraw Graphics suite X7, Cowpland Research Laboratory, Ottawa, Canada). Our micro-bioreactor device, containing two independent circular spaces (radius = 3 mm) at up-stream and four rectangular spaces (10 mm long and 5 mm wide) at down-stream, was fabricated by standard soft-lithographic techniques. The wafer master was photo-lithographically patterned through SU8-2050 negative photoresist (MicroChem) according to the manufacturer’s indications. SU-8 was poured on the wafer and conducted spin-coating as 1700 rpm, 30 s to obtain a final thickness of 100 μm. After spin-coating, wafer was baked at 95 °C for 30 min then SU-8 was polymerized by using 365 nm UV light for 50 s with the light power of 13 mW/cm2. Polymerized SU-8 wafer was baked at 95 °C for 20 min and isopropanol was used to eliminate unexposed SU-8 completely. After degassing, a premixed 10:1 mixture of polydimethylsiloxane (PDMS) pre-polymer and curing agent solutions (Sylgard 184 kit; Dow Corning) was cast on the silicon wafer and cured at 80 °C oven for 3 h. The PDMS mold was cut, peeled off and punched with a biopsy punch to make inlet and outlet holes and were fitted with silicon tubes. The PDMS mold was assembled and sealed to cleaned glass slide by plasma bonding (Diener Electronics, Ebhausen, Germany) ()). The assembled device was cleaned with isopropanol (Sigma-Aldrich) and sterilized by autoclaving. Microfluidic device were filled with collagen solution (0.1% w/v) and incubated 2 h at 4 °C. Before cell seeding, the collagen solution was aspirated and the microfluidic device was incubated at 37 °C and a 5% CO2 atmosphere for 1 h. For dynamic culture (after cell seeding), micro-bioreactor device was connected to syringe pump and a flow rate of 100 μL/h was applied. In order to maintain sterile conditions, a 0.2 μm sterile filter was placed upstream of inlet. The outlet of micro-bioreactor device was connected to waste bottle with silicon tube. A valve was placed in the middle of silicon tube for sampling the medium ().
Figure 1. Schematic fabrication and assembling of micro-bioreactor device platform to study effects of shear stress and cell–cell interaction on hepatic differentiation. (A) A thin uniform layer of SU-8 photoresist is spin-coated on a silicon wafer and then overlaid with photomask. The photomask protects some regions of the SU-8 and exposes others during exposure to high-intensity UV light. Unexposed SU-8 completely removed with isopropanol and microscale pattern remained on the wafer (master mold). PDMS pre-polymer and curing agent solution is cast on the master mold, polymerized and peeled off. The PDMS mold was assembled and sealed to cleaned glass slide by plasma bonding. (B) The fabricated device. (C) Micro-bioreactor device assembling for continuous perfusion of differentiation media and waste collection.
Flow field simulation
Fluid shear stress is one of the mechanical forces applied to the hepatocytes in the dynamic environment of liver and affects many of the morphological and functional properties of the cells. To determine the flow rate that ensures physiological shear stress within our micro-bioreactor device especially at the cell culture surface area, COMSOL Multiphysics (COMSOL Inc.) was used to estimate shear stress and flow field. Standard transport equations were used in the simulations:
where, Q is the flow rate; τw is the shear stress at the walls; μ the fluid viscosity; h is the channel’s height; and w is the channel’s width.
Expansion of induced pluripotent stem cells
Human iPSCs were provided by the research group led by Prof. Masoud Soleimani in Stem Cells Technology Research Center [Citation25] . Human iPSCs were cultured and expanded on a feeder layer of mitomycine-C (Sigma) inactivated mouse embryonic fibroblasts (MEFs) in iPSCs medium [DMEM/F12 supplemented with 20% knockout serum replacement, 0.1 mmol/L non-essential amino acids, 2 mM l-glutamine, 0.1 mM β-mercaptoethanol and 10 ng/mL of recombinant human basic fibroblast growth factor (bFGF), all from Invitrogen in a 5% CO2 and 95% humidity. Before differentiation, in order to embroid body (EB) formation, iPSCs colonies were detached enzymatically using 0.1% collagenase type IV (Invitrogen) and transferred to ultra-low adherent culture plate in EB medium (iPSCs medium without bFGF) for 5 days.
Induction of hepatic differentiation in vitro
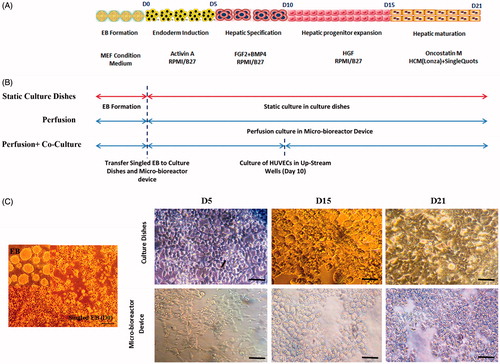
In order to cell seeding, the syringe containing the singled EB cell suspension (5 × 105 cells/mL in MEF-conditioned medium) attached to micro-bioreactor device and cells were loaded by hand to the pre-coated device. Cells remained under static condition for >3 h at 37 °C and 5% CO2 without perfusion, to allow cell adhesion. IPSCs to hepatocytes differentiation protocol was performed as previously described [Citation26]. In the first step, the MEF-conditioned medium was replaced with RPMI1640 medium supplemented with B27 (Invitrogen) and 100 ng/ml activin A (Peprotech) for 5 days and followed with 20 ng/ml BMP4 (Peprotech) and 10 ng/ml FGF-2 (Invitrogen) in RPMI/B27. After 5 days, the medium was replaced with RPMI/B27 supplemented with 20 ng/ml HGF (Peprotech) and finally for 5 days with 20 ng/ml Oncostatin-M (Stemgent) and dexamethasone (Dex, Sigma) (10−7 M), in Hepatocyte Culture Media (Lonza) supplemented with SingleQuots (without EGF). IPSCs differentiation to hepatocytes was performed in three situations ().
In perfusion micro-bioreactor device, as described above, singled EB were cultured in four down-stream rectangular spaces alone. In second group, to begin the cell–cell interaction study, HUVECs were cultured in the two up-stream circular wells at Day 10 (co-cultured group). For the control group, singled EB were cultured on collagen-coated polystyrene dishes without perfusion and were induced by the same differentiation protocol. Cells morphology in static and dynamic cultures was recorded by inverted microscope.
RNA extraction and quantitative real-time PCR
To detect hepatic gene expression, total RNA was extracted from differentiated hiPSC using the RNeasy Mini Kit (Qiagen) following the manufacturer’s protocol. Then, RNA was reverse transcribed to complementary DNA (cDNA) using a cDNA synthesis kit (iNtRON, Daejeon, South Korea) and a random hexamer primer in a total reaction volume of 15 μl. The polymerase chain reaction (PCR) parameters included denaturation at 95 °C for 3 min and then 40 cycles at 95 °C for 20 s, annealing at 60 °C for 30 s and elongation at 72 °C for 30 s. Quantitative real-time PCR was performed using a SYBR-Green kit (Takara, Seoul, Korea) according to the manufacturer’s protocol and using the ABI Light Cycler (ABI step one). The reaction was carried out at 95 °C for 2 min, followed by 40 amplification cycles (each 5 s at 95 °C, 30 s at 60 °C) with fluorescence detection and a final step of melting curve analysis. All reactions were performed in triplicate, and the results were normalized against the Ct (critical threshold) value of the housekeeping gene GAPDH to correct RNA input in reactions. The relative quantification (ΔΔCt) method was used to analyze the data. The primer sequences were designed using AlleleID software (Primer Biosoft), which is illustrated in .
Table 1. Specifications of primer sequences used for quantitative real-time PCR.
Flow cytometry analysis
For flow cytometry analysis, cells cultured in micro-bioreactor device were harvested with 0.25% trypsin–EDTA at different time points of differentiation (Days 5, 15 and 21). Then, cells washed twice with PBS, centrifuged for 5 min at 200g, re-suspended and fixed in 4% (w/v) paraformaldehyde and incubated at room temperature (RT) for 20 min. Subsequently, cells were washed twice with PBS and permeabilized with 0.2% Triton X-100 (Sigma) diluted in PBS for 1 h. After 1 h blocking by goat serum, cells were incubated with primary Sox17, AFP and ALB antibodies (all from R&D) for 1 h at RT and followed by FITC (R&D) and phycoerythrin (PE, R&D) conjugated secondary antibodies for 30 min at RT in darkness. The cells were washed twice with Tween buffer, re-suspended in 300 μl PBS, and analyzed using flow cytometry (Attune™ Acoustic Focusing Cytometer). For each sample, cells were analyzed using FlowJo® software.
Immunofluorescent staining
To evaluate hepatic differentiation, the samples were washed with PBS and fixed with 4% (w/v) paraformaldehyde (Sigma) in PBS at 4 °C for 20 min in different stages of differentiation process. After fixation, the cells were washed with PBS and permeabilized with 0.1% Triton X-100 (Sigma) for 20 min and blocked with 10% goat serum (Sigma) for 1 h at RT. Then, samples were incubated with specific primary antibodies overnight at 4 °C. The primary antibodies include human SRY-box containing gene 17 (SOX17, 1:200; R&D, MAB1924), α-fetoprotein (AFP, 1:200, R&D, MAB1368) and albumin (ALB, 1:200, R&D, MAB1455) were diluted with 1% BSA in PBS. In the following day, the samples were washed three times with PBS and incubated with secondary antibodies include phycoerythrin-conjugated antibody (PE, 1:100; R&D, F0102B) and fluorescein-conjugated antibody (FITC, 1:100; R&D, F0103B) at RT in darkness. After washing thrice with PBS, the samples were incubated with 0.1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI, Sigma) for 5 min, to label the nucleus. The samples were imaged using an optic microscope (Axioplan2; Carl Zeiss Microscopy GmbH, Oberkochen, Germany).
Human albumin ELISA
Conditioned media from the differentiated iPSC-Heps cultured in 2D and micro-bioreactor device were collected every 2 days and frozen at −20 °C until assay. The concentration of albumin secreted into the media was determined using Albumin ELISA Quantitation Kit (albumin ELISA; Orgentech Diagnostica, Mainz, Germany) according to manufacturer’s instructions. Assays were performed on supernatant samples obtained from three independent cultures and were performed in duplicate. Albumin secretion was normalized to the total number of cells.
Urea synthesis assay
The cell culture supernatant was collected every 2 days form outlet side of micro-bioreactor device and stored at −20 °C. Sampling was done in two hours intervals per day (three times per day). As a control, medium samples were taken from 2D static condition. The urea production test was performed using a colorimetric Urea Assay Kit (Pars Azmun Co., Tehran, Iran) according to manufacturer’s instructions. Urea synthesis was normalized to the total number of cells.
Statistical analysis
All experiments were performed at least triplicate independently, and the data are reported as a mean ± standard deviation (SD). Results were analyzed by t-test, two-way analysis of variance (ANOVA), and Bonferroni’s post hoc test was used for comparison of perfusion device with culture dishes by GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA). In between the comparison groups, a value of p < .05 was considered statistically significant.
Results
Design, modelling and assembly of micro-bioreactor device
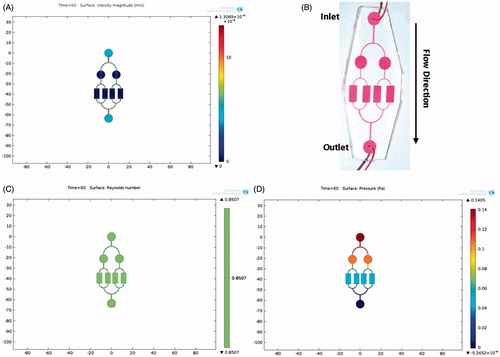
A two layered micro-bioreactor device, consisting of a glass slide and a patterned PDMS, was designed and fabricated (). Since the shear stress generated by the flow rate of the culture medium can influence the differentiation of iPSCs, the perfusion performance in our micro-bioreactor device was initially simulated (). As shown in , the simulated field of velocity distribution demonstrated a uniform flow in the culture region of our micro-bioreactor device. Consistent with the simulation results, distribution of the phenol-red dye showed that the flow field inside the culture regions of device was uniform (). The Reynolds number (Re) of our micro-bioreactor device under 100 μl/h flow rate was 8.5 × 10−1. Since the Re was smaller than 2100, the flow was considered laminar, indicating that under 100 μL/h flow rate, a favourable laminar flow in our device was created (). Finally, under the flow rate of 100 μL/h, the viscosity of RPMI 1640 culture medium 0.001 poised at 37 °C, 4 mm width and 80 μm height, the shear stress on the cell surface was calculated to be 0.5 Pa (5 × 10−1 dyne/cm2) in the micro-bioreactor device (). Based on the previous studies in which the shear stress in normal hepatic sinusoids were reported to reach up to 2 dyne/cm2 [Citation24,Citation27], our micro-bioreactor device could effectively simulate physiological shear stress.
Figure 2. Simulation results of flow field and observation. COMSOL Multiphysics (COMSOL Inc.) was used to model and estimate velocity, Reynolds number and shear stress in cell culture surface areas of the micro-bioreactor device. (A) Simulation of velocity distribution in micro-bioreactor device. The flow field showed a uniform flow in cell seeding part of the micro-bioreactor device. (B) Phenol Red was used as a tracing dye for the actual flow field observation in our device. (C) The Reynolds number evaluation for a 100 μl/h flow rate showed a laminar flow in our micro-bioreactor device. (D) Shear stress on the cell surface was calculated to be 0.5 Pa (5 × 10−1 dyne/cm2) in micro-bioreactor device, which is equivalent to the physiological shear stress in liver sinusoids.
Morphology of iPSCs-Heps cultured in micro-bioreactor device
To generate hepatocytes from hiPSCs, we used a stepwise differentiation protocol as illustrated schematically in ). Singled EBs was loaded to micro-bioreactor device and treated with dynamic differentiation medium. The responsiveness of iPSCs-Heps to shear stress, in terms of change in morphology, was examined using inverted microscope. The differentiating iPSCs took spindle-like shape morphology by Day 5 in the micro-bioreactor device and in culture dishes. During the Days 5–15 (at the intermediate stage), a clear increase in cell numbers was observed and the cells gradually took round or polygonal shapes. By Day 21 in both groups, the morphology of cells was changed into a cuboidal shape having vacuoles within their cytoplasm, known as a typical morphological feature of hepatocyte. These results suggest that the morphology of the cells under 0.5 Pa shear stress in micro-bioreactor device remains identical to those in static culture () indicating that cellular morphology of iPSCs-Heps was not influenced by shear stress generated in micro-bioreactor device.
Figure 3. The hepatic differentiation of human iPS cells. (A) A flow chart showing the stepwise differentiation protocol. (B) Processes of hepatic differentiation of iPSCs in culture dish and micro-bioreactor device (perfusion and perfusion + co-culture). (C) Morphological changes of iPSCs during differentiation process in culture dishes and micro-bioreactor device. Note that the cells first took a round and polygonal shape after which they were converted into cuboidal-shaped cells, a typical morphological feature of hepatocytes. The cell morphology under 0.5 Pa shear stress in micro-bioreactor device remained in the same shape as in static culture. Scale bar = 50 μm.
Hepatic differentiation of hiPSCs in micro-bioreactor device
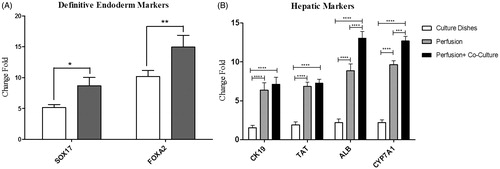
The expression of differentiation markers at the levels of mRNA and protein in iPSCs were examined during their hepatic differentiation in the micro-bioreactor device and compared to those under static condition. Our real-time RT-PCR analysis revealed that at Day 5 (early differentiation stage), the expression levels of major definitive endoderm markers including FOXA2 and SOX17, were significantly increased in cells cultured in the micro-bioreactor device compared to those in the static condition (*p < .05 and **p < 0.01, respectively, ). At Day 21 (later differentiation stage), the expression of human liver-associated genes including albumin, CK19, TAT and Cyp7A1 were significantly increased in iPSCs-Heps co-cultured with HUVECs in the micro-bioreactor device compared to those cultured in the static condition and iPSCs alone (***p < 0.001 and ****p < .0001, ).
Figure 4. Expression of hepatic differentiation markers in iPSCs-Heps cultured in culture dishes and micro-bioreactor device. The cells were cultured in the presence of differentiation medium (as indicated in ) for 5 and 21 days after which the expression of hepatic differentiation markers was examined using real-time PCR. (A) The expression levels of definitive endodermal genes at Day 5. Note that the expression of these markers was significantly increased in iPSCs-derived endodermal cells cultured in the micro-bioreactor device compared to those cultured in dishes. (B) The expression levels of hepatic maturation genes at Day 21. Note that the expression of human liver-associated genes was significantly increased in iPSCs-Heps co-cultured with HUVECs in micro-bioreactor device compared to those cultured in the static condition and iPSCs alone. Relative levels of gene expression were normalized to the undifferentiated hiPSCs. The value is shown on each graph as mean ± SD. *p < .05, **p < .01, ***p < .001, ****p < .0001, Bonferroni’s post hoc test.
Our flow cytometry analysis showed that a high percentage of cells in the micro-bioreactor device express SOX17 (a definitive endoderm marker, 79.9%) at Day 5. Moreover, significant expression of AFP (a hepatoblast marker, 84.3%) and ALB (the hepatic-specific marker, 90%) were observed at Day 15, indicating a homogenous and efficient differentiation of hiPSCs by this time. At Day 21, while expression of ALB was maintained (84%), the expression of AFP was reduced (18.3%), suggesting that the hepatocyte-like cells proceeded to a more mature phenotype (). The expressions of all mentioned markers were confirmed by immunocytochemistry ().
Figure 5. Expression of hepatic-specific differentiation proteins in iPSCs-Heps. (A) The cells were cultured in the presence of differentiation medium (as indicated in ) for 5, 15 and 21 days after which the expression of hepatic-specific differentiation proteins were examined using flow cytometry (A) and immunocytochemistry (B). (A) Flow cytometry analysis of endodermal marker, SOX17, at Day 5 and hepatic maturation markers, AFP and albumin (ALB), at Days 15 and 21 of the cells cultured in micro-bioreactor device and culture dishes. Note the high percentage of AFP and ALB positive cells at Day 15 suggesting homogenous and efficient differentiation. The reduced expression of AFP at Day 21 indicates that the iPSCs-Heps proceed to a more mature phenotype by this time in micro-bioreactor device. (B) Immunostaining of the iPSCs-Heps with antibodies against SOX 17 (red), AFP (green) and ALB (red) at Days 5, 15 and 21, respectively. Note that the expression profile of these protein markers is consistent with flow cytometry results. Scale bar = 25 μm, 10 μm.
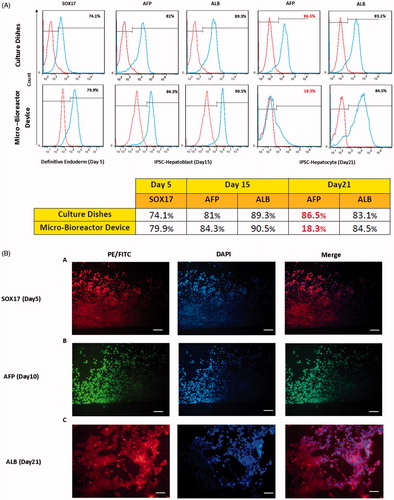
Functional activities of iPSCs-Heps in micro-bioreactor device
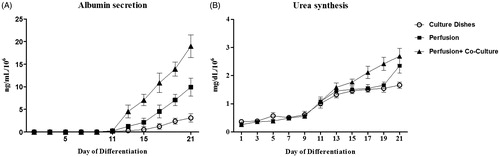
To evaluate the liver-specific functions of iPSCs-Heps in the static culture dishes and the micro-bioreactor device, albumin and urea secretion was examined in the differentiating iPSCs. Our results showed that albumin secretion was increased at early stages of differentiation, starting from Day 11, both in culture dishes and the micro-bioreactor device. At the later stages (Days 15–21), however, albumin secretion in micro-bioreactor device was significantly higher than culture dishes. More interestingly, the addition of HUVECs significantly improved albumin secretion of iPSCs-Heps in micro-bioreactor device when compared to those cultured in the absence of HUVECs and under the static condition (). The rate of urea secretion showed a constant increase in culture dishes and in the micro-bioreactor device during the experiment. However, the value of urea secreted by iPSCs-Heps in micro-bioreactor device was higher than those cultured under the static condition. Consistent with the effects of the HUVECs on albumin secretion, the addition of HUVECs to iPSC-Heps also led to increase the urea secretion during the differentiation, even though the difference was not significant (). Thus, according to the results from the real-time RT-PCR, flow cytometry, and the immunocytochemistry, our functional analysis demonstrated that the cells cultured alone or co-cultured with HUVECs in micro-bioreactor device are efficiently converted from endodermal cells into a hepatic fate.
Figure 6. Functional analysis of iPSCs-Heps cultured in differentiation medium over 21 days. (A) Albumin secretion (especially between Days 15 and 21) in the cells cultured in micro-bioreactor device was significantly higher than culture dishes. Addition of HUVECs significantly improved albumin secretion of iPSCs-Heps in micro-bioreactor device when compared with those cultured in the absence of HUVECs and in the static condition. (B) Urea synthesis of differentiated hiPSCs showed a constant increase in all defined condition during time-course of experiment. While urea synthesis was increased in the cells cultured on the device with or without HUVEC, the difference was not significant. Values were normalized to 1 × 106 seeded cells.
Discussion
In the present study, a perfusion-based micro-bioreactor device was designed to improve the production of differentiated hepatocytes from iPSCs. Our fabricated device showed advantages over standard culture dishes and other similar devices used to induce the differentiation of stem cells to hepatocyte-like cells [Citation21,Citation28,Citation29]. First, the simulated field of velocity distribution inside the culture region of device was uniform due to the rectangular geometry. This feature creates a relatively homogeneous medium distribution within our device suggesting a favourable environment for hepatic differentiation of iPSCs. Second, our micro-bioreactor device mimics the physiological shear stress that theoretically confers advantages over static culture dishes. While the shear stress in normal hepatic sinusoids doesn’t surpass 2 dyne/cm2 [Citation24], for the flow rate of 100 μL/h, the shear stress in cell culture surface area was determined to be 5 × 10−1 dyne/cm2. Previous studies have also reported that the shear stress exerted on cells during medium replacement in static culture can vary from 0.9 to 2.8 dyne/cm2 [Citation3]. Thus, the shear stress exerted on iPSCs-Heps in our micro-bioreactor device is about 2–6 times less than those in static culture dishes during medium replacement. Since the shear stress in our dynamic micro-bioreactor device was adjusted to physiological condition, cellular morphology of iPSCs-Heps was not influenced by shear stress and it is likely this characteristic contributes to the efficient differentiation and retaining cell phenotype and function for a longer time.
The third advantage of our micro-bioreactor device is that unlike other previous designs, it allows co-culture of other cells beside iPSCs-Heps. In the present study, HUVECs were chosen as a well-established and standardized cell source for parenchymal–endothelial cell co-culture. As the umbilical vein is the major afferent vessel in the fetal liver [Citation1,Citation30], HUVECs might thereby be crucial for early liver development. For this reason, in several studies HUVECs we successfully utilized as supporting cells for hepatic differentiation [Citation31–33]. Takebe et al. [Citation31] have shown that iPSCs-derived hepatocytes co-cultured with HUVECs and MSCs, self-organized into liver organoids, thus providing suitable environment that enhance the differentiation of hepatocyte-like cells. Asai et al. [Citation34] established a liver organoid using iPSCs, MSCs and HUVECs, and showed that released soluble factors from MSCs and HUVECs induced a hepatocyte-like phenotype in iPSCs and promoted hepatocyte maturation in liver organoids. Our findings also showed that co-cultivation of iPSCs with HUVECs in our micro-bioreactor device can improve differentiation and maturation of iPSCs to hepatocyte-like cells, confirming the importance of endothelial cells during hepatocyte differentiation and maturation.
During hepatic differentiation, iPSCs were cultured in static culture dishes and the micro-bioreactor device with the same hepatic induction medium gradually changing into polygonal and cuboidal-shaped cell known as a typical shape for hepatocytes. Our micro-bioreactor device, mimicking physiological shear stress in hepatic microenvironment via supplying a continuous flow of nutrients and removing metabolic waste and low-quality loose cells, seems to have led to generate a homogenous population of hepatocyte-like cells.
Our flow cytometry and immunocytochemistry analysis revealed that in the presence of continuous perfusion of hepatic inductive medium, iPSCs sequentially acquired characteristics of definitive endodermal cells (SOX17 positive), hepatic progenitor cells (AFP positive) and mature hepatocyte (ALB positive). Generally, hepatocytes derived from pluripotent stem cells using current static differentiation protocols are immature, verified here by high expression of AFP in the late stages of hepatic differentiation (>85%). Interestingly, in iPSCs-Heps cultured in micro-bioreactor device, we observed nearly five-fold reduction in AFP expression that associated with the stable expression of ALB as a maturation marker.
The expression of hepatic-specific maturation markers (albumin, CK19, Cyp7A1 and TAT) in iPSCs-Heps cultured in our micro-bioreactor device were confirmed by real-time RT-PCR analysis as well. Prominent expression of hepatic maturation markers was observed even more when iPSCs-Heps was co-cultured with HUVECs. These results indicate that co-culturing in such device steady perfusion promotes differentiation and maturation of hepatocyte-like cells. This finding shows great potential of this device for practical applications as mature hepatocytes are critical in drug screening and developing bioartificial liver device [Citation35].
It has been shown that functional activities of primary hepatocytes [Citation3], liver cell lines [Citation22], MSCs-Heps [Citation21] and iPSC-Heps [Citation20] cultured in perfusion devices and/or co-cultured with endothelial cells [Citation31,Citation33,Citation36,Citation37] are higher than those cultured in culture dishes. We also showed that albumin and urea secretion (as a functional hepatic markers) are maintained and increased over time in iPSCs-Heps cultured in the micro-bioreactor device. In addition, a significant increase in albumin secretion was observed during the experiment when iPSCs-Heps were cultured in the presence of HUVECs. However, we did not observed a significant change in urea secretion in iPSCs-Heps cultured in our device in the presence or absence of HUVEC. Since albumin and urea secretion continue after the period of differentiation, more maturation could be achieved in a more prolonged differentiation period.
To the best of our knowledge, this is the first report describing the co-cultivation of iPSCs and HUVECs under physiological shear stress in a micro-bioreactor device to generate hepatocyte-like cells. The results presented here showed that the hepatic differentiation and maturation of iPSCs in our micro-bioreactor device is more efficient than those in culture dishes and other previously reported designs, and thus, the device is shown to be suitable for cell therapy, drug screening and basic research. Not only does our study suggest iPSCs as an alternative source for primary hepatocytes, but it also introduces a device providing biological and physico-chemical conditions to obtain functional hepatocytes. Moreover, this device has the potential to be developed for large-scale production of hepatocyte-like cells toward addressing the high demand in cell therapy industry.
Acknowledgments
This study was supported by University of Tehran and Iranian Council for Stem Cell Sciences and technologies.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Si-Tayeb K, Lemaigre FP, Duncan SA. Organogenesis and development of the liver. Dev Cell. 2010;18:175–189.
- LeCluyse EL, Witek RP, Andersen ME, et al. Organotypic liver culture models: meeting current challenges in toxicity testing. Crit Rev Toxicol. 2012;42:501–548.
- Kang YBA, Sodunke TR, Lamontagne J, et al. Liver sinusoid on a chip: long‐term layered co‐culture of primary rat hepatocytes and endothelial cells in microfluidic platforms. Biotechnol Bioeng. 2015;112:2571–2582.
- Guillouzo A. Liver cell models in in vitro toxicology. Environ Health Perspect. 1998;106(Suppl. 2):511.
- Nahmias Y, Berthiaume F, Yarmush ML. Integration of technologies for hepatic tissue engineering. Adv Biochem Eng Biotechnol. 2007;103:309–329.
- Gerbal-Chaloin S, Funakoshi N, Caillaud A, et al. Human induced pluripotent stem cells in hepatology: beyond the proof of concept. Am J Pathol. 2014;184:332–347.
- Mahmoodinia Maymand M, Soleimanpour-lichaei HR, Ardeshirylajimi A, et al. Improvement of hepatogenic differentiation of iPS cells on an aligned polyethersulfone compared to random nanofibers. Artif Cells Nanomed Biotechnol. 2018;46:853–860.
- Maymand MM, Soleimanpour-Lichaei HR, Ardeshirylajimi A, et al. Hepatogenic differentiation of human induced pluripotent stem cells on collagen-coated polyethersulfone nanofibers. ASAIO J. 2017;63:316–323.
- Enderami SE, Kehtari M, Abazari MF, et al. Generation of insulin-producing cells from human induced pluripotent stem cells on PLLA/PVA nanofiber scaffold. Artif Cells Nanomed Biotechnol. 2018 [Feb 27]; [1–8]. DOI:10.1080/21691401.2018.1443466
- Ghodsizadeh A, Taei A, Totonchi M, et al. Generation of liver disease-specific induced pluripotent stem cells along with efficient differentiation to functional hepatocyte-like cells. Stem Cell Rev Rep. 2010;6:622–632.
- Takayama K, Inamura M, Kawabata K, et al. Efficient generation of functional hepatocytes from human embryonic stem cells and induced pluripotent stem cells by HNF4α transduction. Mol Ther. 2012;20:127–137.
- Nassiri Mansour R, Barati G, Soleimani M, et al. Generation of high-yield insulin producing cells from human-induced pluripotent stem cells on polyethersulfone nanofibrous scaffold. Artif Cells Nanomed Biotechnol. 2018 [Feb 12]; [1–7]. DOI:10.1080/21691401.2018.1434663
- Davidson MD, Ware BR, Khetani SR. Stem cell-derived liver cells for drug testing and disease modeling. Discov Med. 2015;19: 349–358.
- Chiang C-H, Huo T-I, Sun C-C, et al. Induced pluripotent stem cells and hepatic differentiation. J Chin Med Assoc. 2013;76:599–605.
- Matsumoto K, Yoshitomi H, Rossant J, et al. Liver organogenesis promoted by endothelial cells prior to vascular function. Science. 2001;294:559–563.
- Stevens K, Ungrin M, Schwartz R, et al. InVERT molding for scalable control of tissue microarchitecture. Nat Comms. 2013;4:1847.
- Niiya T, Murakami M, Aoki T, et al. Immediate increase of portal pressure, reflecting sinusoidal shear stress, induced liver regeneration after partial hepatectomy. J Hepato-Biliary-Pancreat Sci. 1999;6:275–280.
- Rashidi H, Alhaque S, Szkolnicka D, et al. Fluid shear stress modulation of hepatocyte-like cell function. Arch Toxicol. 2016;90: 1757–1761.
- Tanaka Y, Yamato M, Okano T, et al. Evaluation of effects of shear stress on hepatocytes by a microchip-based system. Meas Sci Technol. 2006;17:3167.
- Giobbe GG, Michielin F, Luni C, et al. Functional differentiation of human pluripotent stem cells on a chip. Nat Methods. 2015;12: 637–640.
- Yen M-H, Wu Y-Y, Liu Y-S, et al. Efficient generation of hepatic cells from mesenchymal stromal cells by an innovative bio-microfluidic cell culture device. Stem Cell Res Ther. 2016;7:120.
- Bhise NS, Manoharan V, Massa S, et al. A liver-on-a-chip platform with bioprinted hepatic spheroids. Biofabrication. 2016;8:014101.
- Bavli D, Prill S, Ezra E, et al. Real-time monitoring of metabolic function in liver-on-chip microdevices tracks the dynamics of mitochondrial dysfunction. Proc Natl Acad Sci USA. 2016;113:E2231–E2E40.
- Domansky K, Inman W, Serdy J, et al. Perfused multiwell plate for 3D liver tissue engineering. Lab Chip. 2010;10:51–58.
- Mahboudi H, Soleimani M, Enderami SE, et al. The effect of nanofibre-based polyethersulfone (PES) scaffold on the chondrogenesis of human induced pluripotent stem cells. Artif Cells Nanomed Biotechnol. 2017 [Nov 6]; [1–9]. DOI:10.1080/21691401.2017.1396998
- Si‐Tayeb K, Noto FK, Nagaoka M, et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297–305.
- Kim L, Toh Y-C, Voldman J, et al. A practical guide to microfluidic perfusion culture of adherent mammalian cells. Lab Chip. 2007;7:681–694.
- Chung BG, Park JW, Hu JS, et al. A hybrid microfluidic-vacuum device for direct interfacing with conventional cell culture methods. BMC Biotechnol. 2007;7:60.
- Guo D-L, Wang Z-G, Xiong L-K, et al. Hepatogenic differentiation from human adipose-derived stem cells and application for mouse acute liver injury. Artif Cells Nanomed Biotechnol. 2017;45: 224–232.
- Collardeau‐Frachon S, Scoazec JY. Vascular development and differentiation during human liver organogenesis. Anat Rec. 2008;291:614–627.
- Takebe T, Sekine K, Enomura M, et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481–484.
- Ma X, Qu X, Zhu W, et al. Deterministically patterned biomimetic human iPSC-derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci USA. 2016;113:2206–2211.
- Freyer N, Greuel S, Knöspel F, et al. Effects of Co-culture media on hepatic differentiation of hiPSC with or without HUVEC Co-Culture. Int J Mol Sci. 2017;18:1724.
- Asai A, Aihara E, Watson C, et al. Paracrine signals regulate human liver organoid maturation from induced pluripotent stem cells. Development. 2017;144:1056–1064.
- Ren S, Irudayam J. Bioartificial liver device based on induced pluripotent stem cell-derived hepatocytes. J Stem Cell Res Ther. 2015;5:263.
- Koui Y, Kido T, Ito T, et al. An in vitro human liver model by iPSC-derived parenchymal and non-parenchymal cells. Stem Cell Rep. 2017;9:490–498.
- Ahmed HMM, Salerno S, Morelli S, et al. 3D liver membrane system by co-culturing human hepatocytes, sinusoidal endothelial and stellate cells. Biofabrication. 2017;9:025022.
