Abstract
Objectives: This study was aimed to further explore whether estradiol (E2) had protective effects on intervertebral disc degeneration (IVDD) in a rat model.
Material/methods: Forty, three-month-old female Sprague Dawley (SD) rats were randomly divided into four groups: Sham, Ovariectomy (OVX), E2 and ICI182780 (ICI). Sham group only underwent the resection of a bit fat; OVX group underwent bilateral ovariectomy; E2 group was treated with E2 based on OVX; ICI group was treated with E2 and pretreated ICI182780 (inhibitor of the estrogen receptor) based on OVX. Radiography, hematoxylin and eosin (HE) staining, immunohistochemistry (IHC), western blot and quantitative real-time PCR (qRT-PCR) were applied to detect the apoptosis characteristics of intervertebral disc cells.
Results: Radiographs showed marked intervertebral disc narrowing and HE staining showed typical apoptosis characteristics of intervertebral disc cells in OVX, which were reversed by E2. Furthermore, the results of IHC, Western blot and qRT-PCR revealed that OVX-induced IVDD was protected by E2 through decreasing the expression of caspase-3 and intracellular matrix metalloproteinases (MMPs), including MMP-3 and MMP-13, while increasing the expression of collagen Type II. All of the detected effects of E2 were abolished after treatment with ICI182780.
Conclusion: 17β-E2 inhibits IVDD by down-regulating MMP-3 and MMP-13 and up-regulating collagen Type II in a rat model.
Introduction
Chronic neck or lower back pain due to intervertebral disc degeneration (IVDD) are common clinical disorders among adults which have a heavy impact on the economy and quality of life of the patients [Citation1–4]. With the application of modern technology, including examination, imaging investigations and trials of intervention, the causes of the syndrome have been studied continuously [Citation5,Citation6]. Multiple factors, including ageing, living conditions, biomechanical loading and genetic factors are related to the degenerative progression of IVDD, the etiology of which is difficult to define precisely. Mild disc degenerative changes were found as early as the second decade and increased in incidence with age [Citation7,Citation8]. Severe disc degeneration leads to loss of boundary between anulus fibrosus (AF) and nucleus pulposus (NP) [Citation9].
Apoptosis, which is involved in many physiological processes, is characterized by histogenesis during prenatal development and maintenance of the immune system and IVDD is considered to be uncontrolled apoptosis [Citation10]. IVDD is considered to begin with changes in the local cellular microenvironment and progresses to destruct their structure and function [Citation11]. It has been reported in previous studies that reduction in active cell numbers and catabolism of the extracellular matrix (ECM), due to excessive disc cell apoptosis, are the key drivers in the process of IVDD in vivo or in vitro [Citation12–15]. The major disc proteoglycan aggrecan becomes degraded; this leads to decreased water-binding capacity and thus to less NP hydration.
Furthermore, aberrant apoptosis and accelerated aging of NP cells (NPCs) have been demonstrated to be two major cellular processes in IVDD, which imposes a profound influence and further compromises normal function of the intervertebral disc (IVD) [Citation16,Citation17]. Previous studies have shown that normal metabolism is imbalanced, which is closely related to IVDD [Citation18]. Intracellular matrix metalloproteinases (MMPs), including MMP-3 and MMP-13 belonging to the family of zinc-dependent endopeptidases, can regulate metabolic processes of ECM by specifically degrading ECM components [Citation19–24]. Degradation of ECM, especially proteoglycan aggrecan, leads to reduced water-binding capacity [Citation25]. The regulation of MMP activity occurs at the stages of gene transcription, translation, proenzyme activation, inhibition of activated enzymes and enzyme degradation.
In previous studies, 17β-Estradiol (E2) has been reported for its function of inhibiting apoptosis induced by interleukin-1 b to protect rat annulus fibrosis cells. NPCs and downregulation of MMP-3 and MMP-13 were involved in the protective mechanism in in vitro studies [Citation26,Citation27]. However, whether E2 has a protective effect in vivo in rats remains unclear. Thus, the aim of the study was to ascertain in a rat-tail model, whether E2 protects against rat intervertebral disc degeneration and to decipher the implication of MMPs’ regulation in this process.
Materials and methods
Ethics statement
Animal protocols were approved by the Institutional Animal Care and Use Committee of the Third Hospital of Hebei Medical University (IACUC number: Z2016–012-1).
Rat coccyx intervertebral disc degeneration model
A total of 40 females, 3-month-old Sprague Dawley (SD) rats with similar size (weighting, 300 ± 18 g) were included in this study. Needle puncture technique was used to induced the animal model of caudal disc degeneration. Each rat tail was punctured with the 20 G needle gauge (Terumo Standard, Hypodermic needles, Somerset, NJ) percutaneously under image guidance (Terumo Medical Corporation, Somerset, New Jersey) at the discs between fifth to eighth coccygeal vertebrae [Citation28,Citation29]. Later the rats were randomly assigned to four groups (n = 10): Sham, ovariectomy (OVX), E2 and ICI. Sham group only underwent the resection of a bit fat; OVX group underwent bilateral ovariectomy; E2 group was treated with E2 (25 μg/kg body weight, Sigma-Aldrich, St. Louis) dissolved in a vehicle of 95% corn oil and 5% benzyl alcohol [Citation28] based on OVX and ICI group was treated with E2 and ICI182780 (25 μg/kg, inhibitor of the estrogen receptor) pretreatment for half an hour based on OVX. As shown in , all the experimental rats received ovariectomy surgery after three days of environmental adaptation [Citation30]. Animals were given drug intervention 28 continuous days and housed under a 12 h light and 12 h dark cycle with water ad libitum free and restricted food consumption (standard laboratory chow diet from the Animal Center of Genetics and Developmental Biology Laboratory, Beijing, China) to minimize weight gain associated with OVX [Citation31]. Two rats were killed by incision infection, two by gastroenteritis and one by anesthetic, finally the Sham and the OVX groups had eight, the ICI had nine and the E2 had all 10 rats left. Lastly, 4 h post the last drug administration fresh rat tails were harvested.
Figure 1. Ovariectomy (OVX). Chloral hydrate was used for rats to be administered general anesthesia; a line was marked as the surgical incision, about 1.5 cm long on the position of 1 cm below the costal margin and 1.5 cm beside the spine. (A) The skin around the surgical incision was shaved; (B) and (C) incised the skin, entered the peritoneal cavity, found the ovaries along with the adipose tissue exposed in the incision and removed the ovaries; (D) sutured the skin.
General anesthesia
Ten percent chloralhydrate (350 mg/kg) was administered as the general anesthesia. To control postoperative pain, buprenorphine (0.01 mg/kg) was used once before recovery and then as needed. Atipamezole hydrochloride (0.2 mg/kg) was administered to reverse anesthesia.
Radiograph analysis
The radiograph was used to evaluate the degree of intervertebral disc degeneration by measuring the disc height index (DHI). Rats were divided into four groups as previously described: Sham, OVX, E2and ICI. With the general anesthesia as described above, all rats were administered for anteroposterior and lateral radiographs of the tails were captured just before the the animals were sacrificed. During the radiography, to avoid disc height errors caused by muscle contractions, 2 L/min oxygen flow and 2% isoflurane concentration for general anesthesia were consistently used to maintain a similar degree of muscle relaxation. Muscle relaxation was determined by examining disappearance of lower extremity muscle tone. The rats were placed in anteroposterior and lateral positions with tails straight and then the radiographs of Co5/Co6, Co6/Co7and Co7/Co8 were taken, scanned (Epson Perfection V750 Pro; Epson, Long Beach, CA) and stored digitally with an image capture software digitally .
All images were measured independently by two surgeons who were blinded to the treatment and group of each trail to avoid biased response. The DHI of the digitalized radiograph was analyzed using the public domain image analysis program of the US National Institutes of Health (Scion software; Scion Corp., Frederick, MD). The average intervertebral disc height of Co5/Co6, Co6/Co7and Co7/Co8 on the anteroposterior radiograph was defined as DHI and data was obtained from the middle portion of adjacent cranial end plate width in each intervertebral disc [Citation32].
Histological analysis
Discs and adjacent vertebrae were harvested from the tails, fixed in 10% neutral formalin, decalcified rapidly with microwave and nitric acid at low temperature, dehydrated and finally embedded in paraffin. The serial midsagittal sections were cut into five-micron through the specimens parallel to the direction of the stab. The sections were stained with hematoxylin and eosin (HE) and the cell morphologic changes of discs tissue were observed under optical microscope. Disc sections for histology were divided into four groups based on the previous rat groups: Sham, OVX, E2and ICI. According to the previously published grading scales, the modified histological grading of HE staining was used to assess morphological changes in both annulus fibrosus (AF) and NP (). The range of grading is from 0–3, where zero point shows normal structure for each portion (NP and AF) and three points represents the most severe degeneration. A randomized blinded method was used to analyze all histological sections by two observers, in keeping with inter-observer reliability.
Table 1. Histological grading scores (H&E) of AF and NP.
Immunohistochemistry
Immunohistochemistry was performed to detect the expression of caspase-3, MMP-3 and MMP-13 in the degenerative intervertebral discs. The paraffin sections of discs and adjacent vertebrae were harvested and divided into four groups as mentioned earlier: Sham, OVX, E2and ICI. First, sections were deparaffinized with xylene and rehydrated with different concentrations of ethanol. Second, sections were treated with 1 × ethylene Diamine Tetra-acetic Acid(EDTA, PH = 8), antigen repair solution by high pressure heat-induced epitope retrieval for 2 min to revealed the activity of endogenous antigens. Third, sections were repaired with 3% hydrogen peroxide in methanol for 15 min in the dark to block the activity of endogenous peroxidase. Fourth, rabbit monoclonal antibody to caspase-3, MMP-3 and MMP-13 were applied at a dilution of 1:100, 1:100, 1:25, respectively, overnight at 4 °C. Fifth, the signal was detected using the diaminobenzidine (DAB) color reagent. Finally, the sections were counterstained with hematoxylin and were observed under light microscope.
Western blot analysis
Western blot analysis were performed to quantitatively detect the expression of caspase-3, MMP-3, MMP-13 and collagen Type II in the degenerative intervertebral discs. This was accomplished by performing the following steps: (1) Fresh intervertebral disc tissues were dissected from the rat tail, then crushed with a mortar and pestle in ice-cold radioimmunoprecipitation assay (RIPA) buffer (0.4 mM phenylmethylsulfonyl fluoride, 1 μM Pepstatin A, 1 mM Iodo and a phosphatase inhibitor cocktail) and were finally placed for 1 h in an ice bath. (2) The lysates were collected after tissue and cell debris was removed using a Heraeus Sepatech Rotor (Marshall Scientific, Hampton, NH) at 11,000 rpm for 15 min. (3) The total protein content was measured with a BCA protein assay and then were degenerated in boiling water bath for 5 min. (4) Later the proteins were separated by 12% polyacrylamide gel electrophoresis under 110 mV and were then transferred to a nitrocellulose membrane with 200 mA for 2 h. (5) The membrane was blocked with 5% nonfat dry milk in phosphate buffered saline with Tween 20 for 1 h at room temperature and primary antibodies of caspase-3, MMP-3 and MMP-13 (Bioworld (St. Louis Park, MN) diluted to 1:1000) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Proteintech (Rosemont, IL) diluted to 1:4000) overnight at 4 °C (6) After rinsing, the membranes were incubated with secondary antibody (anti-rabbit IgG, Bioworld, diluted to 1:4000) marked with horse radish peroxidase (HRP) rabbit for 1 h at room temperature. (7) Digital gel imaging (Chemi Imager 4000, Alpha Innotech, San Leandro, CA ) was used to scan and analyze the result.
Quantitative real-time PCR (qRT-PCR)
The qRT-PCR is used to detect expression level of mRNA, including caspase-3, MMP-3, MMP-13 and collagen Type II. This was accomplished by performing the following steps: (1) Isolation of total RNA with TRIzol (Solarbio, Beijing, China) according to the manufacturer’s instructions. (2) Measurement of total RNA was performed fluorometrically with a CyQuant-Cell Proliferation Assay Kit (Molecular Probes, Thermo Fisher Scientific Co., Waltham, MA). (3) The primers were designed with Primer Premier Version 5.0 software and their efficiency test was performed by sequencing their products of conventional PCR (). (4) mRNA were utilized as templates to perform cDNA synthesis using a ThermoScript RT-qPCR System (Invitrogen, Shanghai, China). (5) Real time PCR was performed with a DyNAmo SYBR Green 2-step RT-qPCR Kit (Promega, Madison, WI) in a total volume of UI following the protocols at 95 °C for two minutes, then 40 cycles of 95 °C for 15 s and finally 60 °C for one minute. (6) Standard curves for each assay was generated to make a linear plot of threshold cycle (Ct) against log (dilution). (7) The amount of target according to the concentration of the standard curve and acquisition of relative Ct value was quantified. (8) Relative gene expression data was calculated according to the 2−ΔΔCT method, with GAPDH as a housekeeping control.
Table 2. Sense and antisense sequences of primers.
Statistical analysis
All statistical analyses were carried out with SPSS software version 13.0 (SPSS, Inc., Chicago, IL). The results are expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was used for the effect of E2 after IVDD on radiographic disc height analysis and histological grading changes, followed by Student-Newman-Keuls test for significant pairwise differences. The statistical significant value was set at p < .05 in the univariate analyses.
Results
E2 treatment prevents intervertebral disc shrinkage in rat model
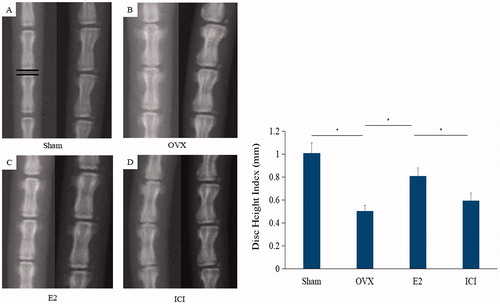
To explore the effects of E2 on the height of intervertebral discs, forty rats were divided into four groups and treated as described in the “Materials and methods” section. Compared with the sham, radiographs showed marked intervertebral disc narrowing in the OVX group (1.186 ± 0.054 vs. 0.494 ± 0.057, p < .05), which was reversed by E2 (0.494 ± 0.057 vs. 1.017 ± 0.056, p < .05). However, the protective effect of E2 on disc height was inhibited by ICI182780 (inhibitor of the estrogen receptor) (1.017 ± 0.056 vs. 0.525 ± 0.028, p < .05; ).
Figure 2. The radiographs were used to measure DHI. On the left is a anteroposterior film and right is lateral film. The average intervertebral disc height of Co5/Co6, Co6/Co7 and Co7/Co8 was defined as DHI. Forty, three-month-old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2 and ICI. Sham group only underwent the resection of a bit of fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX and ICI group was treated with 17β-Estradiol and were also pretreated with ICI182780 (inhibitor of the estrogen receptor) based on OVX (Mean ± SD; n = 10). DHI: Disc Height Index; E2: 17β-Estradiol. *p < .05.
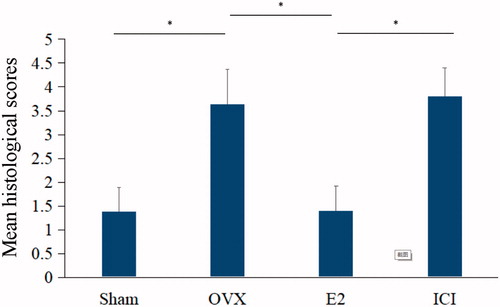
At the histological level, in absence of estradiol for 28 days, a loss of well-distributed pattern, as well as a gradual reduction in the number of NP cells with some areas of clustering, were clearly observed in the OVX group, testifying to the tissue’s degradation. The following specific changes were observed: the percentage of normal cells decreased significantly; the shapes of cells were irregular and some of them were swollen; the nuclear chromatin in the outer nuclear layered gathered toward the center with an uneven distributionand some of the cells were pink without nuclei. Interestingly, these signs of histological deterioration on IVD were reversed by E2 treatment (). Significant differences in histological scores were found between the sham and E2 group (p < .05). However, the anti-degeneration effect of E2 on histological grades was significantly inhibited by ICI182780 (inhibitor of the estrogen receptor, p < .05; ).
E2 regulates the translation and gene expression of proteins involved in ECM homeostasis
Figure 3. The sections of rat intervertebral disc tissues were stained with HE. Forty, 3-month-old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2 and ICI. Sham group only underwent the resection of a bit fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX; ICI group was treated with 17β-Estradiol and were also pretreated with ICI182780 (inhibitor of the estrogen receptor) based on OVX (Mean ± SD; n = 10). HE: hematoxylin and eosin; E2: 17β-Estradiol. * p < .05.
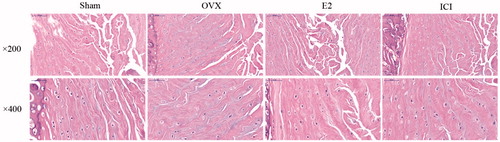
Figure 4. Histological grading scores of rat intervertebral disc tissues were obtained from HE staining. Forty, three-month old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2 and ICI. Sham group only underwent the resection of a bit of fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX and ICI group was treated with 17β-Estradiol and were also pretreated with ICI182780 (inhibitor of the estrogen receptor) based on OVX (Mean ± SD; n = 10). HE: hematoxylin and eosin; E2: 17β-Estradiol. *p < .05.
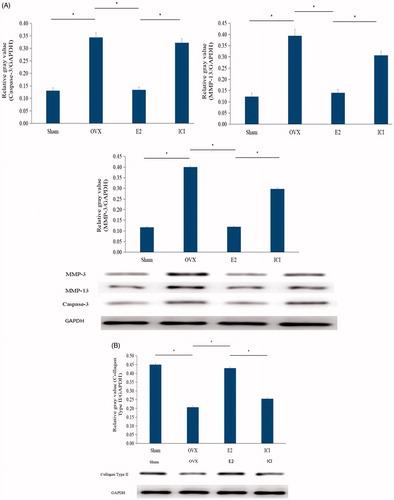
Having found that E2 treatment prevents intervertebral discs narrowing and degeneration as confirmed by radiographic and histological results, we sought to investigate the molecular mechanisms involved in this process. To this end, proteins were harvested from degenerated intervertebral discs and the level of expression of proteins implicated in the ECM regulation, namely caspase-3, MMP-3, MMP-13 and collagen Type II, were quantitatively determined by Western blot experiments. Compared with the sham group, the OVX group showed a significant increase on the expression of caspase-3, MMP-3 and MMP-13 (p < .05), but a decrease in the level of expression of collagen Type II (). On the other hand, E2 downregulated caspase-3, MMP-3 and MMP-13 proteins level but upregulated collagen Type II (). Importantly, treatment with ICI182780 showed comparable results with the OVX group, characterized by an increase in caspase-3, MMP-3 and MMP-13 but decrease in collagen, as shown in (; p < .05). These results indeed suggested that E2 may prevent intervertebral discs degradation via the regulation of these enzymes involved in the homeostasis of the ECM.
Figure 5. Protein level of caspase-3, MMP-3, MMP-13 and collagen Type II. Forty, three-month-old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2 and ICI. Sham group only underwent the resection of a bit of fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX; ICI group was treated with 17β-Estradiol and were also pretreated with ICI182780 (inhibitor of the estrogen receptor) based on OVX (Mean ± SD; n = 10). MMP-3: matrix metalloproteinase-3; MMP-13: matrix metalloproteinase-13; E2: 17β-Estradiol. *p < .05.
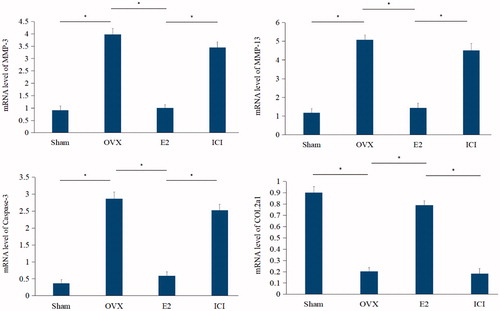
To further investigate these processes at the molecular level, the gene expression of these proteins was ascertained by qRT-PCR experiments. Compared with the sham, the mRNA levels of OVX group presented a significant (p < .05) increase in the expression of the genes caspase-3, MMP-3 and MMP-13, but decrease in COL2a1. The addition of E2 had the opposite effect on mRNA expression levels compared to the OVX group, which was further reversed by the addition of ICI182780 (p < .05) as shown in .
E2 modulates ECM regulators proteins in rat intervertebral discs
Figure 6. Quantitative real-time PCR (qRT-PCR) analysis. The mRNA level of caspase-3, MMP-3, MMP-13 and collagen Type II. Forty, three-month-old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2 and ICI. Sham group only underwent the resection of a bit of fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX and ICI group was treated with 17β-Estradiol and were also pretreated with ICI182780 (inhibitor of the estrogen receptor) based on OVX (Mean ± SD; n = 10). MMP-3: matrix metalloproteinase-3; MMP-13: matrix metalloproteinase-13; E2: 17β-Estradiol. *p < .05.
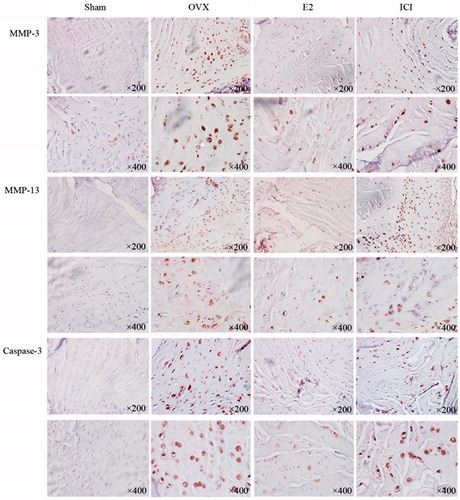
To confirm our findings at histological level, we analyzed caspase-3, MMP-3 and MMP-13 levels by immunohistochemistry staining of all degenerated intervertebral discs. The expression of MMP-3 and MMP-13, which could degrade specific ECM, components was strongly positive in the paracellular area of NP and at the border of NP and AF, whereas the expression of collagen Type II, a major component of the ECM, was weakened. The positive staining of MMP-3 and MMP-13 became significantly weakened, while collagen Type II was strengthened when treated with E2 for 28 days. The expression of caspase-3 was obvious in the paracellular area of NP and AF during the process of degeneration. Little caspase-3 expression was identified within the E2 group. However, the anti-apoptosis effect of E2 was entirely reversed by ICI182780, as shown in .
Figure 7. Immunohistochemistry was performed to detect the expression of caspase-17, MMP-3 and MMP-13. Forty 3-month-old female Sprague Dawley rats were randomly divided into four groups: Sham, OVX, E2, ICI. Sham group only underwent the resection of a bit of fat; OVX group underwent bilateral ovariectomy; E2 group was treated with 17β-Estradiol based on OVX; ICI group was treated with 17β-Estradiol and pretreated ICI182780 (inhibitor of the estrogen receptor) based on OVX ((Mean ± SD; n = 10). MMP-3: matrix metalloproteinase-3; MMP-13: matrix metalloproteinase-13; E2: 17β-Estradiol. *p < .05).
Discussion
In recent years, increased attention has been given to the effects of chronic neck or lower back pain (LBP); these conditions not only bring about physical and psychological pain to the patients, but also result in numerous socio-economic burdens. In natural populations, up to 70–80% of adults have experienced at least one incident of LBP in their lifetime [Citation33]. IVDD is widely recognized in the scholarly literature as a premise and foundation of intervertebral disc herniation. As the exact pathological mechanism of IVDD has not been confirmed, current treatments for IVDD including conservative approaches and surgical procedures such as spine fusion and discectomy only partially relieve symptoms and do not prevent the occurrence and development of disease, which often results in disc disease recurrence and the need for repeated treatment [Citation34,Citation35]. Along with the rapid development of modern cell and molecular biology, the process of IVDD has been thought to have a close relevance to the spontaneous generation of a considerable number of cytokines, such as MMPs and IL-1, tumor necrosis factor and others [Citation13,Citation36,Citation37]. As reported in the previous studies, E2 has been proven to inhibit disc cell apoptosis in vitro [Citation18,Citation27]. In addition, to our knowledge, few studies have revealed effects of E2 in vivo in rats. Therefore, the aim of this article was to further explore the effect and possible mechanism of E2 in vivo in a rat intervertebral disc degeneration model. The results strongly indicate that E2 is a potent inhibitor of IVDD progression.
In this study, our data precisely show that E2 protects against IVDD in vivo in a rat intervertebral disc degeneration model. Results further reveal that the anti-degeneration mechanism is closely related to the down-regulation of MMP-3 and MMP-13, in addition to down-regulation of the protein level of caspase-3 and up-regulation of the protein level of type II collagen. However, no significant difference is observed between MMP-3 and MMP-13 when applying Western blot and immunohistochemistry analysis at the protein level. The result suggests that the degradation effect of MMP-3 and MMP-13 on ECM seems to be equal. Similarly, Yang et al. [Citation27] reported that E2 could inhibit apoptosis of rat nucleus pulposus cells via down-regulation of MMP-3 and MMP-13. Wang et al. [Citation38] found that E2 could protect against apoptosis in human NPCs in vitro. When discs were pretreated with ICI182780, an inhibitor of E2, we found that the protein levels of MMP-3, MMP-13 and caspase-3 were increased; however, the protein level of collagen Type II was reduced, which further confirmed the anti-degenerative effect of E2.
From lateral radiographic and histological analyses, significant differences were found in the E2 groups when compared with control groups after intervention for 28 days. The intervertebral disc height of the E2 group was better than that of the control groups, which suggested that a loss of part of the material used to sustain normal structure led to the loss of thickness of disc at macroscopy. The IVD is composed of the NP, AF and ECM. NP cells play an important role in maintaining the balance and stability of the ECM [Citation38]. It has been reported in previous studies that reduction in active cell numbers and ECM, due to excessive disc cell apoptosis, is the key link in the process of IVDD in vivo or in vitro. The current study shows that, as the main components of the ECM, type II collagen and proteoglycans can play crucial roles in enabling discs to maintain water content, enhance elasticity and toughness and effectively withstand a mechanical stress [Citation38]. Balanced metabolism of ECM is closely correlated to IVDD. The normal morphology and function of ECM are maintained through balancing anabolic metabolism and catabolism. Once the delicate balance metabolism from cells or ECM ever fails, the degeneration occurs and develops continuously. MMPs including MMP-3 and MMP-13 have been proven to have the function of regulating ECM in a large number of in vitro studies [Citation39–41]. In addition, Wang et al. [Citation42] has reported that MMP-3 and MMP-13 have collagenolytic activity to collagen type I–III. Caspase-3, a kind of cysteine proteinase, is considered to be the key enzyme causing apoptosis. Once activated by signaling pathways, caspase-3 can degrade proteins in cells to induce apoptosis. Therefore, increased expression of caspase-3 suggests that chronic irreversible cell death, results in changes in the number and morphology of disc cells during IVDD. As far as we know, few studies have reported on an in vivo rat-tail intervertebral disc degeneration model treated by E2, which was closer to the real physiological process than of that in in vitro studies.
This work still has some limitations. First, the appropriate concentration range and action time of E2 that protected against IVDD has not been explored in the study, which needs to be studied to explore the appropriate concentration range of E2 that protected the NPCs. Second, the optimal size and time of the needle puncture technique were not studied and the best animal models might have more accurate results. Even though we chose in vivo study, we still need a lot of work to assess more comprehensive anti-apoptosis mechanism of signal transduction of E2 in further studies.
Conclusions
E2 protects against rat intervertebral disc degeneration by down-regulating MMP-3 and MMP-13 and up-regulating type II collagen in an in vivo rat-tail model. To the patients and clinicians fighting against the disease, the study demonstrates that MMP-3 and MMP-13 may be important targets for prevention and E2-based treatment of IVDD in both early and advanced stages.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Jha SC, Higashino K, Sakai T, et al. Clinical significance of high-intensity zone for discogenic low back pain: a review. J Med Invest. 2016;63:1–7.
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8–20.
- Beattie PF, Silfies SP. Improving long-term outcomes for chronic low back pain: time for a new paradigm. J Orthop Sports Phys Ther. 2015;45:236–239.
- Samini F, Gharedaghi M, Khajavi M, et al. The etiologies of low back pain in patients with lumbar disk herniation. Iran Red Crescent Med J. 2014;16:e15670.
- Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–187.
- Luoma K, Riihimäki H, Luukkonen R, et al. Low back pain in relation to lumbar disc degeneration. Spine. 2000;25:487–492.
- Niemeläinen R, Battié MC, Gill K, et al. The prevalence and characteristics of thoracic magnetic resonance imaging findings in men. Spine. 2008;33:2552–2559.
- Wang H, Zou F, Jiang J, et al. The correlation between ossification of the nuchal ligament and pathological changes of the cervical spine in patients with cervical spondylosis. Spine. 2014;39:B7–B11.
- Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207.
- Wang SZ, Rui YF, Lu J, et al. Cell and molecular biology of intervertebral disc degeneration: current understanding and implications for potential therapeutic strategies. Cell Prolif. 2014;47:381–390.
- Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology. 2009;48:5–10.
- Ha KY, Koh IJ, Kirpalani PA, et al. The expression of hypoxia inducible factor-1alpha and apoptosis in herniated discs. Spine. 2006;31:1309–1313.
- Séguin CA, Pilliar RM, Roughley PJ, et al. Tumor necrosis factor-alpha modulates matrix production and catabolism in nucleus pulposus tissue. Spine. 2005;30:1940–1948.
- Singh K, Masuda K, An HS. Animal models for human disc degeneration. Spine J. 2005;5:267S–279S.
- Smith LJ, Nerurkar NL, Choi KS, et al. Degeneration and regeneration of the intervertebral disc: lessons from development. Dis Model Mech. 2011;4:31–41.
- Vo NV, Hartman RA, Patil PR, et al. Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016;34:1289–1306.
- Le MCL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007;9:R45.
- Pinto JM, Wroblewski KE, Huisingh-Scheetz M, et al. Global sensory impairment predicts morbidity and mortality in older US adults. J Am Geriatr Soc. 2017;65(12):2587–2595.
- Mavrogonatou E, Angelopoulou MT, Kletsas D. The catabolic effect of TNFα on bovine nucleus pulposus intervertebral disc cells and the restraining role of glucosamine sulfate in the TNFα-mediated up-regulation of MMP-3. J Orthop Res. 2014;32:1701–1707.
- Wang X, Wang H, Yang H, et al. Tumor necrosis factor-α- and interleukin-1β-dependent matrix metalloproteinase-3 expression in nucleus pulposus cells requires cooperative signaling via syndecan 4 and mitogen-activated protein kinase-NF-κB axis: implications in inflammatory disc disease. Am J Pathol. 2014;184:2560–2572.
- Liu H, Pan H, Yang H, et al. LIM mineralization protein-1 suppresses TNF-α induced intervertebral disc degeneration by maintaining nucleus pulposus extracellular matrix production and inhibiting matrix metalloproteinases expression. J Orthop Res. 2015;33:294–303.
- Gu SX, Li X, Hamilton JL, et al. MicroRNA-146a reduces IL-1 dependent inflammatory responses in the intervertebral disc. Gene. 2015;555:80–87.
- Yang K, Palm J, König J, et al. Matrix-metallo-proteinases and their tissue inhibitors in radiation-induced lung injury. Int J Radiat Biol. 2007;83:665–676.
- Liu C, Wan X, Ye T, et al. Matrix metalloproteinase 2 contributes to pancreatic beta cell injury induced by oxidative stress. PLoS One. 2014;9:e110227.
- Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol. 2007;53:4–18.
- Coleto I, de la Peña M, Rodríguez-Escalante J, et al. Leaves play a central role in the adaptation of nitrogen and sulfur metabolism to ammonium nutrition in oilseed rape (Brassica napus). BMC Plant Biol. 2017;17:157.
- Yang SD, Ma L, Gu TX, et al. 17β-Estradiol protects against apoptosis induced by levofloxacin in rat nucleus pulposus cells by upregulating integrin α2β1. Apoptosis. 2014;19:789–800.
- Iwaniec UT, Magee KA, Mitova-Caneva NG, et al. Bone anabolic effects of subcutaneous treatment with basic fibroblast growth factor alone and in combination with estrogen in osteopenic ovariectomized rats. Bone. 2003;33:380–386.
- Keorochana G, Johnson JS, Taghavi CE, et al. The effect of needle size inducing degeneration in the rat caudal disc: evaluation using radiograph, magnetic resonance imaging, histology, and immunohistochemistry. Spine J. 2010;10:1014–1023.
- Wang L, Wu Y, Tan Y, et al. Cytotoxic effects of the quinolone levofloxacin on rabbit meniscus cells. J Appl Toxicol. 2014;34:870–877.
- Iwaniec UT, Moore K, Rivera MF, et al. A comparative study of the bone-restorative efficacy of anabolic agents in aged ovariectomized rats. Osteoporos Int. 2007;18:351–362.
- Han B, Zhu K, Li FC, et al. A simple disc degeneration model induced by percutaneous needle puncture in the rat tail. Spine. 2008;33:1925–1934.
- Madigan L, Vaccaro AR, Spector LR, et al. Management of symptomatic lumbar degenerative disk disease. J Am Acad Orthop Surg. 2009;17:102–111.
- Froud R, Patterson S, Eldridge S, et al. A systematic review and meta-synthesis of the impact of low back pain on people's lives. BMC Musculoskelet Disord. 2014;15:50.
- Phillips FM, Reuben J, Wetzel FT. Intervertebral disc degeneration adjacent to a lumbar fusion. An Experimental Rabbit Model. J Bone Joint Surg Br. 2002;84:289–294.
- Haro H, Komori H, Kato T, et al. Experimental studies on the effects of recombinant human matrix metalloproteinases on herniated disc tissues–how to facilitate the natural resorption process of herniated discs. J Orthop Res. 2005;23:412–419.
- Le MCL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–R745.
- Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23:1057–1070.
- Peck SH, McKee KK, Tobias JW, et al. Whole transcriptome analysis of notochord-derived cells during embryonic formation of the nucleus pulposus. Sci Rep. 2017;7:10504.
- Li Z, Liu B, Zhao D, et al. Omentin-1 prevents cartilage matrix destruction by regulating matrix metalloproteinases. Biomed Pharmacother. 2017;92:265–269.
- Basaran R, Senol M, Ozkanli S, et al. Correlation of matrix metalloproteinase (MMP)-1, -2, -3, and -9 expressions with demographic and radiological features in primary lumbar intervertebral disc disease. J Clin Neurosci. 2017;41:46–49.
- Kang L, Hu J, Weng Y, et al. Sirtuin 6 prevents matrix degradation through inhibition of the NF-κB pathway in intervertebral disc degeneration. Exp Cell Res. 2017;352:322–332.
