Abstract
Prevention of fibrosis and urethral scar formation is critical for a successful urethral reconstruction. We have previously shown that epithelial-differentiated adipose-derived stem cells (EASC) seeded bladder acellular matrix grafts (BAMG) can be used for urethral reconstruction. We have also shown that suppression of tissue inhibitor of metalloproteinases-1 (TIMP-1) reduces epithelial–mesenchymal transition in urethral fibroblasts in vitro and in vivo. However, it is unknown whether suppression of TIMP-1 in EASC seeded BAMG may benefit urethral reconstruction through inhibition of fibrosis. Here, we addressed this question. In a rabbit substitution urethroplasty model, we found that E-cadherin + EASC resulted in wider urethral caliber and formation of less urethral scar tissue, compared to non-purified EASC. Bioinformatics study showed that among all TIMP-1-targeting microRNAs (miRNAs), miR-365 is a conserved one in rabbits and humans, and functionally inhibits TIMP-1 protein translation. MiR-365-transduced E-cadherin + EASC seeded BAMG further reduced fibrosis and increased urethral caliber width during urethral reconstruction in rabbits, compared to E-cadherin + EASC seeded BAMG. Together, these data suggest that EASC seeded BAMG method for urethral reconstruction could be further improved through purification of EASC by E-cadherin and through post-transcriptional inhibition of TIMP-1 via miR-365 in EASC.
Introduction
Inflammation and injuries to ureter may lead to urethral stricture, a fibrotic process that may result in constricted urethral lumen [Citation1]. The molecular mechanisms underlying the formation of urethral stricture include activation of urethral fibroblasts, transformation of fibroblasts into myofibroblasts and augmentation of collagen deposition in extracellular matrix [Citation2]. In these processes, the matrix metalloproteinases (MMPs) family members [Citation3] and tissue inhibitor of metalloproteinases 1 (TIMP-1) have been found to play a pivotal role.
MMPs are calcium-dependent zinc-containing endopeptidases that take charge of degradation of all kinds of extracellular matrix proteins, which is important for cell proliferation, adhesion, migration and differentiation, angiogenesis and immunological responses [Citation4]. MMPs are synthesized as inactive pro-peptides, the activation of which requires catalysis by enzymes like TIMP-1 [Citation5]. TIMP-1 is a ubiquitously expressed glycoprotein that regulates extracellular matrix turnover through control of the activity of MMPs [Citation5–7]. Recently, high levels of TIMP-1 were detected in urethral scar tissues, suggesting that it may play a critical role in the fibrosis during urethral reconstruction [Citation8–11]. We have previously shown that epithelial-differentiated adipose-derived stem cells (EASC) [Citation12–15] seeded bladder acellular matrix grafts (BAMG) can be used for urethral reconstruction [Citation16]. We have also shown that suppression of tissue inhibitor of metalloproteinases-1 (TIMP-1) reduces epithelial–mesenchymal transition in urethral fibroblasts in vitro [Citation17] and in vivo [Citation18]. However, it is unknown whether suppression of TIMP-1 in EASC seeded BAMG may benefit urethral reconstruction through inhibition of fibrosis.
MicroRNAs (miRNAs) are non-coding small RNAs that regress mRNA translation through Watson–Crick pairing to the 3′-untranslated region (3′-UTR) of the target mRNA. Typically, a specific gene could be regulated by a number of different miRNAs, while an individual miRNA could different target mRNAs from a variety of genes [Citation19–22]. Previous studies have revealed several miRNAs that may anchor 3′-UTR of TIMP-1 mRNA, like miR-1293 [Citation23] and miR-29a [Citation24]. However, to the best of our knowledge, a role of miR-365 in control of TIMP-1 protein translation has not been reported.
Thus, in the current study, we addressed these questions. Using bioinformatics tools, molecular biology techniques and a rabbit substitution urethroplasty model, we conclude that EASC seeded BAMG method for urethral reconstruction could be further improved through purification of EASC by E-cadherin and through post-transcriptional inhibition of TIMP-1 via miR-365 in EASC.
Materials and methods
Ethics statement
This investigation was conducted in accordance with the ethical standards and according to the Declaration of Helsinki and national and international guidelines. The study was approved by the Animal Core Facility at Shanghai Jiaotong University.
Animal model
New Zealand white male rabbits (1.5–2.0 kg; SLAC Laboratory Animal, Shanghai, China) were used in the current study. The rabbits were randomly divided into different experimental groups, and each containing 10 animals. All surgeries were performed by the same surgeon, according to a published protocol [Citation18]. Briefly, a ventral urethral mucosal defect (VUMD) with a mean length of 2.0 cm and mean width of 0.8 cm was created in the rabbit penile urethra about 2.0 cm from the external urethral orifice. The grafts seeded bladder acellular matrix grafts (BAMG) was placed over the urethral mucosal defect using 6–0 vicryl sutures (Ethicon, Somerville, NJ). A urethral catheter was placed for 14 days to provide bladder drainage after the surgery. Retrograde urethrography and histology analysis were done 5 months after surgery.
Isolation, culture and epithelial cell differentiation of ASCs
Isolation, culture and epithelial cell differentiation of ASCs were performed as described [Citation16]. Briefly, the fresh adipose tissues were isolated from the dorsocervical subcutaneous region of male New Zealand rabbits and put into culture dishes for digestion with 0.10% collagenase I (Sigma-Aldrich, St. Louis, MO) in a 37 °C shaking for 60 min. The digestion was terminated by the same volume of fetal bovine serum (FBS; Invitrogen, Carlsbad, CA), after which the suspension was filtered through a 200-mm nylon mesh and then centrifuged at 1200 g for 10 min. The pellet was re-suspended and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen, Carlsbad, CA) supplied with 10% FBS. The ASCs of passage 5 were used for the study. For epithelial differentiation of ASCs, a 3D culture system was used as described [Citation16]. Briefly, ASCs were seeded on the upper side of the membrane of a Millicell insert (1.0-mm pore size; Millpore Co., Billerica, MA) coated with 0.10% collagen type IV (Sigma-Aldrich). The culture media are DMEM supplied with 2% FBS, 2.5 mM all-trans retinoic acid (Sigma-Aldrich), 20 ng/mL epidermal growth factor (R&D System, Los Angeles, CA), 10 ng/mL hepatocyte growth factor (R&D System), 10 ng/mL keratinocyte growth factor (R&D System), and 0.5 mg/mL hydrocortisone (Sigma-Aldrich). After 12 days of induction, the epithelial-differentiated adipose-derived stem cells (EASC) are ready for further study.
Cell transfection and transduction
EASC cells were transfected with miR-365 or an antisense (as) of miR-365, or a null sequence (null) as a control (RiboBio Co. Ltd., Shanghai, China). All plasmid constructs also contained a green fluorescence protein (GFP) reporter, to allow the transfected cells being visualized by green fluorescence. MiR-365 sequence: 5′-GCCCACCUAGUGCUACGUUAAAA-3′, as-miR-365 sequence: 5′-UUUUAACGUAGCACUAGGUGGGC-3′. Transfection was performed with Lipofectamine 3000 reagent (Invitrogen), according to the manufacturer’s instructions. The coding sequence of miR-365 or null was cloned into pLVX-ZsGreen1-C1 vector (Clontech, Mountain View, CA). To generate lentiviral particles, NIH HEK293T cells were seeded in a 100 mm dish at 50,000 cells/cm2 and co-transfected with 10 µg of recombinant DNA plasmids and 5 µg each of packaging plasmids (REV, pMDL and VSV-G) using Lipofectamine-3000 (Invitrogen). The supernatant containing lentiviral particles was collected 48 h after transfection and filtered through a 0.45 µm syringe filter.
Bioinformatics and dual luciferase-reporter assay
The target miRNAs for TIMP-1 in mice and humans was determined by TargetScan, using the context++ score system, as described [Citation25]. Determination of the conservation of the miR-365-binding site on the 3′-UTR of TIMP-1 mRNA between rabbits and human was applied using NCBI database. The dual-luciferase reporter plasmids, p3′-UTR-TIMP-1 (containing the wild-type TIMP-1 3′-UTR binding site in luciferase reporter plasmid and p3′-UTR-TIMP-1-mutate (containing the mutant TIMP-1 3′-UTR) were constructed in RiboBio Co. Ltd. For the luciferase assay, the constructed 3′-UTR plasmid (wild-type or mutate) and miR-365/as-miR-365/null were co-transfected into EASC cells using Lipofectamine™ 3000 Reagent (Invitrogen). Then the luciferase activity was detected with the dual-luciferase reporter assay system (Promega, Shanghai, China) after co-transfection cells for 48 h, following the manufacturer’s protocol.
Cell viability assay
For assay of cell viability, cultured cells were seeded into 96 well-plate at 5000 cells per well and subjected to an MTT Kit (Roche, Indianapolis, IN), according to the instruction from the manufacturer. Quantification was done at 540 nm absorbance value (OD). Experiments were performed five times.
Quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using miRNeasy mini kit (Qiagen, Hilden, Germany) for cDNA synthesis and RT-qPCR performed in duplicates with QuantiTect SYBR Green PCR Kit (Qiagen). All primers were purchased from Qiagen. Data were collected and analysed using 2−△△Ct method. Values of genes were first normalized against α-tubulin and then compared to experimental controls.
Western blot, histology and immunocytochemistry
Protein was extracted from the cultured cells or conditioned media by RIPA buffer (Sigma-Aldrich) for Western Blot. Primary antibodies for Western Blotting are anti-TIMP-1 and anti-α-tubulin (Cell Signaling, San Jose, CA). The secondary antibody is HRP-conjugated anti-rabbit (Jackson Labs, Bar Harbor, ME). Images shown in the figure were representative from five repeats. Densitometry of Western blots was quantified with NIH ImageJ software (Bethesda, MA). Masson-trichrome staining was performed using a Trichrome Stain (Masson) Kit (Sigma-Aldrich). For immunohistochemistry, the primary antibody is rat anti-Ki-67 (Abcam, Cambridge, MA) and anti-TIMP-1 (Cell Signalling). Indirect fluorescent staining was performed with Cy3-conjugated donkey anti-rat secondary antibody (Jackson Labs). Nuclear staining was performed with DAPI (Abcam). Quantification of at least 500 cells was done in 5 repeats in each condition.
Statistical analysis
GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA) was used for statistical analyses. One-way ANOVA with a Bonferroni correction was applied for comparison among several groups. Unpaired two-tailed Student t-test was applied for comparison between two groups. Data were represented as mean ± SD and were considered significant if p < .05.
Results
E-cad + EASC have higher proliferative potential than E-cad − EASC
ASC have been differentiated towards epithelial cells to obtain EASC, as described [Citation16]. After epithelial differentiation, we purified E-cadherin (E-cad)+ EASC and E-cad− EASC by flow cytometry (). E-cad + EASC represented purified epithelial cells differentiated from ASC, while E-cad− EASC represented purified non-epithelial cells failed from the epithelial differentiation from ASC. The aim of this purification is to evaluate if purified E-cad + EASC may have a better therapeutic effect than EASC without this purification on urethral reconstruction. RT-qPCR was performed on the E-cad + EASC versus E-cad− EASC, showing a more than 34-fold increase in the E-cad levels in E-cad + EASC population (). These 2 cell populations were then subjected to an MTT assay, and we detected significantly higher growth rate in E-cad + EASC, compared to E-cad− EASC (). The cell proliferation rate was then evaluated by Ki-67 assay. We detected significantly higher percentage of Ki-67 + cells in E-cad + EASC, compared to E-cad− EASC, shown by quantification (), and by representative images (). Together, these data suggest that E-cad + EASC have higher proliferative potential than E-cad− EASC.
Figure 1. E-cad + EASC have higher proliferative potential than E-cad− EASC. (A) E-cadherin (E-cad)+ EASC and E-cad− EASC were separated by flow cytometry. (B) RT-qPCR for E-cad in E-cad + EASC and E-cad− EASC cell populations. (C) An MTT assay for E-cad + EASC and E-cad− EASC cell populations. (D,E) Analysis of Ki-67+ cells by immunohistochemistry in E-cad + EASC and E-cad− EASC cell populations, shown by quantification (D) and by representative images (E). *p < .05. N = 5. Scale bars are 20 µm.
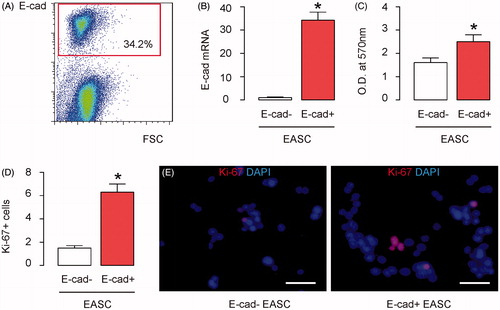
E-cad + EASC seeded BAMG have improved therapeutic effects during urethroplasty in rabbits
Next, a ventral urethral mucosal defect (VUMD) was generated on rabbits, as described [Citation18]. Urethroplasty was then performed with either E-cad + EASC seeded BAMG or EASC (no purification for E-cad) seeded BAMG, as described [Citation16]. The implanted grafts were then evaluated with two parameters. First, retrograde urethrograms were done to examine the width of urethral caliber. Second, Masson-trichrome staining was performed to evaluate the urethral tissue fibrosis and scar formation. We found that compared to VUMD controls (no implants), implants with EASC seeded BAMG increased the width of urethral caliber, shown by representative images (), and by quantification (). Implants with E-cad + EASC seeded BAMG further increased the width of urethral caliber reconstructed by EASC seeded BAMG, shown by representative images (), and by quantification (). Moreover, compared to VUMD controls, implants with EASC seeded BAMG increased the ratio of muscle versus collagen in the reconstructed urethral tissue, which suggests less fibrosis and scar formation, shown by representative images (), and by quantification (). Implants with E-cad + EASC seeded BAMG further increased the ratio of muscle versus collagen in the reconstructed urethral tissue, shown by representative images (), and by quantification (). Together, these data suggest that purification for E-cadherin in EASC improves their therapeutic potential seed BAMG during urethroplasty in rabbits.
Figure 2. E-cad + EASC seeded BAMG have improved therapeutic effects during urethroplasty in rabbits. A ventral urethral mucosal defect (VUMD) was generated on rabbits and then urethroplasty was performed with either E-cad + EASC seeded BAMG or EASC (no purification for E-cad) seeded BAMG. VUMD only (no implants) was used as a control. (A,B) Retrograde urethrograms analysis, shown by representative images (A) and by quantification of the width of urethral caliber (B). (C,D) Masson-trichrome staining for the reconstructed urethral tissue, shown by representative images (C), and by quantification of the ratio of muscle versus collagen (D). *p < .05. N = 10. Scale bars are 50 µm.
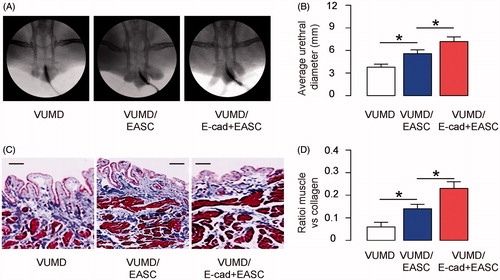
MiR-365 targets 3′-UTR of TIMP-1 to inhibit its protein translation in EASC
Since we have shown that TIMP-1 levels affect the fibrosis during urethral reconstruction [Citation18], and suppression of TIMP-1 reduces epithelial–mesenchymal transition in urethral fibroblasts in vitro [Citation17], we aimed to determine whether suppression of TIMP-1 may be achieved by miRNAs in EASC and if using these modified EASC seeded BAMG may improve urethral reconstruction. Since we used a rabbit urethroplasty and we wanted our study translatable to humans, we screened all TIMP-1-targeting miRNAs that are conserved in rabbits and humans. Specifically, we found that miR-365 was such a miRNA with high score in a bioinformatics analysing system. MiR-365 has a binding site at the 37th–43rd base pair of the 3′-UTR of TIMP-1 mRNA (). Moreover, this binding site is conserved between human and rabbits (). To evaluate whether the binding of miR-365 to 3′-UTR of TIMP-1 mRNA may affect protein translation of TIMP-1, we first transfected E-cad + EASC cells with plasmids carrying miR-365 or as-miR-365 or null as a control. RT-qPCR for miR-365 was performed in transfected cells, which confirmed the effects of miR-365 modification (). Next, the intact 3′-UTR of wild-type TIMP-1 mRNA (wt TIMP-1 3′-UTR) and the 3′-UTR of TIMP-1 mRNA with a mutant at miR-365-binding site (mut TIMP-1 3′-UTR) were respectively cloned into luciferase reporter plasmids. E-cad + EASC cells were then co-transfected with one plasmid from miR-365/as-miR-365/null plasmids and one plasmid from either wt TIMP-1 3′-UTR or mut TIMP-1 3′-UTR, and subsequently subjected to a dual luciferase reporter assay. We found that depletion of miR-365 increased luciferase activity of wt TIMP-1 3′-UTR, while overexpression of miR-365 reduced luciferase activity of wt TIMP-1 3′-UTR but had no effects on mut TIMP-1 3′-UTR (). Alteration of miR-365 levels in E-cad + EASC cells did not alter TIMP-1 mRNA (), but miR-365 overexpression decreased TIMP-1 protein while miR-365 depletion increased TIMP-1 protein, by Western blotting () and by immunocytochemistry (). These results suggest that miR-365 specifically targets 3′-UTR of TIMP-1 mRNA to inhibit its translation in EASC cells.
Figure 3. MiR-365 targets 3′-UTR of TIMP-1 to inhibit its protein translation in EASC. (A) Bioinformatics analysis shows that miR-365 has a binding site at the 36th–42nd base pair of the 3′-UTR of TIMP-1 mRNA. (B) Conservation of the miR-365-binding site on the 3′-UTR of TIMP-1 mRNA between rabbits and human. (C) E-cad + EASC cells were transfected with plasmids carrying miR-365 or as-miR-365 or null as a control. RT-qPCR for miR-365 was performed in transfected cells. (D,E) The intact 3'-UTR of wild-type TIMP-1 mRNA (wild-type TIMP-1 3'-UTR) and the 3'-UTR of TIMP-1 mRNA with a mutant at miR-365-binding site (mutate TIMP-1 3'-UTR) were respectively cloned into luciferase reporter plasmids. E-cad + EASC cells were then co-transfected with one plasmid from miR-365/as-miR-365/null plasmids and one plasmid from either wild-type TIMP-1 3'-UTR or mutate TIMP-1 3'-UTR, and subsequently subjected to a dual luciferase reporter assay. (F) RT-qPCR for TIMP-1. (G) Western blotting for TIMP-1. (H) Immunocytochemistry for TIMP-1. *p < .05. NS: non-significant. N = 5. Scale bars are 20 µm.
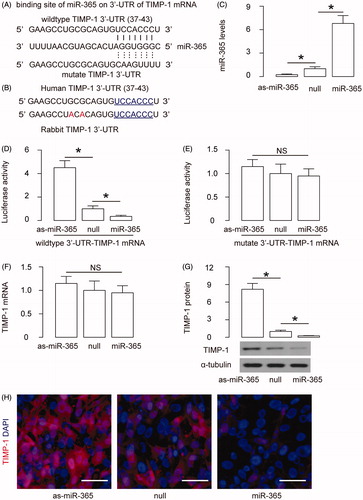
Preparation of miR-365-transduced E-cadherin + EASC
In order to check if the reduction of TIMP-1 by miR-365 in E-cad + EASC cells may further improve their therapeutic potential in urethroplasty, we prepared E-cad + EASC cells that stably express miR-365 by lentiviral transduction. E-cad + EASC cells that were transduced with lentivirus carrying null were used a control. Both lentiviruses have GFP reporter to allow transduced cells to be purified by flow cytometry (). We found that the TIMP-1 mRNA levels were not altered in miR-365-E-cad + EASC cells (). However, the TIMP-1 protein levels were significantly reduced in miR-365-E-cad + EASC cells, compared to null-E-cad + EASC cells, by Western blotting () and by immunocytochemistry (). Thus, these cells were used in the urethroplasty study.
Figure 4. Preparation of miR-365-transduced E-cadherin + EASC. E-cad + EASC cells were transduced with lentivirus expressing miR-365 or null as a control. Both lentiviruses have GFP reporter to allow transduced cells to be purified by flow cytometry. (A) Representative flow charts for miR-365 or null-transduced E-cad + EASC cells. (B) RT-qPCR for TIMP-1. (C) Western blotting for TIMP-1. (D) Immunocytochemistry for TIMP-1. *p < .05. NS: non-significant. N = 5. Scale bars are 20 µm.
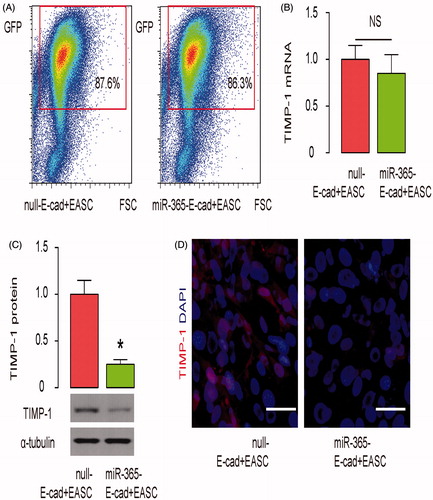
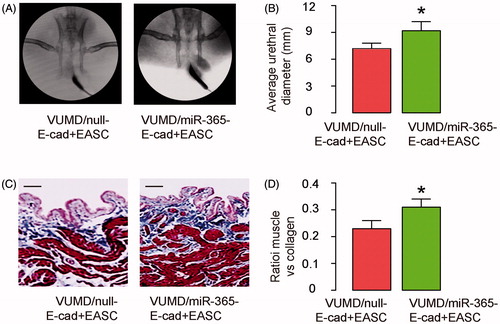
MiR-365-transduced E-cad + EASC seeded BAMG have further improved therapeutic effects during urethroplasty in rabbits
Next, VUMD was generated on rabbits and urethroplasty was then performed with either null-E-cad + EASC seeded BAMG or miR-365-E-cad + EASC seeded BAMG. The implanted grafts were then evaluated. We found that compared to null-E-cad + EASC seeded BAMG, implants with miR-365-E-cad + EASC seeded BAMG further increased the width of urethral caliber, shown by representative images (), and by quantification (). Moreover, compared to null-E-cad + EASC seeded BAMG, implants with miR-365-E-cad + EASC seeded BAMG further the ratio of muscle versus collagen in the reconstructed urethral tissue, shown by representative images (), and by quantification (). Together, these data suggest that miR-365-transduced E-cad + EASC seeded BAMG have further improved therapeutic effects during urethroplasty in rabbits.
Figure 5. MiR-365-transduced E-cad + EASC seeded BAMG have further improved therapeutic effects during urethroplasty in rabbits. VUMD was generated on rabbits and urethroplasty was then performed with either null-E-cad + EASC seeded BAMG or miR-365-E-cad + EASC seeded BAMG. The implanted grafts were then evaluated. (A,B) Retrograde urethrograms analysis, shown by representative images (A) and by quantification of the width of urethral caliber (B). (C,D) Masson-trichrome staining for the reconstructed urethral tissue, shown by representative images (C), and by quantification of the ratio of muscle versus collagen (D). *p < .05. N = 10. Scale bars are 50 µm.
Discussion
The present study is the first to evaluate the application of a combined graft with acellular BSM collagen matrix and genetically modified EASC through miRNA for urethral reconstruction. Previously, we have shown that suppression of TIMP-1 reduces epithelial–mesenchymal transition in urethral fibroblasts in vitro [Citation17] and in vivo [Citation18]. However, we used shRNA to decrease the expression of TIMP-1 in our previous studies and the target cells were fibroblasts in the urethral tissue. Here, we addressed another target, the implanted ASC other than cells from the animals themselves. The reason that here we chose miRNAs rather than shRNA to knockdown TIMP-1 is due to that we surprisingly found that the shRNA that had been used to level down TIMP-1 in our previous studies did not appear to be very effective in ASC (data not shown). This finding suggests that the regulation of TIMP-1 may be not identical in different cell types. Compared to shRNA method, using miRNAs has a similar advantage that the transgene is very small and can be well packaged into lentivectors.
Here, our study showed several novel findings. First, purification of E-cad + EASC appeared to improve the effects on rabbit urethroplasty. Epithelial cells are the most important cells that play crucial roles in the urethral reconstruction. The growth of E-cad + EASC through proliferation provides adequate cell sources for the formation of the urethral wall, and depletion of E-cad− EASC should remove unnecessary cells in the regeneration niche, and thus favour the connection of epithelial cells to form cell–cell junctions for a tightly organized regenerated urethral wall. Moreover, non-epithelial cells in the regeneration site may cause inflammation and result in fibrosis. Indeed, a purification step here showed significant improvement in the outcome of the implant therapy.
Second, miR-365 is found to effectively suppress TIMP-1 protein translation in EASC. To the best of our knowledge, this is the first study to show the presence of the regulation. Indeed, Li et al. recently showed that TIMP-1 was a target of miR-1293 in HEK293 cells [Citation23]. However, no other studies have ever addressed a TIMP-1-regulatory miRNA. Here, our study specifically identified miR-365 as a powerful suppressor of TIMP-1 protein translation in EASC, which are widely used in urethral reconstruction.
Third, based on the findings of the current study and our previous work [Citation16–18], we feel that EASC seeded BAMG method can be a very useful and promising method for urethral reconstruction, and this method could be further improved through purification of EASC by E-cadherin and through post-transcriptional inhibition of TIMP-1 via miR-365 in EASC. In addition, blocking of both endogenous TIMP-1 in remaining urethral tissue and exogenous TIMP-1 in the grafts may be an attractive strategy to be evaluated.
Besides TIMP-1, miR-365 has been shown to target some other proteins. For example, miR-365 was reported to suppress cell growth by targeting CyclinD1 in vascular smooth muscle cells [Citation26,Citation27]. In addition, miR-365 was found to inhibit growth, invasion and metastasis of malignant melanoma by targeting neuropilin1 [Citation28]. Although not tested here in EASC, it may be possible that miR-365 could affect cell cycle to some extent, and this question deserves to be answered in future studies.
Disclosure statement
The authors have declared that no competing interests exist.
References
- Kim BS, Kwon TG. Urethral reconstruction using autologous vein grafts for the management of urethral strictures. Curr Urol Rep. 2015;16:467.
- Zarei F, Soleimaninejad M. Role of growth factors and biomaterials in wound healing. Artif Cells Nanomed Biotechnol. 2018 [cited 2018 Feb 15]; [6 p.]. DOI: 10.1080/21691401.2018.1439836
- Shirzad M, Hamedi J, Motevaseli E, et al. Anti-elastase and anti-collagenase potential of Lactobacilli exopolysaccharides on human fibroblast. Artif Cells Nanomed Biotechnol. 2018 [cited 2018 Feb 27]; [11 p.]. DOI: 10.1080/21691401.2018.1443274
- Eivazy P, Atyabi F, Jadidi-Niaragh F, et al. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231). Artif Cells Nanomed Biotechnol. 2017;45:889–896.
- Ries C. Cytokine functions of TIMP-1. Cell Mol Life Sci. 2014;71:659–672.
- Bodden MK, Windsor LJ, Caterina NC, et al. Analysis of the TIMP-1/FIB-CL complex. Ann N Y Acad Sci. 1994;732:84–95.
- Arthur MJ, Iredale JP. Hepatic lipocytes, TIMP-1 and liver fibrosis. J R Coll Physicians Lond. 1994;28:200–208.
- Huang X, Wei DP, Yang YR. [Detection of collagenase activity and tissue inhibitor of metalloproteinase-1 expression level in the urethral scar tissue]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2003;17:433–435.
- Chen B, Wen Y, Zhang Z, et al. Menstrual phase-dependent gene expression differences in periurethral vaginal tissue from women with stress incontinence. Am J Obstet Gynecol. 2003;189:89–97.
- Chen B, Wen Y, Wang H, et al. Differences in estrogen modulation of tissue inhibitor of matrix metalloproteinase-1 and matrix metalloproteinase-1 expression in cultured fibroblasts from continent and incontinent women. Am J Obstet Gynecol. 2003;189:59–65.
- Gobet R, Bleakley J, Cisek L, et al. Fetal partial urethral obstruction causes renal fibrosis and is associated with proteolytic imbalance. J Urol. 1999;162:854–860.
- Casagrande S, Tiribuzi R, Cassetti E, et al. Biodegradable composite porous poly(dl-lactide-co-glycolide) scaffold supports mesenchymal stem cell differentiation and calcium phosphate deposition. Artif Cells Nanomed Biotechnol. 2017 [cited 2017 Dec 21]; [11 p.]. DOI: 10.1080/21691401.2017.1417866
- Dadashpour M, Pilehvar-Soltanahmadi Y, Mohammadi SA, et al. Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells. Artif Cells Nanomed Biotechnol. 2018;46:819–830.
- Guo DL, Wang ZG, Xiong LK, et al. Hepatogenic differentiation from human adipose-derived stem cells and application for mouse acute liver injury. Artif Cells Nanomed Biotechnol. 2017;45:224–232.
- Jun-Jiang C, Huan-Jiu X. Vascular endothelial growth factor 165-transfected adipose-derived mesenchymal stem cells promote vascularization-assisted fat transplantation. Artif Cells Nanomed Biotechnol. 2016;44:1141–1149.
- Li H, Xu Y, Xie H, et al. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: an animal model. Tissue Eng A. 2014;20:774–784.
- Sa Y, Li C, Li H, et al. TIMP-1 induces alpha-smooth muscle actin in fibroblasts to promote urethral scar formation. Cell Physiol Biochem. 2015;35:2233–2243.
- Guo H, Sa Y, Huang J, et al. Urethral reconstruction with small intestinal submucosa seeded with oral keratinocytes and TIMP-1 siRNA transfected fibroblasts in a rabbit model. Urol Int. 2016;96:223–230.
- Zhu P, Zhang J, Zhu J, et al. MiR-429 induces gastric carcinoma cell apoptosis through Bcl-2. Cell Physiol Biochem. 2015;37:1572–1580.
- Zhang T, Tian F, Wang J, et al. Atherosclerosis-associated endothelial cell apoptosis by MiR-429-mediated down regulation of Bcl-2. Cell Physiol Biochem. 2015;37:1421–1430.
- Sun DK, Wang JM, Zhang P, et al. MicroRNA-138 regulates metastatic potential of bladder cancer through ZEB2. Cell Physiol Biochem. 2015;37:2366–2374.
- Song W, Li Q, Wang L, et al. Modulation of FoxO1 expression by miR-21 to promote growth of pancreatic ductal adenocarcinoma. Cell Physiol Biochem. 2015;35:184–190.
- Li P, Ma Y, Wang Y, et al. Identification of miR-1293 potential target gene: TIMP-1. Mol Cell Biochem. 2013;384:1–6.
- Ciechomska M, O'Reilly S, Suwara M, et al. MiR-29a reduces TIMP-1 production by dermal fibroblasts via targeting TGF-beta activated kinase 1 binding protein 1, implications for systemic sclerosis. PLoS One. 2014;9:e115596.
- Agarwal V, Bell GW, Nam JW, et al. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4. DOI:10.7554/eLife.05005.
- Zhang P, Zheng C, Ye H, et al. MicroRNA-365 inhibits vascular smooth muscle cell proliferation through targeting cyclin D1. Int J Med Sci. 2014;11:765–770.
- Kim MH, Ham O, Lee SY, et al. MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1. J Cell Biochem. 2014;115:1752–1761.
- Bai J, Zhang Z, Li X, et al. MicroRNA-365 inhibits growth, invasion and metastasis of malignant melanoma by targeting NRP1 expression. Cancer Biomark. 2015;15:599–608.
