Abstract
Science of drug delivery has achieved tremendous milestones in the last few decades. Emergence of novel drug delivery techniques and the most popular nanotechnology directed the drug delivery to another level. Without any doubt, present technology holds the proficiency to approach even the intercellular targets. Between all these success auras, there lies wads of giant challenges. One such challenge is delivering the molecule directly to the blood stream. Parenteral route is considered as the most effective route for delivering active pharmaceutical substances, but is associated with major disadvantages of painful drug delivery. Modern drug delivery suggests several approaches to outstrip this painful phenomenon. In the present article, we represent a new systematic vision to understand the ability and desirability of mucosal sites to achieve painless drug delivery. Human mucosa presents supreme proximity to the blood circulation that one can even observe with naked eye. Advances in drug delivery provide numerous approaches to exploit the mucosa for systemic reach. However, the revolutionary success is still unapproachable, with an understandable reason of associated complexities and challenges. This manuscript summarizes the significance of each mucosal site, on the basis of anatomical-physiological grounds. Particular attention is given to rationalize the selection of disease and a suitable drug delivery approach for its treatment.
Introduction
Effective drug delivery has been a major challenge in medical science to achieve therapeutic success. The need of delivering an active molecule to the systemic circulation is significant in most of the cases. Except topical therapeutics, systemic reach in optimum unaltered form is the prerequisite for any drug molecule or antigenic moiety to elicit the targeted response. Generally oral, parenteral and topical routes hold the whole stake of current drug delivery. Bioavailability (BA) is considered as the superiority sign of any route and parenteral is considered as the standard with 100% bioavailability. Various parenteral routes, like subcutaneous, intramuscular, intravenous have been used for drug delivery but due to their invasiveness through the skin epithelium, a significant momentum and attention generated in developing alternate route of administration.
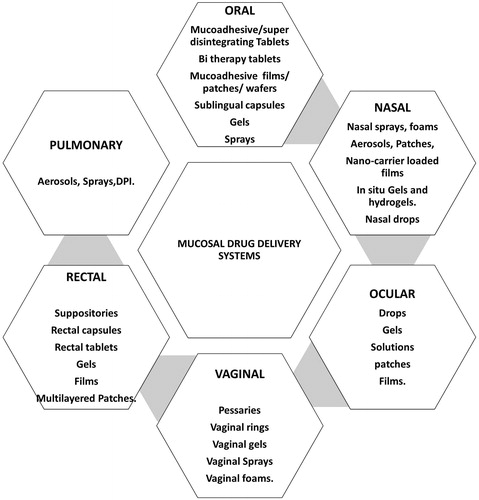
In this context, mucosal sites viz. oral, nasal, ocular, pulmonary mucosa, rectal and vaginal mucosa presents logically best alternates. Anatomical make up of these sites with thin epithelium lining, trans-membrane network and rich blood supply sets an impressive track for huge variety of therapeutic molecules to get into blood stream [Citation1,Citation2]. These sites offer versatile anatomical and physiological milieus leading to an ease as well as challenge to select a suitable mucosal site () [Citation3–12]. We present a systematic insight to the different conditions related to all these sites determining their positive and negative impact on delivering drug molecules to circulation. Selection of appropriate delivery systems in this regard again puts a question upfront. Conventional as well as advanced approaches such as nano drug delivery and physical techniques are being discussed explaining their suitability and challenging aspects [Citation13].
Table 1. The brief specification including mucosal thickness, pH and biological secretion’s at various transmucosal sites.
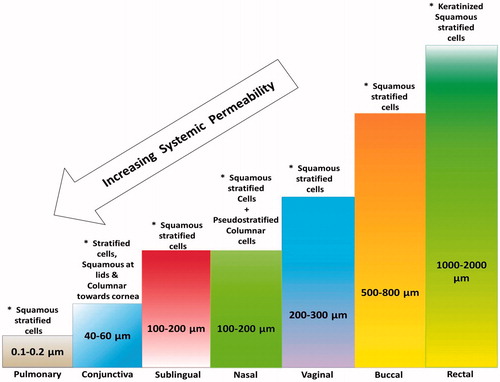
Varied physiological conditions of transmucosal site is the major challenge for systemic delivery drugs including differences in pH of physiological fluids; presence of different microbial flora and enzymes; thickness of mucosal layers; differences in blood connectivity and their distribution; changes in physiological condition with respect to age, sex and disease condition; changes in volume and area of dissolution. Similarly, limited volume and area; and presence of enzyme and their clearance mechanism is limiting factor for drug delivery through nasal and ocular route. In addition, with the advantages of large surface area and excellent blood supply of pulmonary track, it is also still underutilized because of complex bronchopulmonary segment, mucocilliary clearance and lower uptake of the drugs through pulmonary route. It is essential to understand this critical balance between the problems associated and advantages offered by different mucosal sites before selecting as delivery route [Citation14–17].
Rationale behind the selection of appropriate mucosal site
A need-based approach is required while selecting a mucosal site for a particular drug therapy. Various factors such as the anatomy or cellular proximity, reservoir of diseases, age, sex, kinetics and longevity of therapy are among the key questions to be assumed before a rational route selection. One of the important criteria for selection of appropriate route for delivery of drugs should be based on types of diseases, diseased condition, types of patient and severity of disease. Local delivery of drugs under diseased condition of mucosal site such as tuberculosis, anthrax and influenza, etc. at lungs; buccal cancer, candidiasis, mouth ulcer at buccal cavity; vaginitis, candidiasis, UTI, STD at vaginal mucosa; conjunctivitis, glaucoma, uveitis at eye site, etc. could be better alternative approach for fast effective treatment of diseased condition. Local delivery of drug to diseased site not only helps to treat disease but also reduce the unwanted toxicity of drugs for other site. Similarly, mucosal sites can also be explored for selected diseased site for treating disease condition such as use of nasal mucosa for brain delivery of drugs; use of sublingual route for cardiovascular disease; use of pulmonary route for instant systemic delivery of drugs; use of rectal route for the systemic delivery of drug to unconscious, sensitive and paediatric patient. Time and again and in most of the cases, the aim is to achieve a therapeutic drug concentration in plasma from which drug enters the tissue. Bioavailability of drugs with extensive hepatic first pass metabolism may be increased by appropriate selection of mucosal route. Further, selection of mucosal site for vaccines delivery is also an important aspect for infection occurred through mucosal route [Citation1,Citation18]. illustrates these decisive factors, which impart logic behind the site selection protocol.
Drug delivery approaches in various mucosal site
Various approaches for systemic drug delivery via these mucosal sites include a variety of drug delivery systems on the basis of their advantages and disadvantages as well as their suitability in achieving desired action ().
Figure 2. Suitability of various delivery systems at different mucosal sites according to their anatomical and physiological considerations. The numerous options present for drug delivery via these mucosal sites conclude the possibility for beneficial outcomes with exploring future of these routes.
Conventional dosage foams
Tablets/lozenges/capsules
For systemic drug delivery through mucosal sites, various conventional drug delivery systems like tablets, lozenges and capsules were successfully used. The tablets administered via oral cavity include buccal tablets, sublingual tablets, lozenges, troches and dental cones. Whereas tablets administered via other routes are implantable or depot tablets and vaginal tablets or inserts. They vary in their shape and size, as per the requirement of mucosal site. Various reports endorse their efficiency for treating several diseases. The buccal tablets are reported to treat CVS diseases using diltiazem, and carvedilol-loaded microspheres [Citation19]. An improvement in the bioavailability and therapeutic efficacy was reported, where pharmacokinetic studies in rabbits revealed considerably higher blood concentration and overall bioavailability in comparison to carvedilol oral tablet. Fentanyl buccal tablet (FBT) provided a commendable solution for patients’ underlying persistent cancer pain. A rapid absorption was observed with shorter Tmax and plasma concentration reached 80% of Cmax inside 25 min, providing consistent plasma concentrations for 2 h [Citation20]. Another study compared the efficacy and safety of FBTs and oral dose of morphine in cancer patients (a randomized, crossover, controlled study with equivalent opioid dose regimen in 263 episodes). On a therapeutically relevant basis, FBT presented a clear advantage in pain management in first half an hour [Citation21]. The choice of excipients plays a very essential role and desired activity can be achieved using super disintegrants, pH modifiers, binders, glidants, taste masking agents, chelating and complexing agents. Many scientists have focused on their specific role in range of formulations [Citation19,Citation22]. Flat-faced core buccal tablets of chlorpheniramine maleate (CPM) were designed for transmucosal sustained-delivery. The plasma concentration versus time profiles in rabbit model showed the considerably dependent effect of an excipient, i.e. mucoadhesive gum Hakea gibbosa (Hakea), on the overall bioavailability of different formulation’s compositions [Citation23]. Not only this, nicotine replacement therapy (NRT) was also delivered through the use of buccal tablets [Citation11]. Carbopol®974P, alginic acid sodium salt and hydroxypropyl methylcellulose based bioadhesive buccal tablet formulations were developed for the delivery of nicotine [Citation24]. In another study, chitosan and carbomer (at different ratios) based bioadhesive buccal tablets of nicotine hydrogen tartrate were developed for NRT. The pharmacokinetic profile comparison of these buccal tablets with a transdermal patch showed no significant difference in the maximum plasma nicotine concentrations Cmax (p > .05) [Citation25]. Another opioid (morphine) based study revealed an interesting outcome, where the absolute bioavailability for morphine was assessed to be 23.9% (oral solution), 22.4% (controlled release oral tablet, MST-Continus) and 18.7% (controlled release buccal tablet) in six healthy volunteers. No significant variance in plasma levels of morphine-6-glucuronide (M6G, an active metabolite of morphine) were observed after intravenous, oral or buccal administration [Citation26]. Safety and efficacy transbuccal testosterone therapy was successfully studied in men using a randomized, double blind, placebo-controlled study in a parallel design. Results suggested it satisfactory to uphold normal sexual function though reducing the overall exposure time to raised circulating serum testosterone levels [Citation27]. Polymeric combination (carbopol, hydroxypropyl methylcellulose, sodium alginate, sodium carboxymethyl cellulose and guar gum) based, buspirone hydrochloride loaded sustained release buccal mucoadhesive tablets were reported to have 5.6 fold improved systemic bioavailability in human volunteers when compared to commercial oral Buspar® tablets [Citation28]. Ritodrine hydrochloride buccal tablets with excipients such as alginate, lactose and microcrystalline cellulose (with or without), were reported for the treatment of premature labor. The results of the in vivo buccal absorption in rats with different formulation combinations revealed variable results, where only few of them were able to raise the plasma drug concentration above the human minimal effective level [Citation29]. Extensive hepatic metabolism and short half-life of tizanidine HCl make it a good candidate for delivering it systemically by buccal route. In a study, chitosan lactate beads cross-linked with sodium tripolyphosphate were used as the carrier for the controlled drug delivery of tizanidine HCl. These beads were then pressed and delivered in the form of wafer for the application in buccal mucosa. Formulation provided a significant encapsulation efficiency of 56.5% and controlled drug release pattern over 8 h, whereas pharmacokinetic studies revealed significantly increased bioavailability and controlled release [Citation30].
The dental cones are the minor tablets placed in the sockets after tooth extractions to prevent bacterial growth by sustained release of antibacterial agents. The implantation tablets are for subcutaneous delivery, however for delivery, they need surgery, so exhibit poor patient compliance system [Citation31]. The vaginal tablets or inserts are pear shaped or somewhat ovoid, for comfortably placing in vaginal or rectal cavity. These tablets are buffered to optimize the pH formulation to favour the action of antiseptic agents [Citation32]. These tablets are used to deliver antibacterial, anti-retroviral and steroidal agents. While comparing nifidipine sublingual capsules with tablets and films, better release was obtained with capsules [Citation33]. The systemic drug delivery using capsules is possible via rectal, sublingual and vaginal mucosae where soft gelatin capsules containing medicaments are inserted.
Patches/films
These are the drug delivery systems, made up of biodegradable hydrophilic polymers and loaded with active moiety and administered by transmucosal or transdermal routes. The preparation technique for the films, patches includes hot melt extrusion, solvent casting and electrospinning. Various excipients added in them include sweetening agents, flavouring agents, penetration enhancers, disintegrants and plasticizers. These delivery systems have excellent swelling index, mucoadhesion and tensile strength. However, there are some limiting factor that affects the performance of patches and films, which includes limited absorptive membrane area and types of administration, continuous release of saliva and organoleptic and physicochemical properties of encapsulated drugs [Citation34]. Buccal soluble film of fentanyl provides an interesting example of this dosage form, where pharmacokinetic studies in healthy volunteers showed appreciable plasma concentration profiles when equivalent doses are delivered by single or multiple dosage units [Citation35]. In another study of this fentanyl buccal soluble film, the pharmacokinetic profiling of formulations at different pHs values (pH 6, 7.25 and 8.5) produces greater peak plasma concentrations in comparison with that of oral transmucosal fentanyl citrate. Study was done on 12 enrolled subjects under randomized, open-label, single-dose, four-period, Latin-square crossover. Specifically, the pH 7.25 formulation in that case revelled the best pharmacokinetic parameters [Citation36]. A COX-2 inhibitor “valdecoxib” loaded mucoadhesive buccal films based on chitosan and HPMC K4M as polymers, were developed for oral submucous fibrosis. A localized drug released at the target site of action, as revealed by pharmacokinetic studies and very poor systemic absorption was absorbed [Citation37].
Nowadays, multi-layered films/patches are explored in market with unidirectional (backing layer), bidirectional or multidirectional release. Multipolymeric monolayered films for ARV therapy are also reported [Citation38]. Tri-layered buccal mucoadhesive patch of nicotine to prevent first pass metabolism are also developed with excellent efficiency. Giovino et al., 2013 have developed polymeric insulin nanoparticle impregnated buccal films using PEG-b-PLA as polymers for insulin encapsulation, chitosan as mucoadhesive polymer and glycerol as plasticiser. Developed mucoadhesive nanoparticle impregnated insulin film have demonstrated better retention at the site of application and yielded 1.8-fold better insulin permeation compared to pure drug in ex-vivo conditions. Buprenorphine buccal film formulations were studied for their pharmacokinetic profile. Two open-label studies were performed in this case on enrolled healthy volunteers receiving oral naltrexone concurrently to reduce adverse events. Studies revealed 46–51% absolute bioavailability, respectively through 16-fold dose range, suggesting the potential for this dosage form [Citation39]. Similarly, sublingual soluble film of buprenorphine/naloxone has been developed for the treatment of opioid dependency. Developed film is safe, effective and provide unit dose with rapid dissolution and suitable palatability. Several drugs in the form of films and patches have been delivered by buccal route including glibenclamide, salbutamol, nifedipine, nitroglycerine, etc. with desired therapeutic effect. Another study reported the 2.29-fold increase in the bioavailability of carvedilol from the polymer-based buccal patches when compared to its oral solution [Citation40]. A mucoadhesive buccal of prednisolone were prepared using hydroxyl propyl methyl cellulose (K100), Carbopol and/or Eudragit polymer showed greater bioavailability and higher plasma concentration as compared to oral suspension [Citation41].
Gels and ointments
These conventional delivery systems is less suited for immediate onset of action, rather they are preferred for extended release, especially ointments. The ointments contain matrix of high melting point solid hydrocarbons and waxy substances like mineral oil, petroleum and polyethylene glycol. Gels are clear, transparent, semisolid systems that can be used for systemic delivery, containing a liquid phase within three-dimensional polymeric matrices and are cross-linked, either physically or chemically. The various biodegradable polymers like natural gums, pectins, carrageenan, carbapol and methylcellulose are used. The bioadhesive polymers provide adhesion of formulations to mucosal sites via numerous hydrogen bonding groups. They have plastic rheological behaviour so they remain on the surface of application for a reasonable time period before being washed off. They exhibit significantly prolonged residence time, which improve bioavailability. Many studies have reported the benefits of bioadhesive ointments and gels. Partially neutralized polymethacrylic acid methyl ester was used to prepare bioadhesive ointment. Developed bioadhesive ointment has shown pseudoplastic rheological properties and better mucoadhesion. Dexamethasone ointment has also been developed for the treatment of mouth ulcer [Citation42]. Efficacy of ointment was also compared with solution for ocular delivery of chloramphenicol, tetracycline hydrochloride, sodium sulfacetamide and fluorescein sodium. Studies have suggested that both types of dosage form shows almost same types of systemic absorption but ointment provide better contact time and slow release of medicaments compared to solution. Similarly, various mucoadhesive ointment bases have also been compared for the oromucosal delivery of benzyl nicotinate including orabase, carbopol 935P, and polymethyl methacrylate and miglyol. Among them, polymethyl methacrylate has demonstrated significantly better effect [Citation43,Citation44].
Nowadays, hydrogels and in-situ gels are explored due to their outstanding advantage [Citation45,Citation46]. Hydrogels have high capacity to absorb enormous water due to polymers like carbapol 934 for meloxicam, zaleplon, timolol sparfloxacin and other drugs. All such systems were administered by mucosal route and demonstrated better retention and efficacy of encapsulated drugs.
Aerosols, foams and sprays
These delivery systems are one of the suitable systems for rapid onset of action, mainly through oral, nasal and pulmonary mucosa and also reported to deliver drug via vaginal and rectal mucosa. The aerosol systems are pressurized package containing medicament, and the delivery depends on the power of a compressed or liquefied gas, which creates a mist of particles after expulsion. These delivery systems are not that suitable for all mucosal sites except the nasal and oral and vaginal cavities. The suitability of this delivery system depends on the excipients like propellants, surfactants containing fluorocarbons, which may cause irritation to sensitive regions. The foams are also a type of aerosols that have bubbles and expand in large area. These are not much used for systemic drug delivery. Mucoadhesive foam-based benzocaine has been developed that provide better pain relief and reduction in oedema. Treatment with foam was demonstrated to be more effective than benzocaine spray with greater pain relief and reduction of oedema as shown by lower patient need of supplemental pain-relief measures [Citation47]. Similarly, daily administration of budesonide foam compared with plain formulation has shown complete mucosal healing of ulcerative colitis [Citation48].
The aerosol sprays deliver drug on mucosal surface as fine particles or droplets and drug absorption exhibits lag time [Citation49]. Polysaccharide gellan has been used to prepare mucoadhesive nasal spray. Gellan has strong gelling capacity that makes gels with low concentrations. Several studies reported use of acyl gellan for the nasal delivery of drugs. Recently, a fluid gel system has been prepared using low- and high-molecular weight acyl gellan. Developed fluid gel system consists of gelled microparticles within ungelled polymeric solution that provide better spraying and mucoadhesive properties at nasal cavity for the delivery of drugs [Citation50]. Sodium hyaluronate, xylitol and sodium bicarbonate based mucoadhesive oral spray has been developed that control the lowering of salivary pH and helpful to prevent dental caries and erosion [Citation51]. Similarly, combination of cyclodextrins with gum ghatti based mucoadhesive spray has been explored for the treatment of stomatitis using irsogladine maleate. Developed spray is retained for longer period of time to the administered site and overcome the problem associated with conventional gel and ointments like application requires a finger, multiple spitting, content uniformity and spreadability [Citation52].
Various dosage forms have been used to deliver medicament topically including pressurized or propellant-driven metered dose inhalers, dry powder inhalers, foam and nebulizers/nebules. These products have been used for the treatment of chronic obstructive pulmonary disease, cystic fibrosis, diabetes, and a range of neurological disorders. One such example is Exubera®-inhaled insulin, which initially was approved and subsequently withdrawn [Citation53]. These delivery systems can administer drug in mechanically ventilated patients during respiratory failure. The other example is aerosolized antibiotics in treating ventilator-associated pneumonia. Sakagami et al., 2001 have developed hydroxypropylcellulose based powder inhaler to access its potential for improved pulmonary drug absorption. Studies have suggested that high viscous hydroxypropylcellulose based powder inhaler demonstrated better bioavailability, absorption and lower mucociliary clearance compared to less viscous formulations [Citation54]. A phase-I, open label, four-way crossover study was designed to study the pharmacokinetic parameters of cannabis-based medicine extract (CBME) when administered in the form of liquid spray on different areas of the buccal mucosa. Results demonstrated that buccal pharmacokinetic parameters are not as promising when compared with other compared routes such as sublingual and oropharyngeal [Citation55].
Suppositories and pessaries
Suppositories are the solid dosage foams intended for containing medicament and used via vaginal, rectal and sometimes urethral route. The rectal and urethral suppositories have vehicles that melt or soften at body temperature while vaginal suppositories, also called pessaries, are made as compressed tablets and they disintegrate in body fluids. The suppositories contain ingredients like suppositories base: cocoa butter, fat and wax combinations with cocoa butter, glycerinated bases and water soluble polyethylene glycol type bases. These systems not only used for drug delivery but also for vaginal vaccination to stimulate the immune system. The vaginal suppositories weigh 3–5 g. and moulded in globular or oviform shape, or tablets pressed in to modify conical shapes. The urethral suppositories or bougies are pencil shaped and pointed at one end, 100–150 mm long and 2–4 g in weight. These dosage forms are used for the treatment of constipation and haemorrhoids. Suppositories containing plasmid coding for glycoprotein D-mediated DNA immunization via intravaginal delivery against bovine herpesvirus-1 in cattles are also developed [Citation56]. Nanocarrier-loaded suppositories for better targeting exhibited better results. Poly (lactide-co-glycolide) nanoparticles containing the lipophilic fluorescent dye coumarin-6 and pDNA/PEI-complex loaded liposomes were delivered through suppositories, which revealed desired therapeutic effect [Citation57]. Poloxamer-based solid suppository containing poloxamer 124 and poloxamer 188 is used to deliver diclofenac sodium in rats. Poloxamer-based suppositories have shown significantly higher bioavailability of encapsulated drug compared to PEG-based suppository and provide mucoadhesion at the site application [Citation58].
Drops
Drops are the sterile solution of medicaments intended for instillation into eyes, especially in corneal membrane for delivering drug to the site of action. The sterility must be maintained in their case for prevention of mucosa from microorganisms. The excipients added in these formulations include preservatives, pH stabilizers and tonicity modifiers. Researchers reported the effectiveness of drops loaded with liposomes [Citation59], the efficacy of N-acetylcarnosine lubricant eye drops for prevention and reversal of cataracts or carcinine biologics [Citation60].
Nano carriers
Nanotechnology is the understanding and control of matter at dimensions of roughly 1–100 nm, where unique phenomenon enables novel application. Any structure less than 100 nm possess unique properties for therapeutics delivery. The advantages of these small magical particles are the modulation of pharmacokinetic properties of incorporated molecules. Nanotechnology can provide versatility, as all drugs can be encapsulated and the toxicity profile depends on the excipients used. They have ability to incorporate, protect and promote the absorption via oral and other routes [Citation61]. The bioavailability of drugs increases as nanosystems protects the incorporated drug from metabolism. Targeting, which is the major problem and main requirement for delivering the therapeutics, can be achieved by nanosystems. The targeting is achieved by either active or passive mode. Passive targeting depends on the inherent properties of nanosystem, including particle size, shape, surface charge and found to enhance its bioavailability, biodistribution and targeting potential. While in active targeting, the nanosystems are modified by attaching specific ligand on the surface to modify surface properties for recognising target sites [Citation62]. Polymeric nanoparticles have been used for oral delivery of small and macro synthetic and biomolecules [Citation63]. Not only this, they can escape bioelimination processes like efflux pumps (particularly p-glycoprotein) present on BBB endothelium, which increases the bioavailability as well as drug residence time at the site [Citation64]. Increasing bioavailability significantly reduces dose, dosing frequency and provide a better regimen with reduced side effects. Keeping all these advantages in mind, various researchers formulated nano drug delivery carriers able to deliver therapeutics via suitable mucosal site in systemic circulations. Acyclovir-loaded nanospheres were proposed to improve systemic bioavailability via buccal route. These nanospheres are prepared by double emulsion solvent evaporation technique and further embedded into buccoadhesive films. Pharmacokinetic studies in rabbits revealed a significant enhancement in bioavailability of acyclovir when compared to the oral route [Citation65]. In another study where selegiline loaded poly(lactide-co-glycolide) nanospheres, impregnated on polymeric buccal film was proposed to enhance the systemic bioavailability of selegiline by preventing extensive hepatic metabolism. Pharmacokinetic studies in rabbits comparing these buccal films with oral solution showed significant increase in absorption and improved bioavailability suggesting a likely alternative strategy for Parkinson’s disease treatment [Citation66]. Phospholipid-bile salts-mixed micelles were explored as another such nanoscale carrier to deliver Cucurbitacin B. These nanoscale carriers in the integration with mucoadhesive buccal films were reported to improve the drug delivery aspects along with its therapeutics and clinical implications. Pharmacokinetic studies revealed a superior and extended drug release from the micelle-based films compared to that of plain polymeric films and oral marketed tablet. Drug absorption from micelle-based films was 2.69-fold high in contrast to the marketed tablets and 10.46-fold higher when compared to drug-loaded plain polymeric films [Citation67]. These nano drug delivery carriers target numerous diseases, including diabetes mellitus, Alzheimer’s, cancer, AIDS, hypertension, Parkinson, epilepsy and many more ().
Table 2. Nanoparticulate and microparticulate systems by various mucosal sites for systemic drug delivery.
Physical methods (electroporation, sonophoresis, iontophoresis, laser radiation, photomechanical waves and needleless injection)
The need for fast onset of action and improved absorption of therapeutics, led to the invention of advanced physical techniques for drug delivery. The drug delivery through mucosal membrane carries great future perspective [Citation68]. The various barriers in mucosal route hinder the drug delivery in an efficient way and these limitations can be removed by physical methods including eutectic formation, supersaturation, electroporation, sonophoresis, iontophoresis, laser radiation, photomechanical waves and needleless injection (nanoneedles and microneedles) () [Citation69]. However, selection of ideal technique for desired site is very crucial. Though the physical methods could be suitable for drug delivery through keratinized epithelial tissues, they are not widely applied for drug delivery through mucosal membranes. However, they carry a great future potential to deliver medicaments through these routes [Citation70].
Figure 3. Impact of mucosal thickness and the cell types (*) on the factors associated with the systemic delivery from the different mucosal sites.
Sonophoresis
Sonophoresis is a process of enhancing skin permeation of various low- and high-molecular weight compounds, including heparin and insulin. The ultrasound waves induce the microvibrations in membrane. As a result, topical agents get kinetic energy for better permeation by microstreaming, cavitation and heating. The formulation is applied with coupling agents to transfer ultrasonic waves from ultrasound transducer. This technique gained attention, as evident from increase in patents filed day by day [Citation69]. The use of this technique is also reported in delivering drug via few mucosal sites like oral and ocular mucosa. Therapeutic delivery to facilitate permeability of drug through ocular barriers, such as the cornea and sclera was also reported [Citation71]. Various scientists suggested the potential use of physical methods for oral cavity. Ultrasound-assisted techniques were used for transbuccal delivery of drugs, proteins, essential organic, inorganic compounds, amino acid and vitamins for systemic action. Studies have suggested that frequency and intensity of ultrasonic energy and use of chemical enhancer affect the permeation of therapeutics.
Iontophoresis
Iontophoresis is a process of improving permeation of ions of soluble salts in the tissue of body by passing electric current for delivering medicaments. The externally applied potential difference, by placing cationic agent under anode or vice versa, causes the movement of ions across the membrane. The ions permeate through the pores with least electrical resistance [Citation72]. The Caco-2 monolayer as a model for trans-epithelium iontophoric delivery of drugs was investigated on the basis of transepithelial electrical resistance (TEER), transmission electron micrographs (TEM) and confocal laser scanning microscopy (CLSM). Results reveal that fluxes of various drug enhanced when electric field is applied [Citation73]. Iontophoresis is explored for targeted local delivery of 5-flurouracil and leucovorin for the treatment of head and neck cancer. Efficacy of short duration cathodal iontophoresis of both drugs was evaluated in solution and in hydroxyethyl cellulose gel using bovine mucosa. Studies have suggested that this technique may be utilized for localized targeted delivery of drugs [Citation74]. Similarly, mucoadhesive hydrogels of local anaesthetic drugs, i.e. prilocaine and lidocaine have also been delivered by iontophoresis. Permeation of drugs was significantly improved by iontophoresis compared to passive diffusion. Further, accumulation of both drugs was significantly higher in combination when compared to individual drugs [Citation75]. Instead of combined delivery, chemical enhancer also showed better diffusion across oral mucosa. Studies have suggested that passive diffusion of lidocaine and nicotine in the presence of iontophoresis and chemical enhancers was two-fold increase when compared to individual approaches (iontophoresis/chemical enhancers) [Citation76]. Several chemical enhancers along with iontophoresis was studied for delivery of lidocaine, nicotine and diltiazem including dodecyl 2-(N,N-dimethylamino) propionate, dodecyl-2-(N,N-dimethylamino) propionate hydrochloride, N-(4-bromobenzoyl)-S,S-dimethyliminosulfurane and laurocapram [Citation77].
Electroporation
Electroporation is a process based on the temporary loss of semipermeability of cell membrane, under the influence of electric pulses of intensity in kilovolts per centimetre, of duration in microseconds to milliseconds. This led to leakage of ions and metabolites, as well as increased uptake of drugs, molecular probes and DNA [Citation78]. Different amplitudes of electric pulses (70–570 V) in electroporation has been optimized for the delivery of fluorescein-isothiocyanate labeled dextran, doxorubicin or fentanyl molecules into cells and tissues, including the skin. Study suggested that non-invasive multi-array electrodes under controlled electric pulse amplitude provide improved delivery of therapeutics within skin [Citation79]. The DNA vaccine encoding influenza virus nucleoprotein (NP) of influenza H1N1 was delivered through oral and vaginal mucosal surface. Results revealed minimal tissue damage and robust and sustainable humoral as well as cellular immune responses obtained [Citation80]. Electroporation has been used to deliver genetic material in ocular site for regulating the activity of retinal pigment epithelium cells in the adult mouse. Studies have suggested that both subretinal injection and electroporation was efficacious but some technical challenges require skill on the part of the surgeon to prevent untoward damage to the eye [Citation81]. This technique was also used to transfer genetic material into the dental tissues of rat. It can be useful for treatment of genetic disorder associated with dental tissue [Citation82]. Local delivery of chemotherapeutic agent under electroporation was also evaluated to provide safe and effective localized control of tumor. Electrochemotherapy is a combined therapeutic intervention where chemotherapeutic drugs were simultaneously administered with electroporation. Clinical studies have demonstrated that intratumoral administration of bleomycin with electroporation therapy have improved intracellular accumulation of chemotherapeutics [Citation83].
Microneedles
Needleless injections are the new discoveries in the field of drug delivery, which can be programmed to deliver a series of doses to varying skin depths. Microneedles were manufactured by microfabrication technique and are used in one of the following ways: (a) pretreated skin by solid microneedles, (b) drug-coated microneedles, which dissolve in skin, (c) microneedles with encapsulated drug in polymer and fully biodegradable, and (d) drug infusion through hollow microneedle [Citation84]. They are reported to deliver low- to high-molecular weight drugs and vaccines. The administration of influenza vaccine through mucosal membrane with the use of coated solid microneedles was reported that maintain the stability of vaccine [Citation85]. The successful vaccine delivery via oral mucosa using coated microneedles to induce systemic and mucosal immunity was also achieved. Microneedles were coated with sulforhodamine, ovalbumin and two HIV antigens. To confirm the insertion of microneedle, histological study was performed. The immune response was characterized by immunoglobulin G (IgG) in serum and immunoglobulin A (IgA) present in saliva. Results were noted to be significant [Citation86].
Photomechanical waves
Photomechanical waves generated by irradiating a solid material with high-power nanosecond laser pulse can also alter the integrity of membrane to facilitate the delivery of drugs through it. These waves were produced by confined ablation with a Q-switched ruby laser. The assay for macromolecule delivery was done by fluorescence microscopy of frozen biopsies. These waves do not cause any pain or changes in histology of tissue [Citation87].
Future prospectives
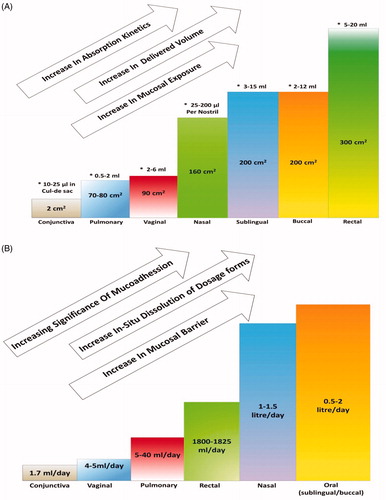
The literature indicates a very promising alternative approach for painless systemic delivery in the form of various mucosal sites but their practical relevance has not been explored to required extent. This space between theoretical potential and practical reality is due to lack of understanding of many related factors. Various studies done so far proved their efficiency in different ways but no ultimate inference could be drawn till date. More studies are required for understanding the relevant details of each site. Anatomical and physiological aspects, mucoadhesive understandings are important for achieving prominent and precise results. The interconnectivity network of various mucosal sites and their relevance with one another will be a crucial factor for systemic bioavailability of drugs and the expected results. Every mucosal site has specific thickness, pH and amount of volume that can be delivered, biological secretions and surface area. These varying parameters have different effects on factors like dissolution, kinetics, permeability, absorptions and mucosal exposure. These can be better understood by observing , which show the relations between mucosal thickness, surface area and biological secretions with systemic permeability, dissolution, mucoadhesion, absorptions and release kinetics.
Figure 4. (A) Impact of surface area and the range of volume that can be delivered (*) from the different mucosal sites, on the factors associated with the systemic delivery of the drugs. (B) Impact of the levels of the biological secretions from different mucosal sites on the factors associated with the systemic delivery of the drugs.
The presence of barriers in mucosal membranes are big hurdles for drug delivery as they prevent the entry of any foreign substance via this route. They act in a similar manner to the therapeutic substances too and are rate determining step for drug delivery. The vascularity in close proximity to mucosal site makes it more efficient in bringing onset of action by the drug. The extent of blood circulation decides the accessibility of a site. This is exemplified by the pulmonary route that has very rich blood supply in close proximity to alveolar epithelium. So the availability of drug to the area of profuse blood supply ultimately increases. To address the problems related to poor drug penetration by mucosal barriers, penetration enhancers were extensively used. Excipients were found to cause irritation in mucosal membranes. This can be substituted with non-irritating polymers like PVA, PCL, CAP, PEO were even proposed. Advanced nanocarrier systems like liposomes, niosomes, nanoparticles, microparticles, microspheres, nanofibres and scaffolds were also used to solve permeability related problems. The first step in delivering a drug is to hold the system on mucosal site for a required time, which is the major challenge. The prolonged retention time can be achieved by using mucoadhesive polymers like gelatin, mucin, chitosan, PVA, etc. so that sufficient bioavailability of drug in systemic circulation can be ensured. Various mucosal sites offer specific similarities and differences from one another, so suitability varies as per the requirement of a particular therapy. So, before choosing a specific site, one should be very clear about the aspects such as anatomical/cellular proximity of diseases, reservoir of the diseases, age/sex, kinetics and longevity of therapeutic regimen and dose dependency. The advanced drug delivery approaches like nanofibres and scaffolds for targeting diseases via these routes have shown better permeability and retention capacity on mucosal sites. But further improvement in these systems are still needed for enhancing permeability with minimal toxic effects, cost effective and satisfactory patient compliance systems.
Conclusion
The mucosal sites have their potential to be a suitable alternative for painless systemic drug delivery. However, this potential could be explored after understanding their advantages, disadvantages and other factors. To understand these key factors, we need to have thorough knowledge of the anatomical and physiological aspects of these mucosal sites and their associated preferences and challenges for drug delivery. Most important is the rationale behind selection of the mucosal site, which is governed by various conditions like age, sex, therapy time period and disease proximity. Various reports endorse the fact that mucosal sites carry potential for effective painless systemic drug delivery. The same can further be improved by the use of nanotechnology and physical methods like iontophoresis, sonophoresis, etc. With the available details of all mucosal sites, we can conveniently establish the preference for choosing specific mucosal site in a particular situation.
Acknowlegement
Author acknowledges the discerning discussions with DBT Lab research fellows and for their active support in concluding the script in a more productive way.
Disclosure statement
No potential conflict of interest was reported by the authors.
Reference
- Breustedt A. Age-induced changes in the oral mucosa and their therapeutic consequences. Int Dent J. 1983;33:272–280.
- Farage M, Maibach H. Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol. 2011;40:9–19.
- Harris D, Robinson JR. Drug delivery via the mucous membranes of the oral cavity. J Pharm Sci. 1992;81:1–10.
- Hussain A, Ahsan F. The vagina as a route for systemic drug delivery. J Control Release. 2005;103:301–313.
- Illum L. Nasal drug delivery: new developments and strategies. Drug Discov Today. 2002;7:1184–1189.
- Illum L. Nasal drug delivery—possibilities, problems and solutions. J Control Release. 2003;87:187–198.
- Patton JS, Fishburn CS, Weers JG. The lungs as a portal of entry for systemic drug delivery. Proc Am Thorac Soc. 2004;1:338–344.
- Urtti A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv Drug Deliv Rev. 2006;58:1131–1135.
- Friend DR. Colon-specific drug delivery. Adv Drug Deliv Rev. 1991;7:149–199.
- Gaurav C, Goutam R, Rohan KN, et al. In situ stabilized AgNPs and (Cu-Cur) CD dispersed gel, a topical contraceptive antiretroviral (ARV) microbicide. RSC Adv. 2015;5:83013–83028.
- Malik T, Chauhan G, Rath G, et al. “Fusion and binding inhibition” key target for HIV-1 treatment and pre-exposure prophylaxis: targets, drug delivery and nanotechnology approaches. Drug Deliv. 2017;24:608–621.
- Malik T, Chauhan G, Rath G, et al. Efaverinz and nano-gold-loaded mannosylated niosomes: a host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif Cells Nanomed Biotechnol. 2017;1–12.
- Hillaireau H, Le Doan T, Chacun H, et al. Encapsulation of mono- and oligo-nucleotides into aqueous-core nanocapsules in presence of various water-soluble polymers. Int J Pharm. 2007;331:148–152.
- Kumar K, Dhawan N, Sharma H, et al. Bioadhesive polymers: novel tool for drug delivery. Artif Cells, Nanomed Biotechnol. 2014;42:274–283.
- Goyal P, Gill S, Gupta U, et al. Development and characterization of rifampicin loaded floating microspheres. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:330–334.
- Aas JA, Paster BJ, Stokes LN, et al. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol. 2005;43:5721–5732.
- Ungell AL, Nylander S, Bergstrand S, et al. Membrane transport of drugs in different regions of the intestinal tract of the rat. J Pharm Sci. 1998;87:360–366.
- Farage MA, Maibach HI. Morphology and physiological changes of genital skin and mucosa. In: Surber C, Elsner P, Farage MA, editors. Current problems in dermatology. Topical applications and the mucosa. Vol. 40, Basel, Switzerland: Karger Publishers; 2011. p. 9–19.
- Yedurkar P, Dhiman MK, Petkar K, et al. Mucoadhesive bilayer buccal tablet of carvedilol-loaded chitosan microspheres: in vitro, pharmacokinetic and pharmacodynamic investigations. J Microencapsul. 2012;29:126–137.
- Darwish M, Hamed E, Messina J. Fentanyl buccal tablet for the treatment of breakthrough pain: pharmacokinetics of buccal mucosa delivery and clinical efficacy. Perspect Med Chem. 2010;4:11–21. PMC. S3928.
- Mercadante S, Adile C, Cuomo A, et al. Fentanyl buccal tablet vs. oral morphine in doses proportional to the basal opioid regimen for the management of breakthrough cancer pain: a randomized, crossover, comparison study. J Pain Symptom Manage. 2015;50:579–586.
- Shahab L, Brose LS, West R. Novel delivery systems for nicotine replacement therapy as an aid to smoking cessation and for harm reduction: rationale, and evidence for advantages over existing systems. CNS Drugs. 2013;27:1007–1019.
- Alur HH, Pather SI, Mitra AK, et al. Transmucosal sustained-delivery of chlorpheniramine maleate in rabbits using a novel, natural mucoadhesive gum as an excipient in buccal tablets. Int J Pharm. 1999;188:1–10.
- Ìkinci G, Şenel S, Wilson C, et al. Development of a buccal bioadhesive nicotine tablet formulation for smoking cessation. Int J Pharm. 2004;277:173–178.
- Ikinci G, Şenel S, Tokgözoğlu L, et al. Development and in vitro/in vivo evaluations of bioadhesive buccal tablets for nicotine replacement therapy. Die Pharmazie Int J Pharm Sci. 2006;61:203–207.
- Hoskin P, Hanks G, Aherne G, et al. The bioavailability and pharmacokinetics of morphine after intravenous, oral and buccal administration in healthy volunteers. Br J Clin Pharmacol. 1989;27:499–505.
- Dobs AS, Hoover DR, Chen M-C, et al. Pharmacokinetic characteristics, efficacy, and safety of buccal testosterone in hypogonadal males: a pilot study. J Clin Endocrinol Metab. 1998;83:33–39.
- Kassem MA, ElMeshad AN, Fares AR. Enhanced bioavailability of buspirone hydrochloride via cup and core buccal tablets: formulation and in vitro/in vivo evaluation. Int J Pharm. 2014;463:68–80.
- Onishi H, Yumoto K, Sakata O. Preparation and evaluation of ritodrine buccal tablets for rational therapeutic use. Int J Pharm. 2014;468:207–213.
- El-Mahrouk GM, El-Gazayerly ON, Aboelwafa AA, et al. Chitosan lactate wafer as a platform for the buccal delivery of tizanidine HCl: in vitro and in vivo performance. Int J Pharm. 2014;467:100–112.
- Passalacqua G, Canonica GW. Sublingual immunotherapy: focus on tablets. Ann Allergy Asthma Immunol. 2015;115:4–9.
- Dostálová M, Rabiskova M. Mucoadhesive oral tablets–a modern dosage form with controlled drug release. Ceska Slov Farm. 2000;49:55–61.
- Save T, Shah MU, Ghamande A, et al. Comparative study of buccoadhesive formulations and sublingual capsules of nifedipine. J Pharmacy Pharmacol. 1994;46:192–195.
- Lindert S, Breitkreutz J. Oromucosal multilayer films for tailor-made, controlled drug delivery. Expert Opin Drug Deliv. 2017;14:1265–1279.
- Vasisht N, Gever LN, Tagarro I, et al. Single-dose pharmacokinetics of fentanyl buccal soluble film. Pain Med. 2010;11:1017–1023.
- Vasisht N, Gever LN, Tagarro I, et al. Formulation selection and pharmacokinetic comparison of fentanyl buccal soluble film with oral transmucosal fentanyl citrate. Clin Drug Investig. 2009;29:647–654.
- Averineni RK, Sunderajan SG, Mutalik S, et al. Development of mucoadhesive buccal films for the treatment of oral sub-mucous fibrosis: a preliminary study. Pharm Dev Technol. 2009;14:199–207.
- Jones E, Ojewole E, Kalhapure R, et al. In vitro comparative evaluation of monolayered multipolymeric films embedded with didanosine-loaded solid lipid nanoparticles: a potential buccal drug delivery system for ARV therapy. Drug Dev Ind Pharm. 2014;40:669–679.
- Bai SA, Xiang Q, Finn A. Evaluation of the pharmacokinetics of single-and multiple-dose buprenorphine buccal film in healthy volunteers. Clin Ther. 2016;38:358–369.
- Vamshi Vishnu Y, Chandrasekhar K, Ramesh G, et al. Development of mucoadhesive patches for buccal administration of carvedilol. Curr drug deliv. 2007;4:27–39.
- Kumria R, Nair AB, Goomber G, et al. Buccal films of prednisolone with enhanced bioavailability. Drug Deliv. 2016;23:471–478.
- Keenan AV. Promising results for dexamethasome ointment for treatment of recurrent aphthae. Evid Based Dent. 2012;13:75.
- Scruggs J, Wallace T, Hanna C. Route of absorption of drug and ointment after application to the eye. Ann Ophthalmol. 1978;10:267–271.
- Sah AK, Suresh PK. Medical management of glaucoma: focus on ophthalmologic drug delivery systems of timolol maleate. Artif Cells Nanomed Biotechnol. 2017;45:448–459.
- Chopra V, Chauhan G, Kumar R, et al. Nanogels in the diagnosis and treatment of tuberculosis. Nanogels for Biomedical Applications UK: Royal Society of Chemistry; 2017. p. 53–76.
- Goyal G, Garg T, Malik B, et al. Development and characterization of niosomal gel for topical delivery of benzoyl peroxide. Drug Deliv. 2015;22:1027–1042.
- Kaur G, Narang R, Rath G, et al. Advances in pulmonary delivery of nanoparticles. Artif Cells Blood Substit Biotechnol. 2012;40:75–96.
- Naganuma M, Aoyama N, Suzuki Y, et al. Twice-daily budesonide 2-mg foam induces complete mucosal healing in patients with distal ulcerative colitis. J Crohn’s Colitis. 2015;10:828–836.
- Kaur P, Garg T, Rath G, et al. In situ nasal gel drug delivery: a novel approach for brain targeting through the mucosal membrane. Artif Cells Nanomed Biotechnol. 2016;44:1167–1176.
- Mahdi MH, Conway BR, Smith AM. Development of mucoadhesive sprayable gellan gum fluid gels. Int J Pharm. 2015;488:12–19.
- Abbate G, Levrini L, Caria M. Salivary pH after a glucose rinse: effect of a new mucoadhesive spray (Cariex®) based on sodium bicarbonate and Xylitol. J Clin Dent. 2014;25:71–75.
- Kawano Y, Imamura A, Nakamura T, et al. Development and characterization of oral spray for stomatitis containing irsogladine maleate. Chem Pharm Bull. 2016;64:1659–1665.
- Patel VF, Liu F, Brown MB. Advances in oral transmucosal drug delivery. J Control Release. 2011;153:106–116.
- Sakagami M, Sakon K, Kinoshita W, et al. Enhanced pulmonary absorption following aerosol administration of mucoadhesive powder microspheres. J Control Release. 2001;77:117–129.
- Guy G, Robson P. A Phase I, open label, four-way crossover study to compare the pharmacokinetic profiles of a single dose of 20 mg of a cannabis based medicine extract (CBME) administered on 3 different areas of the buccal mucosa and to investigate the pharmacokinetics of CBME per oral in healthy male and female volunteers (GWPK0112). J Cannabis Ther. 2004;3:79–120.
- Loehr B, Rankin R, Pontarollo R, et al. Suppository-mediated DNA immunization induces mucosal immunity against bovine herpesvirus-1 in cattle. Virology. 2001;289:327–333.
- Eley J, Pujari V, McLane J. Poly (lactide-co-glycolide) nanoparticles containing coumarin-6 for suppository delivery: in vitro release profile and in vivo tissue distribution. Drug Deliv. 2004;11:255–261.
- Yong CS, Oh Y-K, Kim Y-I, et al. Physicochemical characterization and in vivo evaluation of poloxamer-based solid suppository containing diclofenac sodium in rats. Int J Pharm. 2005;301:54–61.
- Takashima Y, Tsuchiya T, Igarashi Y, et al. Non-invasive ophthalmic liposomes for nucleic acid delivery to posterior segment of eye. Yakugaku Zasshi J Pharm Soc Japan. 2012;132:1365–1370.
- Babizhayev MA, Khoroshilova‐Maslova IP, Kasus‐Jacobi A. Novel intraocular and systemic absorption drug delivery and efficacy of N-acetylcarnosine lubricant eye drops or carcinine biologics in pharmaceutical usage and therapeutic vision care. Fundam Clin Pharmacol. 2012;26:644–678.
- Wertz P, Hoogstraate A, Squier C. Biochemical basis of the permeability barrier in skin and oral mucosa. Drugs Pharm Sci. 1996;74:27–49.
- Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10.
- Modgill V, Garg T, Goyal AK, et al. Permeability study of ciprofloxacin from ultra-thin nanofibrous film through various mucosal membranes. Artif Cells Nanomed Biotechnol. 2016;44:122–127.
- Kreuter J. Nanoparticulate systems for brain delivery of drugs. Adv Drug Deliv Rev. 2001;47:65–81.
- Al-Dhubiab BE, Nair AB, Kumria R, et al. Formulation and evaluation of nano-based drug delivery system for the buccal delivery of acyclovir. Colloids Surf B Biointerfaces. 2015;136:878–884.
- Al-Dhubiab BE, Nair AB, Kumria R, et al. Development and evaluation of buccal films impregnated with selegiline-loaded nanospheres. Drug Deliv. 2016;23:2154–2162.
- Lv Q, Shen C, Li X, et al. Mucoadhesive buccal films containing phospholipid-bile salts-mixed micelles as an effective carrier for Cucurbitacin B delivery. Drug Deliv. 2015;22:351–358.
- Singh D, Pradhan M, Nag M, et al. Vesicular system: versatile carrier for transdermal delivery of bioactives. Artif Cells Nanomed Biotechnol. 2015;43:282–290.
- Rao R, Nanda S. Sonophoresis: recent advancements and future trends. J Pharm Pharmacol. 2009;61:689–705.
- Giannola LI, Sutera FM, De Caro V. Physical methods to promote drug delivery on mucosal tissues of the oral cavity. Expert Opin Drug Deliv. 2013;10:1449–1462.
- Souza JG, Dias K, Pereira TA, et al. Topical delivery of ocular therapeutics: carrier systems and physical methods. J Pharm Pharmacol. 2013;66:507–530.
- Rawat S, Vengurlekar S, Rakesh B, et al. Transdermal delivery by iontophoresis. Indian J Pharm Sci. 2008;70:5.
- Leonard M, Creed E, Brayden D, et al. Evaluation of the Caco-2 monolayer as a model epithelium for iontophoretic transport. Pharm Res. 2000;17:1181–1188.
- Gratieri T, Kalia YN. Targeted local simultaneous iontophoresis of chemotherapeutics for topical therapy of head and neck cancers. Int J Pharm. 2014;460:24–27.
- Cubayachi C, do Couto RO, de Gaitani CM, et al. Needle-free buccal anesthesia using iontophoresis and amino amide salts combined in a mucoadhesive formulation. Colloids Surf B Biointerfaces. 2015;136:1193–1201.
- Wei R, Simon L, Hu L, et al. Effects of iontophoresis and chemical enhancers on the transport of lidocaine and nicotine across the oral mucosa. Pharm Res. 2012;29:961–971.
- Hu L, Silva SM, Damaj BB, et al. Transdermal and transbuccal drug delivery systems: enhancement using iontophoretic and chemical approaches. Int J Pharm. 2011;421:53–62.
- Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60:297–306.
- Blagus T, Markelc B, Cemazar M, et al. In vivo real-time monitoring system of electroporation mediated control of transdermal and topical drug delivery. J Control Release. 2013;172:862–871.
- Kichaev G, Mendoza JM, Amante D, et al. Electroporation mediated DNA vaccination directly to a mucosal surface results in improved immune responses. Hum Vaccin Immunother. 2013;9:2041–2048.
- Nickerson JM, Goodman P, Chrenek MA, et al. Subretinal delivery and electroporation in pigmented and nonpigmented adult mouse eyes. In: Shu-Zhen Wang, editor. Retinal Development. New York: Springer; 2012. p. 53–69.
- Yao S, Beckley ML, Liu D. Delivery of plasmid DNA into dental tissues of developing rat teeth by electroporation. In: Shulin Li, editor. Electroporation Protocols. New York: Springer; 2014. p. 179–188.
- Landström FJ, Nilsson CO, Reizenstein JA, et al. Electroporation therapy for T1 and T2 oral tongue cancer. Acta Oto-Laryngol. 2011;131:660–664.
- Shende P, Sardesai M, Gaud R. Micro to nanoneedles: a trend of modernized transepidermal drug delivery system. Artif Cells Nanomed Biotechnol. 2018;46:19–25.
- Kang S-M, Song J-M, Kim Y-C. Microneedle and mucosal delivery of influenza vaccines. Expert Rev Vaccines. 2012;11:547–560.
- Ma Y, Tao W, Krebs SJ, et al. Vaccine delivery to the oral cavity using coated microneedles induces systemic and mucosal immunity. Pharm Res. 2014;31:2393–2403.
- Lee S, McAuliffe DJ, Flotte TJ, et al. Photomechanical transdermal delivery: the effect of laser confinement. Lasers Surg Med. 2001;28:344–347.

