?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The major challenge for the treatment of inflammatory bowel disease (IBD) is the incompetence to deliver the drug molecule selectively at the site of inflammation. Taking this into consideration, we proposed development of mannosylated nanostructured lipid carrier system (Mn-NLCs) for active targeting and site-specific delivery of budesonide to the inflamed tissues. The developed Mn-NLCs were characterized for particle size and size distribution, zeta potential, %entrapment efficiency, FTIR and TEM analysis. Furthermore, to ensure delivery of developed cargo to the colonic region, the Mn-NLCs were encapsulated using Eudragit® S100 coated pellets. The in vivo evaluation of developed system was performed by employing oxazolone colitis rat model. The average particle size of Mn-NLCs (301.7 ± 2.88 nm) was found to be more than that of unconjugated NLCs (284.0 ± 4.53 nm) with marginally reduced % entrapment efficiency (90.88 ± 3.86%). The in vitro cytotoxicity studies using J774A.1 cell line revealed that Mn-NLCs were non-toxic as compared to pure drug. The in vivo evaluation depicted that Mn-NLCs showed significant reduction in clinical activity scoring, macroscopic and microscopic indexing, colonic myeloperoxidase activity and inflammatory cytokines. In conclusion, the developed Mn-NLCs appear to be promising for the treatment of IBD by selectively targeting inflamed colonic region as compared to unconjugated nanoparticulate system.
Graphical Abstract
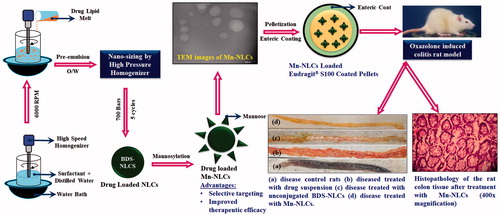
Introduction
Inflammatory bowel disease (IBD) is an idiopathic inflammatory disorder involving the mucosa and sub-mucosa of the colon [Citation1,Citation2]. In order to control the acute attacks and prevent recurrence of the disease, anti-inflammatory agents (both steroidal and non-steroidal) and immunosuppressive agents like corticosteroids are used as mainstay [Citation3,Citation4]. However, corticosteroids have many systemic side-effects, and hence, it is wise to have a targeted delivery of these agents to colon. For an adequate therapeutic effect, the delivery system should be such that it enables delivery of appropriate concentration of the drug to the proximal part of the colon without any major loss. Nonetheless, majority of the presently available therapeutic approaches lack this targetability [Citation5].
A colon targeted drug delivery system (CoDDS) could prove to be advantageous in terms of delivering drug locally to the site of inflammation. Since more than two decades, various approaches have been proposed for achieving CoDDS viz; pH-dependent CoDDS, time-dependent CoDDS, microbially triggered CoDDS, and pressure sensitive CoDDS [Citation6–12]. However, these CoDDS when used, may not target the drug molecule to the site of inflammation because of change in GI physiological condition of patients suffering from IBD. The most common challenge in the treatment of IBD is diarrhoea, which results into rapid clearance of large-sized dosage forms, thus, leading to reduced efficacy and increased adverse drug reactions. Hence, development of a novel targeted drug delivery systems (by taking into consideration the pathophysiological conditions of IBD), which can release the drug molecule selectively to the inflamed tissues (without affecting normal cells) is the need of an hour. Therefore, the most important step in this direction would be development of nano-sized dosage forms, which will get retained in colonic region for longer duration of time, and thus, improve biodistribution of drugs [Citation12,Citation13].
Nano-sized dosage forms demonstrated a site-specific targeting of drugs to the inflamed colonic region, thereby reducing the dose of drugs and their related side effects. This could be attributed to the fact that (i) the macrophages and neutrophils (of inflammation) readily uptakes nanoparticulate systems, and (ii) the nano-sized dosage forms easily penetrates through the intestinal mucosal layer [Citation12]. In addition, nano-sized dosage forms can also be delivered by means of active (surface engineering of particles) as well as passive (physicochemical properties of particles) targeting approaches. These explicit characteristics of nano-sized dosage forms ultimately leads to prolonged retention of drug at the site of inflammation [Citation12].
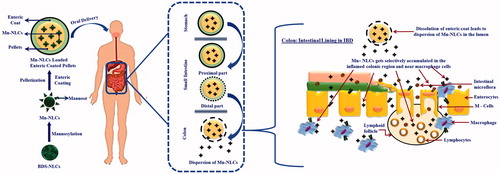
Looking into the advantages offered by lipid nanoparticulate systems over other colloidal carriers, a budesonide (BDS) loaded nanostructured lipid carrier systems (BDS-NLCs) was developed for treatment of IBD. Owing to its excellent topical efficacy and minimal systemic side effects, BDS was selected as a drug molecule. Further, it has been reported that about 70–80% of drug administered gets eliminated through feces, thus leading to poor therapeutic outcome [Citation14]. Hence, a stronger interaction with targeted tissues is therefore desirable and could lead to a noteworthy enhancement in therapeutic outcome. Taking this into consideration, a surface engineered BDS-NLCs was developed by employing mannose as ligand moiety. The mannose receptors [175-kDa transmembrane protein of the C-type lectin family (CLR)] are exclusively overexpressed on the surface of macrophages present at the site of inflammation [Citation15,Citation16]. Upon contact with this receptor system, the mannosylated BDS-NLCs will get rapidly internalized through receptor-mediated endocytosis, thus leading to targeted delivery of developed BDS-NLCs. Further, in order to achieve CoDDS of drug molecules, an enteric-coated (Eudragit® S100) pellet system carrying mannosylated BDS-NLCs was developed. Eudragit® S100 will protect the developed mannosylated BDS-NLCs, and prevent early release of drug in the upper part of gastrointestinal tract (GIT) [Citation17]. In colonic region, upon dissolution of an enteric coat, disintegration of pellets will take place, followed by dispersion of mannosylated BDS-NLCs at the site of action ().
Materials and methods
Materials
Budesonide was received as a kind gift from Zydus Healthcare, Ahmedabad, India. Compritol 888 ATO and Labrafac WL 1349 were received as a gift sample from Gattefosse India Private Limited, Mumbai, India. Eudragit® S100 was supplied as a gift sample from Evonik Röhm GmbH, Darmstadt, Germany. Poloxamer 188 was obtained as a gift sample from BASF India Ltd., Mumbai, India. Stearylamine and Polysorbate 80 were purchased from Central Drug House (Pvt) Ltd., New Delhi, India. d-mannose was purchased from Otto Chemie Pvt Ltd., Mumbai, India. Oxazolone (4-ethoxymethylene-2-phenyl-2-oxazolin-5-one) was procured from Sigma-Aldrich Co. LLC, St. Louis, MO, USA. Hexadecyltrimethylammonium bromide (CTAB) and o-dianisidine dihydrochloride was purchased from HiMedia Laboratories, Mumbai, India. Other excipients used were of standard pharmaceutical grade. All chemicals and reagents used were of analytical reagent grade.
Development of BDS-NLCs
High-pressure homogenization technique was used to develop drug-loaded NLCs. Briefly, BDS (10% w/w) was dissolved in lipid melt (heated at 10 °C above the melting point of solid lipid) consisting of Compritol 888 ATO (Saint-Priest, France) and Labrafac WL 1349 (Saint-Priest, France) (85:15), stearylamine (New Delhi, India) (10% w/w), and Span® 80 (New Delhi, India) (20% w/w). Simultaneously, an aqueous surfactant solution (Polysorbate (New Delhi, India) 80: 6%; Poloxamer (Ludwigshafen, Germany) 188: 0.05%) was prepared by heating at same temperature as that of lipid phase. Further, the melted lipid phase was added to the hot aqueous surfactant solution and was subjected to high-speed mechanical stirring at 6000 rpm (Remi Instruments Ltd., Mumbai, India). This leads to the formation of a pre-emulsion, which was subsequently homogenized using a high-pressure homogenizer (Panda Plus, Italy; GEA Niro Soavi, Düsseldorf, Germany) for 5 homogenization cycles at a pressure of 700 bar to obtain BDS-NLCs. The prepared BDS-NLCs were further lyophilized (Labfreeze, Beijing, China) at −80 °C for 48 h by using mannitol as cryoprotectant [Citation17].
Preparation of mannosylated BDS-NLCs (mn-NLCs)
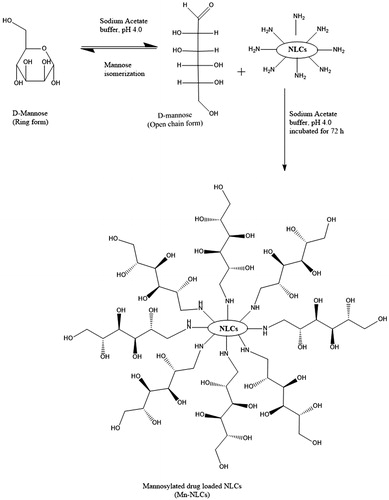
Mannose coating on nanoparticles was performed by employing the method previously reported by Kumar et al. [Citation18] with some modifications (). To summarize, d-mannose (50 mM) was dissolved in sodium acetate buffer (pH 4.0; 0.1 M), and subsequently added to uncoated (plain) BDS-NLCs. The mixture was continuously stirred on a magnetic stirrer (Remi, Mumbai, India) at an ambient temperature for 72 h to ensure the completion of reaction. The resulting Mn-NLCs was purified by dialyzing against double distilled water using a dialysis tube (molecular weight cut-off 12–14 kDa) for 24 h to remove unreacted mannose followed by lyophilization (Labfreeze, Beijing, China) [Citation19]. Further, the FTIR spectroscopic studies were performed to characterize the synthesis of Mn-NLCs.
In vitro drug release study
A dialysis membrane method was employed to determine the in vitro drug release from developed formulations. Briefly, 10 ml drug loaded NLCs dispersion were placed in the regenerated dialysis membrane (MWCS 12000–14000 Da, Himedia Ltd., Mumbai, India). These dialysis membrane were further placed in 50 ml pH 5.0 acetate buffer (mimic the acidic pH conditions during IBD) containing 2% w/v sodium lauryl sulphate maintained at 37 ± 0.5 °C in shaking water bath at 100 rpm. At given time intervals, 5 ml samples were withdrawn from the beaker and was replaced with the same volume of fresh medium. BDS content in the dissolution samples were estimated using UV/VIS spectrophotometer (Shimadzu UV 2450, Shimadzu Corporation, Kyoto, Japan) at 243 nm. The cumulative percentage release for BDS was calculated (mean ± SD, n = 3) over the sampling time points using Beer Lambert’s curve generated for the dissolution medium [Citation17].
Characterization of formulation
Particle size and zeta potential measurements
The size of the developed NLCs was determined using dynamic light scattering technique by employing Zetasizer Nano ZS (Malvern Instruments Ltd., Worcestershire, UK) at an angle of 90°. The zeta potential measurements were performed at 25 °C with electric field strength of 23 V/m [Citation17].
% Entrapment efficiency (%EE) and % drug loading (%DL)
The entrapment efficiency of NLCs was determined using Sephadex G-50 MP Biomedicals, California, USA minicolumn. The formulation was passed through a Sephadex G-50 minicolumn by centrifuging at 3000 rpm for 3 min in order to remove the unentrapped drug from NLCs dispersion. The drug-loaded NLCs were then lysed using 0.1% v/v Triton-X 100, and the amount of drug entrapped was determined using UV/VIS spectrophotometer at 243 nm [Citation17].
(1)
(1)
(2)
(2)
Fourier transform infrared spectroscopy (FTIR)
FTIR spectra was recorded over a wavenumber region of 4000–400 cm−1 using FTIR spectrometer (Shimadzu Corporation, Tokyo Japan). Briefly, a small amount of sample was mulled with an anhydrous potassium bromide (KBr) pellets, and the spectra was recorded.
Preparation of Mn-NLCs loaded CoDDS
Preparation of Mn-NLCs loaded CoDDS
Mn-NLCs loaded pellets (Batch MNP) were prepared by employing extrusion-spheronization technique (). The pellets were further enteric coated by employing 5% w/v Eudragit® S100, using pan coating technique. The coating process was performed till the desired coating level (15% w/w) was achieved [Citation17]. The pellets were cured in the coating pan for 15 min and then in a tray drier at 40 °C for 24 h. The percentage coating level of the pellets after coating was considered to be indicative of the coat thickness [Citation17].
Table 1. Composition of Mn-NLCs loaded pellets (mean ± SD, n = 3).
In vitro dissolution study of CoDDS
The in vitro dissolution studies of enteric-coated Batch MNP was performed by employing 900 ml dissolution medium at 100 rpm, 37 °C ± 0.5 °C temperature using USP dissolution apparatus I (basket method, Electro lab, TDT-06 T, Mumbai, India). Pellets (∼9 mg BDS) were filled in dialysis membrane, which was further placed in basket of dissolution apparatus. A sequential pH change method was applied to determine drug release behaviour from the developed CoDDS. Briefly, the CoDDS was subjected to pH 1.2 (2 h; stomach pH), pH 6.0 (1 h; duodenum pH), pH 7.2 (2 h; ileum pH), and pH 5.0 (acetate buffer; mimicking IBD condition) [Citation17,Citation20–26]. Samples were withdrawn at regular time intervals and were estimated using UV/VIS spectrophotometer at 243 nm for BDS content. The cumulative percentage drug released was calculated (mean ± SD, n = 3) using Beer Lambert’s curve generated in respective medium [Citation17].
In vitro studies
Cell culture
RPMI 1640 media 1640 supplemented with 10% fetal bovine serum, penicillin 10 IU/ml, and 100 μg/ml streptomycin was used to maintain the J774A.1 macrophage cell line. Cells were grown in T-25 cm2 flasks (Thermo Fischer Scientific, waltham, MA, USA) in an atmosphere of 5% CO2/95% air (v/v) at 37 °C.
Effect of developed formulations on cell viability
The effect of the developed formulations on cell viability was estimated using 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) dye assay. Briefly, J774A.1 cells (1.5 × 105 cells/well) were seeded in 96-well plates (Nest Scientific USA Rahway, NJ, USA) and maintained for 24 h at 37°C and 5% CO2 for cell attachment. The pure drug, blank NLCs (without drug) and Mn-NLCs were added in different concentrations (10–1000 µg/mL) and incubated for 48 h. Following this, the cells were washed and incubated for 2 h with 5 mg/ml MTT in RPMI. After 2 h, the medium was removed, and 200 μl DMSO was added to stop the reaction and absorbance was measured at 560 nm using a using ELISA plate reader (M. P. Biomedicals, Santa Ana, CA, USA, Model MR96A).
In vivo efficacy studies
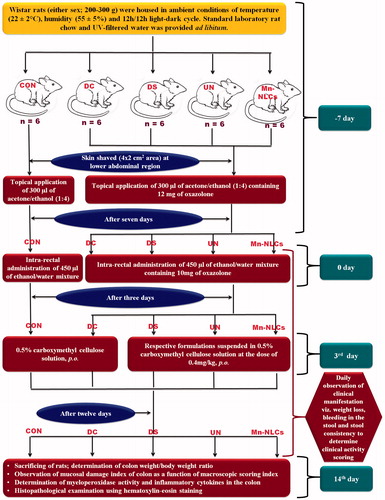
The ethical committee approval was taken from Institutional Animal Ethics Committee (Protocol No. IP/PCEU/MPH/18/029). Oxazolone-induced colitis rat model [Citation4] was used for in vivo efficacy study (). The quantification of myeloperoxidase (MPO) activity in colon was determined as described by Lamprecht et al. [Citation27]. The concentrations of pro-inflammatory cytokines (TNF-α and IL-1β) in the colon were determined by using a sandwich-type ELISA kits (Krishgen Biosystems, Mumbai, India) as per instructor’s manual using ELISA plate analyzer after determination of the total protein [Citation28]. For the histopathological assessment of the colonic inflammation, small sections of the colon were fixed in 4% formalin buffer overnight, washed with 70% ethanol and subsequently embedded in paraffin. Sections were taken and stained using hematoxylin-eosin dye. The histological score was determined in a blind fashion according to scoring of colitis [Citation29].
Figure 3. Schematic representation of oxazolone induced colitis rat model and treatment protocol. CON: normal control receiving only vehicle; DC: disease control; DS: diseased treated with drug suspension; UN: disease treated with enteric-coated pellets containing BDS-NLCs; Mn-NLCs: disease treated with enteric-coated pellets containing mannosylated NLCs.
Statistical analysis
Statistical analysis was performed with GraphPad InStat software (V. 3.00, GraphPad Software, San Diego, CA, USA). Statistical differences between the means of the various groups were evaluated using one-way analysis of variance (ANOVA) followed by Tukey’s test. Data were considered statistically significant at p values <.05.
Results and discussion
Preparation of BDS-NLCs
BDS-NLCs were prepared by employing hot high-pressure homogenization technique. Compritol ATO 888 and Labrafac® WL 1349 (medium chain triglyceride; MCT) was used as solid and liquid lipid, respectively (at ratio of 85:15). Further, it has been reported that MCT plays a significant role in inhibiting TNF-α and IL-8 [Citation30,Citation31]. Thus, apart from improving the solubility of BDS, MCT can also play a beneficial role in minimizing inflammatory responses in the treatment of IBD [Citation17,Citation30,Citation31]. Stearylamine was added to provide amine tailored surfaces on BDS-NLCs, which play a crucial role in subsequent steps of mannosylation.
Preparation of Mn-NLCs
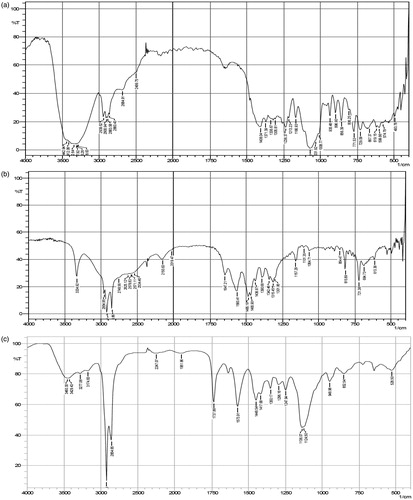
Mannosylation was executed by ring opening of mannose and subsequent reaction of its aldehyde group with free amine functionalities present over the surface of BDS-NLCs in acetate buffer (pH 4.0). This leads to formation of Schiff’s base, and mannose became bonded over the surface of BDS-NLCs. The presence of hydroxyl group (O–H) and carbonyl group (C–O) in pure mannose was confirmed by broad intense peaks around 3442.94–3288.63 cm−1 and 1070.49 cm−1 respectively (). The FTIR spectrum for pure stearylamine () revealed characteristic bands of the amine groups at 3334.92 cm−1, 1157.29 cm−1 and 815.89 cm−1. depicts N–H bending of secondary amine at 1573.91 cm-1 and C=N stretch at 1448.54 cm−1, thus revealing the formation of Schiff’s base (RCH=N–R bond), and confirming the formation of a linkage between mannose ligand and amine termination of the BDS-NLCs [Citation19]. Also, broad O–H stretch of mannose at 3460.30–3174.83 cm−1 and strong C–O stretch at 1124.5 cm−1 proved the presence of hydroxyl groups (in large number) of mannose in Mn-NLCs. The conjugation was also confirmed by the disappearance of peaks at 3332.76 cm−1, 1157.21 cm−1 and 815.83 cm−1 that depicts the presence of strong amine group.
Characterization of developed formulation
Entrapment efficiency
The %EE of developed Mn-NLCs (90.88 ± 2.45%) was found to be similar to that of uncoated BDS-NLCs (92.66 ± 3.42%) revealing very low loss of drug during conjugation process (). This could be attributed to the hydrophobic nature of drug molecule, which is molecularly dispersed in the lipid matrix structure of BDS-NLCs [Citation17], and which is insoluble in sodium acetate buffer (pH 4.0) (was used as a medium for mannosylation of BDS-NLCs) (refer supplementary data for %EE details).
Table 2. Characterization of drug loaded NLCs (mean ± SD, n = 3).
Particle size determination
The particle size analysis of developed Mn-NLCs was found to be great than that of BDS-NLCs (). An increment in size may be attributed to the conjugation of mannose on the surface of BDS-NLCs (refer supplementary data for particle size details). These results are in agreement with that of TEM analysis, which also indicates increase in the size of Mn-NLCs due to mannosylation of BDS-NLCs.
Zeta potential
The zeta potential of the developed BDS-NLCs was found to be 12.8 ± 0.50 mV. This could be due to the presence of amine functionalities at the surface of uncoated BDS-NLCs (). However, upon mannosylation, the zeta potential of Mn-NLCs gets lowered to 7.51 ± 0.71 mV. This could be attributed to masking of positively charged amine groups present on the surface of BDS-NLCs.
Morphology
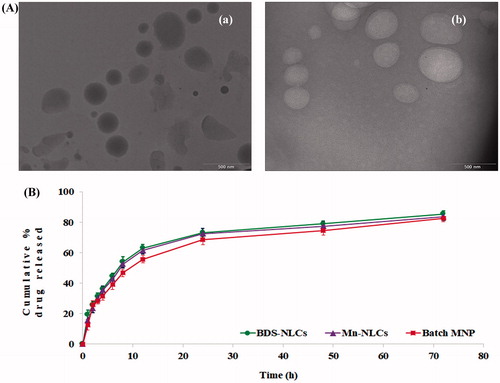
The TEM images of developed BDS-NLCs and Mn-NLCs revealed spherical shape of both the systems. Also, an augmentation in size due to mannosylation of BDS-NLCs could be revealed from the photomicrographs ().
In vitro drug release studies
Dissolution profile of BDS-NLCs and Mn-NLCs was found to be similar () with a similarity factor (f2) of 83.908. This resemblance in drug release profile revealed integrity of BDS-NLCs during mannosylation process.
Kinetics of drug release
In order to determine the kinetics of drug release from the prepared Mn-NLCs, the dissolution profile data of Mn-NLCs was fitted to zero-order, first-order, Higuchi, Weibull, Korsemeyer–Peppas, and Hixson–Crowell models. The release profiles of Mn-NLCs fitted best to the Weibull model, (showed the least F-value compared with other models) (). Thus, it may be concluded that the drug release from BDS-NLCs is best explained by Weibull model, i.e. the drug release mechanism involves dissolution, diffusion and mixed dissolution – diffusion rate limited processes [Citation17].
Table 3. F-values of Mn-NLCs using different kinetic models.
Preparation of Mn-NLCs loaded CoDDS
In vitro evaluation of Mn-NLCs loaded pellets
Dissolution profile of Mn-NLCs and Mn-NLCs loaded pellets (batch MNP) was found to be similar () with a similarity factor (f2) of 70.842. This resemblance in drug release profile revealed integrity of the pelleted Mn-NLCs during extrusion and spheronization process. Mn-NLCs, if broken during pelletization process, would have led to increase in the amount of drug released with reduction in f2 value [Citation17].
Further, Mn-NLCs loaded pellets were enteric coated by employing Eudragit® S100 (ES) at coating level of 15% w/w [Citation17]. The in vitro drug release studies revealed that a coating level of 15% w/w imparts lag time of 5 h (transit time to reach colon) (data not shown), adequate enough to prevent premature release of drug in the upper part of GIT.
Effect of nanoparticles on cell viability
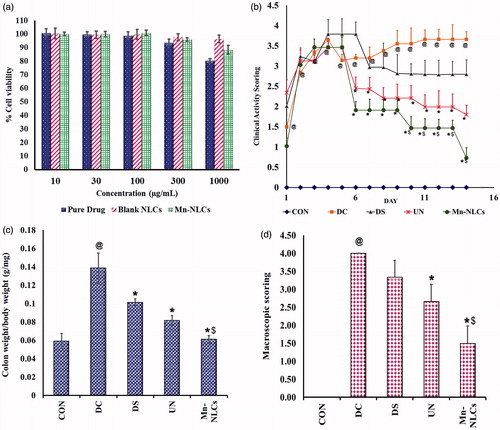
J774A.1 cell line is a well-established macrophage cell line [Citation32]. To study the effect of NLCs on cell viability, MTT assay was carried out on J774A.1 cell line. The results revealed that BDS, blank NLCs and Mn-NLCs did not exhibit cytotoxicity up to 100 µg/mL. At concentrations as high as 1000 µg/mL concentration, BDS showed ∼80% cell viability while blank NLCs and Mn-NLCs showed ∼96% and ∼88% cell viability respectively (). More than 95% cell viability by blank NLCs suggests that the developed nanocarrier is safe and non-toxic.
Figure 6. (a) Cell viability studies on J774A.1 cells after 48 h of exposure, (b) Clinical Activity Scoring during the period; no body weight reduction was scored as 0 point, 1–5% as 1 point, 5–10% as 2 points and more than 20% as 4 points. Scaling of stool consistency involved a 0 score for well-formed pellets, 2 points were assigned to pasty and semi-formed stools that did not stick to anus, and 4 points were given to liquid stool that stuck to the anus. Rectal bleeding was scored as 0 score for no blood, 2 for traces of blood, and 4 points to severe bleeding. The mean of all the individual scores formed the clinical activity scoring ranging 0–4, (c) colon weight to body weight ratio, and (d) macroscopic scoring index at the end of treatment period; where score was 0 if there was no damage to intestinal mucosa; score was 1 for mild hyper anaemia and oedema without ulcer or erosion of mucosal surface; score was 2 for moderate hyper anaemia and oedema with ulcer or erosion of mucosal surface; and score was 3 for severe hyper anaemia and oedema necrosis and ulcer appearing of mucosal surface with the major ulcerative area < 1 cm. @ significantly different than control group (p < .05); *significantly different than disease control group (p < .05); $ significantly different than BDS-NLCs treated group (p < .05). CON: normal control receiving only vehicle; DC: disease control; DS: diseased treated with drug suspension; UN: disease treated with enteric-coated pellets containing BDS-NLCs; Mn-NLCs: disease treated with enteric-coated pellets containing mannosylated NLCs. Data are presented as Mean ± SD (n = 6).
In vivo studies
Oxazolone-induced colitis is widely accepted model for IBD [Citation33]. Hence, the efficacy of the developed formulations was evaluated using oxazolone-induced colitis rat model.
Clinical activity scoring
Patients with IBD experience weight loss, diarrhoea, bloody stools, malnutrition, anaemia and abdominal cramps [Citation34,Citation35]. In order to study these hallmark symptoms of IBD, the clinical activity scoring was determined () (refer supplementary data for body weight). The clinical activity scoring steadily increased upto day 4 in all the groups, except in control group. The drug suspension reduced the clinical activity scoring from day 7 to 14; however, it was not statistically significant when compared to disease control group. Treatment with BDS-NLCs and Mn-NLCs produced a significant (p < .05) reduction in the scoring from day 6 to day 14 as compared to disease control group. Moreover, the reduction in clinical activity scoring in Mn-NLCs treated group was significantly (p < .05) different than BDS-NLCs treated group from day 10 to 14.
CLRs are one of the pattern recognition receptors, which are involved in first line of host defense. They are reported to be present on macrophages, monocytes, dendritic cells in small intestine as well as in the colon [Citation36], and they bind to the carbohydrates like mannose, α-mannan, β-glucan, etc. [Citation37]. Thus, targeting CLRs using Mn-NLC might have resulted in internalization of budesonide due to receptor-mediated endocytosis and producing better improvement than BDS-NLCs in clinical hallmarks of IBD in terms of weight loss, diarrhoea and bloody stools.
Colon weight/body weight ratio
During IBD conditions, decreased colon weight/body weight ratio has been reported due to failure in maintaining a normal growth velocity [Citation38]. Hence, these factors are used to identify the severity of inflammation. Colon weight to body weight ratio was seen to be significantly (p < .05) increased in disease control animals as compared to normal control animals. A significant (p < .05) decrease in the ratio was observed in drug suspension, BDS-NLCs and Mn-NLCs treated animals as compared to disease control. Reduction by Mn-NLCs was also significantly (p < .05) different than BDS-NLCs. This indicates that due to better selective targeting potential (at inflamed region) and drug accumulation, the Mn-NLCs have a better effect than BDS-NLCs in IBD ().
Macroscopic indexing
Macroscopic indexing helps to determine the extent of damage in intestinal mucosa. depicts gross morphology of the colon at the end of treatment period. The normal control group showed no mucosal damage of the colon. In disease control animals, a significant (p < .05) increase in macroscopic score marked by oedema, necrosis, ulceration and hyperaemia was observed as compared to normal control animals. In the drug suspension treated animals and BDS-NLCs treated animals, moderate hyperaemia and oedema with ulcer and erosion of mucosal surface was observed. Mn-NLCs treated animals were found to have patchy inflamed colon or minimal inflammation. The macroscopic index in Mn-NLCs treated animals was significantly (p < .05) different than disease control group and it was also significantly (p < .05) different than BDS-NLCs ().
Figure 7. Gross morphology of the colon at the end of treatment period (n = 6); (a) disease control rats, (b) diseased treated with drug suspension, (c) disease treated with enteric -coated pellets containing BDS-NLCs, and (d) disease treated with enteric-coated pellets containing mannosylated NLCs.
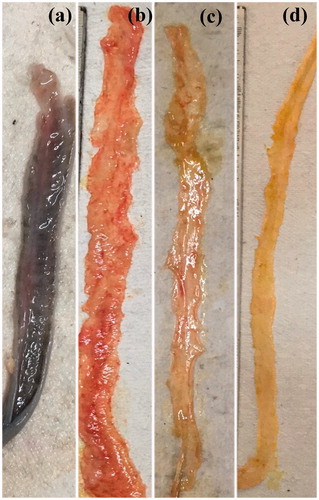
Macroscopic indexing reveals that the condition of the animals treated with ligand appended NLCs was significantly improved than those animals, which were treated with drug suspension or unconjugated BDS-NLCs due to more efficient therapeutic activity.
Tissue inflammatory markers
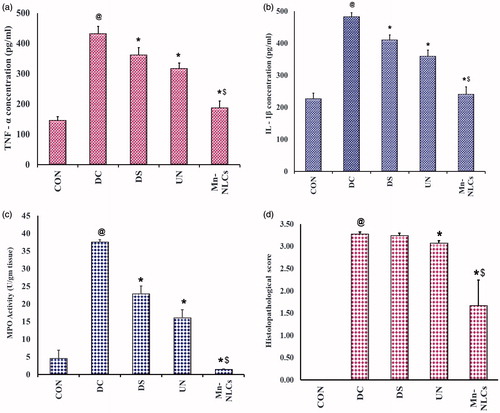
During active inflammatory disease, the activation of macrophages causes secretion of the pro-inflammatory cytokines in large quantity. It has been reported that the levels of TNF-α are increased in IBD, which is induced by NF-kB [Citation39]. In addition, NF-kB activation leads to increased expression of IL-1β in both ulcerative colitis [Citation40], and Crohn's disease [Citation41]. Therefore, the study of the levels of these cytokines, namely TNF-α and IL-1β, help to determine the extent of inflammation treated by the prepared formulation.
There was a significant (p < .05) increase in tissue TNF-α and IL-1β levels in disease control group as compared to normal control group. Treatment with drug suspension, BDS-NLCs and Mn-NLCs produced a significant (p < .05) decrease in these cytokine levels indicating therapeutic efficiency. The decrease in the tissue TNF-α and IL-1β levels by Mn-NLCs was significantly (p < .05) different than BDS-NLCs treated animals (). Thus, the reduction in the pro-inflammatory cytokine levels by Mn-NLCs suggests that it is possible to target the inflamed area by the aid of ligand appendation.
Figure 8. (a) Tissue TNF-α, (b) Tissue IL-1β, (c) MPO activity, and (d) histopathological scoring; where 0: no structural change; 1: inflammation visible; 2: leukocytes present in lamina propria: 3: leucocytes present in epithelium (3.1: < 5% crypts involved; 3.2: < 50% crypts involved; 3.3: > 50% crypts involved); 4: crypt destruction. @ significantly different than control group (p < .05); * significantly different than disease control group (p < .05); $ significantly different than BDS-NLCs treated group (p < .05); Each bar represents Mean ± SD (n = 6). CON: normal control receiving only vehicle; DC: disease control; DS: diseased treated with drug suspension; UN: disease treated with enteric-coated pellets containing BDS-NLCs; Mn-NLCs: disease treated with enteric-coated pellets containing mannosylated NLCs.
Myeloperoxidase (MPO) activity
MPO is used as a standard marker in IBD, since it is one of the most abundant protein in neutrophils. In inflammatory condition, it reflects the influx of neutrophils into the colon and hence the level of MPO activity can directly be correlated with number of neutrophils present [Citation42].
A significant (p < .05) increase in the MPO activity was found in disease control group as compared to normal control animals, which was significantly (p < .05) decreased by treatment with drug suspension, BDS-NLCs and Mn-NLCs. The decrease in MPO activity in group treated with Mn-NLCs was significantly (p < .05) different than BDS-NLCs treated animals. Thus, this indicates that the level of neutrophils in the colon of rats treated with Mn-NLCs is less, which again signifies active targeting of drug at the inflamed site of colon ().
Histopathological studies
Histopathological scoring depicts the level of loss of structural changes, presence of crypts, crypts destruction and mucosal surface changes and the scoring pattern is well-recognized method to understand the extent of inflammation [Citation43]. Thus, this study helps us understand the actual changes occurring during induction and treatment and hence gives us an idea of how efficient the formulation is.
Normal control group exhibited normal mucosa with no structural modifications of colon. In disease control group, there was a significant (p < .05) increase in histopathological scoring as compared to normal control group, as shown by more than 50% crypts destruction, presence of leucocytes and neutrophil infiltration. The histopathological microscopy of the rats treated with drug suspension also showed few ulceration. The histopathological microscopy of rats treated with BDS-NLCs depicted that less than 50% of the crypts showed the presence of leucocytes in the epithelium, which was significantly (p < .05) less as compared to disease control group. As compared to disease control group, the treatment with Mn-NLCs caused in significant (p < .05) reduction in the crypt destruction and neutrophil levels and this was significantly (p < .05) reduced when compared to BDS-NLCs group ().
indicates the representative figures of histology of colon tissue in which normal colon is made of well-designed, intact crypts along with the linings of muscularis and sub-mucosa. Crypts are normal tubular structure composed of cells that extend into the walls of the intestine. These crypts contain the cells that give rise to all of the other cells that migrate out of the crypts and then line the inner surface of the intestine. Inflammation of the crypts is known as cryptitis. The inflammation creates tiny sores called ulcers on the lining of the colon. It is a microscopical manifestation of several different diseases, which was seen in the diseased animals in the current study (). The histopathological microscopy of the rats treated with drug suspension showed ulceration hence proving lack of complete healing action of budesonide at the inflamed colon (). The colon of BDS-NLCs treated rats () when observed under microscope showed lesser ulceration as compared to the diseased rats. The crypts were almost completely formed when compared to the diseased rats. The rats treated with Mn-NLCs () showed re-formation of the crypts with leucocytes present in the lamina propia of the crypts, which symbolizes superficial damage to colon.
Figure 9. Representative figures of histopathology of the colon tissue after hematoxylin and eosin staining from (a) normal control rats, (b) disease control rats, (c) diseased treated with drug suspension, (d) disease treated with enteric-coated pellets containing BDS-NLCs, and (e) disease treated with enteric-coated pellets containing mannosylated NLCs; at [I] 100× magnification and [II] 400× magnification.
![Figure 9. Representative figures of histopathology of the colon tissue after hematoxylin and eosin staining from (a) normal control rats, (b) disease control rats, (c) diseased treated with drug suspension, (d) disease treated with enteric-coated pellets containing BDS-NLCs, and (e) disease treated with enteric-coated pellets containing mannosylated NLCs; at [I] 100× magnification and [II] 400× magnification.](/cms/asset/8a0de227-ba9f-4df9-afdc-177205158da4/ianb_a_1463232_f0009_c.jpg)
The improvement in histopathological studies can be attributed to the fact that CLR activation is reported to have beneficial effect in IBD due to its virtue of improvement in epithelial regeneration. CLRs are documented to enhance intestinal epithelial cell proliferation and differentiation and thereby repairing the damaged mucosa in IBD [Citation44]. In addition, CLRs activation causes increased production of IL-22, which in turn is responsible for regeneration of epithelial layer and strengthening of mucous barrier [Citation45,Citation46]. Altogether, it is possible that in addition to efficient targeting of the Mn-NLCs, activation of CLR due to mannosylated NLC, there is significant improvement in colitis condition.
Treatment of IBD continue to be challenging despite of advancements in research being carried out. For several years, strategies like development of microspheres [Citation47], liposomes [Citation48], microemulsions [Citation49], nanocores [Citation50], etc. have been adopted for targeted delivery of drug in the treatment of IBD. However, use of nanoparticulate drug delivery systems have been on the rise due to several advantages they offer in various diseases including IBD [Citation51]. Furthermore, targeting mannose receptor for selective drug delivery in IBD has recently gained importance. Xiao et al. [Citation15] developed nanoparticulate system targeting mannose receptor for IBD using siRNA. However, the author has performed only ex-vivo experiments to prove the efficacy of developed system and no in vivo animal testing was performed to provide proof of concept. Further, in previous study from our laboratory, a budesonide-loaded NLCs (unconjugated), systematically optimized by applying the concept of design of experiment (DoE) has been developed [Citation17]. In extension to this, in current investigation, we prepared mannose appended budesonide loaded NLCs, and have carried out detailed in vivo studies to provide the proof of concept for the treatment of IBD.
Conclusions
Mannose conjugated NLCs as an active targeting system for selective and site-specific delivery of BDS was developed for the treatment of IBD. Furthermore, in order to target the developed BDS-NLCs to colonic region, pellets were prepared successfully from Mn-NLCs without affecting the integrity of the nanoparticles. The pellets were enteric coated with Eudragit® S100 and were found to prevent premature release of drug in the upper part of GIT. The in vivo evaluation revealed that the developed Mn-NLCs get selectively accumulated in the inflamed tissues compared to unconjugated BDS-NLCs as depicted from reduction in clinical activity scoring, macroscopic and microscopic indexing, colonic myeloperoxidase activity, colonic inflammatory cytokine levels and improvement in histopathological architecture of the colon. Thus, it can be concluded that the developed Mn-NLCs appears to be more promising in the treatment of IBD by selectively targeting inflamed colonic region compared to unconjugated nanoparticulate system.
Supplemental_file.docx
Download MS Word (277.4 KB)Acknowledgements
The authors are thankful to Nirma University, Ahmedabad, India for providing necessary facilities to carry out the research work.
Disclosure statement
The authors report no declarations of interest.
Additional information
Funding
References
- Makhlof A, Tozuka Y, Takeuchi H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur J Pharm Biopharm. 2009;72:1–8.
- Klein S, Stein J, Dressman J. Site-specific delivery of anti-inflammatory drugs in the gastrointestinal tract: an in-vitro release model. J Pharm Pharmacol. 2005;57:709–719.
- Rodrigeuz M, Antunez JA, Taboada C, et al. Colon-specific delivery of budesonide from microencapsulated cellulosic cores: evaluation of the efficacy against colonic inflammation in rats. J Pharm Pharmacol. 2001;53:1207–1215.
- Lamprecht A, Yamamoto H, Takeuchi H, et al. Nanoparticles enhance therapeutic efficiency by selectively increased local drug dose in experimental colitis in rats. J Pharmacol Exp Ther. 2005;315:196–202.
- Lamprecht A. Multiparticulate systems in the treatment of inflammatory bowel disease. Curr Drug Targets Inflamm Allergy. 2003;2:137–144.
- Patel M, Shah T, Amin A. Therapeutic opportunities in colon specific drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2007;24:147–202.
- Patel M, Amin A. Recent trends in microbially and/or enzymatically driven colon-specific drug delivery systems. Crit Rev Ther Drug Carrier Syst. 2011;28:489–552.
- Patel MM. Cutting-edge technologies in colon-targeted drug delivery systems. Expert Opin Drug Deliv. 2011;8:1247–1258.
- Patel MM. Colon targeting: an emerging frontier for oral insulin delivery. Expert Opin Drug Deliv. 2013;10:731–739.
- Patel MM. Getting into the colon: approaches to target colorectal cancer. Expert Opin Drug Deliv. 2014;11:1343–1350.
- Patel MM. Colon: a gateway for chronotherapeutic drug delivery systems. Expert Opin Drug Deliv. 2015;12:1389–1395.
- Patel MM. Micro/nano-particulate drug delivery systems: a boon for the treatment of inflammatory bowel disease. Expert Opin Drug Deliv. 2016;13:771–775.
- Youshia J, Lamprecht A. Size-dependent nanoparticulate drug delivery in inflammatory bowel diseases. Expert Opin Drug Deliv. 2015;4:1–14.
- Moulari B, Béduneau A, Pellequer Y, et al. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J Control Release. 2014;188:9–17.
- Xiao B, Laroui H, Ayyadurai S, et al. Mannosylated bioreducible nanoparticle-mediated macrophage-specific TNF-α RNA interference for IBD therapy. Biomaterials. 2013;34:7471–7482.
- Wileman TE, Lennartz MR, Stahl PD. Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci USA. 1986;83:2501–2505.
- Sinhmar KG, Shah NN, Chokshi NV, et al. Process, optimization, and characterization of budesonide loaded nanostructured lipid carriers for the treatment of inflammatory bowel disease. Drug Dev Ind Pharm. 2018. DOI:10.1080/03639045.2018.1434194
- Kumar PV, Asthana A, Dutta T, et al. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J Drug Target. 2006;14:546–556.
- Jain A, Agarwal A, Majumder S, et al. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J Control Release. 2010;148:359–367.
- Patel MM, Amin AF. Process, optimization and characterization of mebeverine hydrochloride loaded guar gum microspheres for irritable bowel syndrome. Carbohydr Polym. 2011;86:536–545.
- Patel MM, Amin AF. Formulation and development of release modulated colon targeted system of meloxicam for potential application in the prophylaxis of colorectal cancer. Drug Deliv. 2011;18:281–293.
- Patel MM, Amin AA. Design and optimization of colon targeted system of theophylline for chronotherapy of nocturnal asthma. J Pharm Sci. 2011; 100:1760–1772.
- Patel MM, Amin AF. Development of a novel tablet-in-capsule formulation of mesalamine for inflammatory bowel disease. Pharm Dev Technol. 2013;18:390–400.
- Patel MM, Patel SL, Bhadani MN, et al. A synchronous colon specific drug delivery system for orally administered mesalamine. Acta Pharm Sci. 2009;51:251–260.
- Patel MM, Shah TJ, Amin AF, et al. Design, development and optimization of a novel time and pH-dependent colon targeted drug delivery system. Pharm Dev Technol. 2009;14:62–69.
- Patel MM. Formulation and development of di-dependent microparticulate system for colon-specific drug delivery. Drug Deliv Transl Res. 2017;7:312–324.
- Lamprecht A, Ubrich N, Yamamoto H. 1990. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J Pharmacol Exp Ther. 2001;299:775–781.
- Beloqui A, Coco R, Alhouayek M, et al. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int J Pharm. 2013;454:775–783.
- Geboes K, Ridell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409.
- Hoshimoto A, Suzuki Y, Katsuno T, et al. Caprylic acid and medium-chain triglycerides inhibit IL-8 gene transcription in Caco-2 cells: comparison with the potent histone deacetylase inhibitor trichostatin A. Brit J Pharmcol. 2002;136:280.
- Kono H, Enomoto N, Connor HD, et al. Medium-chain triglycerides inhibit free radical formation and TNF-alpha production in rats given enteral ethanol. Am J Physiol Gastrointest Liver Physiol. 2000;278:G467–G476.
- Ralph P, Nakoinz I. Phagocytosis and cytolysis by a macrophage tumour and its cloned cell line. Nature. 1975;257:393–394.
- Boirivant M, Fuss IJ, Chu A, et al. Oxazolone colitis: a murine model of T helper cell type 2 colitis treatable with antibodies to interleukin 4. J Exp Med. 1998;188:1929–1939.
- Panaccione R. Mechanisms of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9:529–532.
- Gasche C, Lomer MCE, Cavill I, et al. Iron, anaemia, and inflammatory bowel diseases. Gut. 2004;53:1190–1197.
- Lech M, Susanti HE, Römmele C, et al. Quantitative expression of C-type lectin receptors in humans and mice. Int J Mol Sci. 2012; 13:10113–10131.
- Lepenies B, Lee J, Sonkaria S. Targeting C-type lectin receptors with multivalent carbohydrate ligands. Adv Drug Deliv Rev. 2013;65:1271–1281.
- Ekstrom GM. Oxazolone-induced colitis in rats: effects of budesonide, cyclosporine A and 5- aminosalicylic acid. Scand J Gastroenterol. 1998;33:174–179.
- Yasukawa K, Tokuda H, Tun X, et al. The detrimental effect of nitric oxide on tissue is associated with inflammatory events in the vascular endothelium and neutrophils in mice with dextran sodium sulfate-induced colitis. Free Radic Res. 2012;46:1427–1436.
- Gan HT, Chen YQ, Ouyang Q. Sulfasalazine inhibits activation of nuclear factor-kappaB in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005;20:1016–1024.
- Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429.
- Mendoza JL, Abreu MT. Biological markers in inflammatory bowel disease: practical consideration for clinicians. Gastroenterol. Clin Biol. 2009;33:S158–S173.
- Marchal Bressenot A, Riddell RH, Boulagnon-Rombi C, et al. Review article: the histological assessment of disease activity in ulcerative colitis. Aliment Pharmacol Ther. 2015;42:957–967.
- Zhang YW, Ding LS, Lai MD. Reg gene family and human diseases. World J Gastroenterol. 2003;9:2635–2641.
- Carvalho A, Giovannini G, De Luca A, et al. Dectin-1 isoforms contribute to distinct Th1/Th17 cell activation inmucosal candidiasis. Cell Mol Immunol. 2012;9:276–286.
- Mizoguchi A. Healing of intestinal inflammation by IL-22. Inflamm Bowel Dis. 2012;18:1777–1784.
- Dadwal NA, Hallan SS, Sharma S, et al. Development of enteric-coated microspheres of embelin for their beneficial pharmacological potential in ulcerative colitis. Artif Cells Nanomed Biotechnol. 2017;45:1–9.
- Rahman M, Kumar V, Beg S, et al. Emergence of liposome as targeted magic bullet for inflammatory disorders: current state of the art. Artif Cells Nanomed Biotechnol. 2016;44:1597–1608.
- Zong S, Pu Y, Li S, et al. Beneficial anti-inflammatory effect of paeonol self-microemulsion-loaded colon-specific capsules on experimental ulcerative colitis rats. Artif Cells Nanomed Biotechnol. 2018;1–12. DOI:10.1080/21691401.2017.1423497
- Parihar AKS, Srivastava S, Patel S, et al. Novel catalase loaded nanocores for the treatment of inflammatory bowel diseases. Artif Cells Nanomed Biotechnol. 2017;45:981–989.
- Jaiswal P, Gidwani B, Vyas A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif Cells Nanomed Biotechnol. 2016;44:27–40.