Abstract
Chitosan-coated ISCOMATRIX nanoparticles co-administrated with PR8 influenza virus were successfully developed via a lipid film hydration method to evaluate their in vivo immuniadjuvant potential in immunization against influenza. The prepared ISCOMATRIX (ISC) and chitosan-coated ISCOMATRIX (ISC-CIT) showed a particle size of 171 and 233 nm with a zeta potential of –9.47 and +5.65, respectively. Furthermore, ISC-CIT formulations were co-administered with PR8 antigen (PR8-ISC-CIT) and their immunogenicity was investigated after intranasal and intramuscular immunization of BALBc/mice. The PR8-ISC formulation elicited more IFN-γ after intranasal or intramuscular administration compared with PR8-ISC-CIT formulation. In contrast, although PR8-ISC-CIT formulation administered by intranasal route secreted more IFN-γ, it significantly decreased the IgG2a/IgG1 ratio and a less immune response was induced. Altogether, the ISC-adjuvanted influenza PR8 antigen could be considered as a powerful intramuscular antigen delivery system for producing a variety of prophylactic and therapeutic vaccines.
Introduction
During the past 30 years, enormous efforts have been performed for development of new adjuvants to be able to induce both humoral and cellular immune responses, particularly those with critical criteria including safety, immunogenicity and clinical efficacy for human vaccines. Natural and synthetic polymers are also utilized to get nanoparticles carrier systems for development of nasal mucosal antibodies [Citation1]. For the first time, Morein et al. [2] in 1984 described the immunostimulating complex or “iscom”. Iscoms are usually caged-like structures that composed of phospholipid, cholesterol and saponin with mean diameter of ∼40 nm [Citation2–5]. Over the past 20 years, many progresses on development of human vaccines based on ISCOM adjuvant technology have been achieved (i.e. usually referred to as ISCOM®, a registered trademark of ISCOTEC AB, a CSL Limited company, Sweden) [Citation6]. ISCOM™-based vaccines have the requisite characteristics to be used as a novel prophylactic and therapeutic agents to induce strong cellular immune response and/or antibody response, including CTL (CD8 + T-cell) responses. They showed to be safe, highly immunogenic and efficacious that induced broad immune responses in human clinical trials resulted in 10- to 100-fold lower doses of antigen than is required with most of adjuvants, including aluminium [Citation7]. It has been also shown that ISCOM™-based vaccines are potent inducers of IgG2a antibody responses in mice resulted in a Th1-type immune response. Th1 immune response is generally considered as a critical factor for producing a variety of prophylactic and therapeutic vaccines, especially those are to prevent or treat viral infections and intracellular pathogens [Citation8,Citation9]. Although ISCOM™-based vaccines consist of saponin as an adjuvant; however, ideal CTL induction is reached when antigen is also physically incorporated into their structures. The rationale behind this is that the ISCOM™-based vaccines have a particulate nature by which more efficiently taken up by immune cells such as antigen-presenting cells (APCs) or dendritic cells. Furthermore, the antigen is rapidly processed and presented to CD8 + T-cells [Citation6,Citation10,Citation11]. Antibody induction has been proved after immunization with ISCOM™-based vaccines containing influenza virus antigens [Citation12,Citation13]. Also, antigen-specific proliferative T-cell responses have been reported in a variety of animal species immunized with an ISCOM™-based vaccine formulation with influenza virus antigens [Citation14]. In addition, it has been shown that ISCOM™-based vaccines that were physically associated with influenza virus antigens could prime T cells and result in proliferation of these cells and cytokine secretion (IL-4 and IFN-γ) [Citation15]. Also, immunological outcomes clearly indicated significant improvement in humoral as well as cellular immune responses after pulmonary immunization against mycobacterium tuberculosis with Antigen 85 complex (Ag85)-loaded ISCOMs in mice [Citation16]. ISCOM™-based vaccines were traditionally prepared with incorporation of viral membrane proteins with a hydrophobic nature [Citation17,Citation18]. However, incorporation of hydrophilic antigens into the ISCOM structures was not successful [Citation3]. This defect is mainly rooted from difficulty in manufacturing procedures. To overcome this limitation, ISCOMATRIX adjuvant (also called ISCOMATRIX™, a trademark of ISCOTEC AB) was introduced. ISCOMATRIX adjuvants are also usually cage-like structures consisting of phospholipid, cholesterol and saponin, but without the inclusion of antigen. These could be simply prepared by admixing the antigens with pre-formed ISCOMATRIX™ adjuvants [Citation11]. One of the most important physical properties of the ISCOMATRIX adjuvants is their negatively charged surface, help them to associate with positively charged materials such as chitosan.
Chitosan is a biocompatible and biodegradable cationic polymer derivatized from naturally occurring chitin, is composed of randomly distributed N-acetylglucosamine and b-(1,4)-linked glucosamine residues [Citation19,Citation20]. Numerous studies have established that chitosan is a penetration enhancer polymer for large hydrophilic compounds across the mucosal surfaces that not only increase the local therapeutic activity, but also increase the systemic availability of the drugs by increasing the residence time at the site of application [Citation21,Citation22]. The proposed mechanism is opening of intracellular tight junctions. Therefore, in this study, chitosan was used to 1 – act as a penetration enhancer and increase the trans-mucosal drug delivery; 2 – physically link the ISCOMATRIX to negatively charged antigens; and 3 – act as an adjuvant [Citation23]. Nasal influenza immunization has distinct features compared with intramuscular vaccines, providing protection at the pathogen’s entry site, higher levels of mucosal antibodies, cross-protection and needle-free application [Citation1]. Human influenza A/Puerto Rico/8//1934 (H1N1) virus (PR8) was the most common cause of human influenza at 2009 that is a subtype of influenza A virus [Citation24,Citation25]. Therefore, the PR8 antigen was mixed with ISCOMATRIX structures to evaluate the in vivo immunoadjuvant potential of these promising adjuvants.
Materials and methods
Materials
Low molecular weight chitosan (deacetylation degree: 95%, intrinsic viscosity (1% solution: 11 cP) was donated by Primex (Siglufjordur, Iceland). IgG1 and IgG2a secondary antibodies were obtained from Zymed Inc. (South San Francisco, CA, USA). Coating and detection mAb antibodies for IFN-γ and IL-4 as well as streptavidin-HRP were obtained from Mabtech (Nacka Strand, Sweden). Concanavalin A was obtained from Sigma Aldrich (St. Louis, MO, USA). Fetal calf sera (FCS), RPMI1640 culture medium and penicillin–streptomycin solution were purchased from Sigma Aldrich (St. Louis, MO, USA).
BALB/c mice and PR8 antigen were purchased from the Pasteur Institute of Iran. Mice were treated based on the guidelines of the Institutional Ethical Committee of Mashhad University of Medical Sciences.
Preparation of chitosan-coated ISCOMATRIX NPs
A lipid film hydration method was used to prepare the ISCOMATRIX (ISC) formulation [Citation26]. The 2:2:1 (w/w/w) ratios of the EPC (Egg phosphatidylcholine) lipid: Quil-A saponin: cholesterol were used. Firstly, 8 mg EPC and 4 mg cholesterol were dissolved in chloroform, followed by the removal of chloroform under reduced pressure by a rotary evaporator (Hettich, Germany). Next, a mixture of tert-butanol and sucrose solution (100 mg/ml) was added to the dried lipid film (4 ml, v/v 1:1) and stirred for 5 min. The resulting lipid phase was flash-frozen on dry ice and then freeze-dried overnight at –60 °C (Lyph-Lock 12; Labconco Corp, Kansas City, MO, USA). To hydrate the solid matrices, a PBS solution (0.01 M, pH 7.4) of Quil A (8 mg/4 ml) was poured into the lipid phase and was subsequently subjected to short bath sonication for 10 min to facilitate the dispersion. Finally, the ISCOMATRIX dispersion was extruded through the polycarbonate membranes with pore diameters between 100 and 400 nm (Avestin, Ottawa, Canada). The total lipid concentration in the obtained formulation was 3 mg/ml.
In the next step, Chitosan-coated ISC (ISC-CIT) formulation was prepared by physical interaction between chitosan and ISC formulation [Citation27,Citation28]. One part (w) of ISC was simply mixed with two parts (w) of chitosan. Each component was dissolved in phosphate buffer (PB, 8 mM, pH 6). Finally, PR8 antigen was added to the ISC-CIT formulation and simply mixed to prepare the PR8-ISC-CIT formulation. The PB dispersion of ISC-CIT formulations showed a pH of 6.2 that had a good colloidal stability for two weeks at 2–8 °C in PB solution. Therefore, all of ISCOMATRIX formulations were prepared in PB and stored at 2–8 °C until use.
Particle size distribution and morphology
Dynamic light scattering analysis (NANO-ZS, Malvern, UK) and transmission electron microscopy (Philips CM120; Philips Electron Optics, Holland) were used to determine the particle size distribution as well as the morphological of ISC nanoparticles.
In vivo vaccination protocol
The in vivo evaluation of immunoadjuvant potential of prepared ISCOMATRIX formulations was investigated in male BALB/c mice. Eight groups were immunized by intramuscular (Im) or intranasal (In) routes with: 1: PBS solution as a negative control (only subcutaneously injection), 2 and 3: PR8 antigen (15 µg/mouse), 4 and 5: PR8-ISC formulation (15 µg PR8 antigen +25 µg ISC/mouse), 6 and 7: PR8-ISC-CIT formulation (15 µg PR8 antigen +25 µg ISC +20 µg chitosan/mouse) and 8 and 9: Blank ISC formulation (25 µg ISC/mouse). Mice (6 per group) were injected three times in two-week intervals with these formulations. For nasal immunization, an intraperitoneal injection of ketamine and xylazine (100 and 10 µg/g body weight, respectively) were used to anesthetize the mice and a total volume of 5 µl of each formulation was administered into the two separated nostrils. For Im immunization, a total volume of 100 µl of each formulation was administered.
In vitro cytokine production
An ELISA method was used to detect the levels of IFN-γ and IL-4 produced by spleen cells. Ten days after the last booster injection, 3 mice in each group were sacrificed and the spleens were removed, aseptically. To obtain a single-cell suspension, the spleen tissues were homogenized and then the mononuclear cells were removed using ammonium chloride [Citation29]. Next, the washed splenocytes were resuspended in RPMI 1640 culture medium and seeded in 96-well plates at a density of 2 × 105 cells/well. Then, the spleen cells were stimulated with either culture medium alone as negative control, PR8 antigen (10 µg/ml), or Concanavaline A (Con A) as positive control and incubated at 37 °C for 72 h. After that, the concentration of IL-4 and IFN-γ were determined in the supernatants using ELISA technique based on the manufacturer’s instructions (Mabtech, Nacka, Sweden).
Antibody isotype assay
Ten days after the last booster injection, the mice blood samples were obtained by heart puncture and retro orbital bleeding. The blood was allowed to coagulate at 4 °C and then the serum was collected by centrifuging for 10 min at 14,000 rpm. The serum samples were and kept at −20 °C [Citation30]. The sera of vaccinated BALB/c mice were used to titrate IgG1 and IgG2a antibodies using an ELISA technique [Citation31]. Briefly, 96-well plates were coated with 50 μl (10 µg/ml in bicarbonate buffer, pH 9.6) of PR8 antigen and kept overnight at 4 °C. After washing the plates, they were blocked with 300 µl of 2.5% BSA in PBS-Tween per well for 1 h at 37 °C. Different dilutions of serum were added to the plates for 75 min at 37 °C. After washing with PBS-Tween solution, plates were treated with IgG1 and IgG2a secondary antibodies based on the manufacturer’s instructions (Zymed Inc., South San Francisco, CA, USA). Optical density was measured by using a microplate reader (StatFax® 4200 microplate reader, NEOGEN® Corporation, USA) at 450 nm, with a reference wavelength of 630 nm.
Statistical analysis
Statistical analysis was performed using the version 6 of the GraphPad Prism (La Jolla, CA, USA). The data were analyzed by a Tukey’s multiple comparison tests and a one-way analysis of variance (ANOVA).
Results
Characterization of ISCOMATRIX formulations
In this study, ISCOMATRIX nanoparticles were prepared by a lipid film hydration method. The mean diameter of ISC formulation was 171.0 nm, as measured by DLS method (). The morphology of ISC NPs was depicted by TEM (). Coating of ISC NPs with chitosan significantly increased the particle size to 233.9 nm. Chitosan has a positive charge, thus resulting in higher zeta potential for the chitosan-coated ISC formulation compared to that of ISC alone. The obtained results are summarized in .
Figure 1. Particle size distribution and morphology and of ISC nanoparticles. (A) and (B) show the particle size and morphology of ISC nanoparticles using DLS and TEM analysis, respectively.
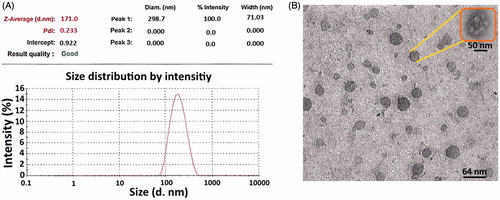
Table 1. Particle size, zeta potential and PDI of ISC formulation.
Cytokine assay
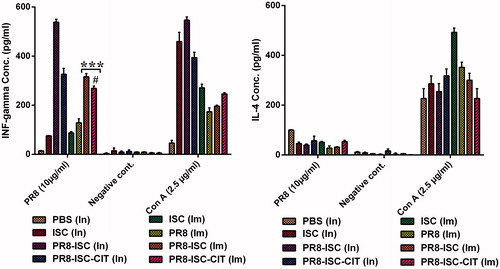
Splenocytes of mice immunized with different formulations were cultured. The concentration of IL-4 and IFN-γ in the supernatant of cell cultures were determined by an ELISA method (). The results show that a more significant IFN-γ response was induced following In administration of PR8-ISC formulations as compared with Im delivery of these formulations (p < .001). Also, a significant reduction of IFN-γ concentration was observed when the PR8-ISC formulations were coated with CIT. The difference between IFN-γ concentrations induced by PR8-ISC-CIT formulation after nasal administration was more significant than Im injection. Altogether, ISCOMATRIX NPs as efficient immunoadjuvant could significantly increase the IFN-γ response to the PR8 whole virus after In (p < .001) and Im administration (p < .001). Additionally, no significant secretion of IL-4 was observed after immunization with these formulations.
Figure 2. BALB/c mice were injected with different formulations. Splenocyets were cultured 10 days after the last booster injection and stimulated in vitro with either PR8 antigen (10 µg/ml), Con A as positive control and culture medium alone as negative control incubated for 72 h. ELISA technique was used to determine the concentration of IFN-γ and IL-4 in supernatants. Significant differences between the PR8-ISC (Im) and PR8-ISC-CIT (Im) groups with the PR8 (Im) group marked as ***(p < .001). Significant differences between PR8-ISC-CIT (Im) and PR8-ISC-CIT (In) groups labelled with #(p < 0.05). Data expressed as mean ± SD (n = 6).
Antibody response
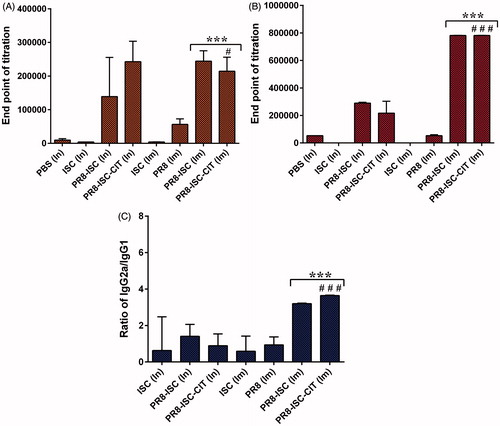
In this study, the adjuvanticity of ISCOMATRIX NPs was investigated by immunization of BALB/c mice [Citation30]. The sera of vaccinated BALB/c mice were used to titrate IGG1 and IgG2a antibodies using an ELISA technique [Citation31]. ISCOMATRIX NPs could significantly increase both IgG1 and IgG2a antibody responses against PR8 whole virus after In and Im administration (). Generally, no significant difference was observed between anti-PR8 IgG antibody titres of CIT-coated formulations and non-coated ones after In or Im administration. The results also show that after Im administration, the IgG2a antibody titres are significantly higher than In administration, both after administration of PR8-ISC and PR8-ISC-CIT formulations (p < .001) (). Also, the IgG2a/IgG1 ratio was significantly higher in intramuscularly administered PR8-ISC and PR8-ISC-CIT formulations to those induced with intranasally delivered formulations (p < .001) ().
Figure 3. The level of IgG1 (A), IgG2a (B) antibodies and the IgG2a/IgG1 ratio (C) of BALBc/mice immunized by In and Im routes, ten days after the last booster injection with different formulations. The assays were performed using an ELISA method in triplicate at different dilutions of serum samples. Significant differences between the PR8-ISC (Im) and PR8-ISC-CIT (Im) groups with the PR8 (Im) group marked as ***(p < .001). Significant differences between PR8-ISC-CIT (Im) and PR8-ISC-CIT (In) groups labelled with #(p < .05), ##(p< .01) and ###(p < .001). Data expressed as mean ± SD (n = 6).
Discussion
Although aluminium salts are used in almost of the currently adjuvanted human vaccines, they usually induce a Th2 antibody dominated response. Accordingly, they are not suitable adjuvants for vaccination against intracellular pathogens, chronic infections or cancers. To overcome this limitation, ISCOM and ISCOMATRIX adjuvants have shown good potentials [Citation6,Citation7]. ISCOMATRIX-adjuvanted vaccines are simply prepared by admixing the antigen with preformed ISCOMATRIX nanoparticles. Sjölander et al. [Citation32] showed that a CTL response as well as strong mucosal and systemic immune responses was induced when PR8 antigen was co-administered with ISCOM formulations and delivered by the In route in BALBc/mice.
In this study, it has been shown that ISCOMATRIX NPs as efficient immunoadjuvant could significantly increase the IFN-γ response to the PR8 whole virus, after both In (p < .001) and Im administration (p < .001). Helgeby et al. [Citation33,Citation34] incorporated both the cholera toxin A1 (CTA1)-DD and PR8 influenza virus into the ISCOM and demonstrated that the immunogenicity of PR8 virus is significantly augmented after In immunization of BALBc/mice when compared with PR8 virus alone.
Both ISCOMATRIX and PR8 are negatively charged. With a hypothesis that connection between antigen and ISCOMATRIX could increase the potential of co-delivery of the both particles to the same APC or macrophage and increase the immune responses, ISCOMATRIX NPs were coated with chitosan as a cationic polymer. It is supposed that this cationic coat could attach to the PR8 and help to co-delivery. However, after co-administration of chitosan coated ISCOMATRIX NPs with PR8 virus (PR8-ISC-CIT), the IFN-γ concentration was less than PR8-ISC group. However, the negative results (p < .01) could be attributed to the agglomeration of chitosan-coated PR8-ISC (PR8-ISC-CIT) and lower uptake of this formulation by M cells and APCs. After nasal administration, the difference between IFN-γ concentrations induced by these two formulations was more significant (p < .001) than im injection (p < .01). After nasal administrations, formulations should first pass through or be uptaken by epithelial, and mainly microfold cells. The possible aggregation of chitosan-coated PR8-ISC (PR8-ISC-CIT) could adversely affect this step and result in lower uptake and lower immune responses. But after Im injection, because of the absence of this epithelial barrier, both PR8-ISC-CIT and PR8-ISC formulations are similarly uptaken by immune cells, and no significant differences were observed between the immune responses induced by PR8-ISC and PR8-ISC-CIT formulations.
It has been also shown that influenza antigens are potent inducers of IgG2a antibody responses in mice and resulted in a Th1-type immune response that is generally considered as a critical factor for producing a variety of prophylactic and therapeutic vaccines, especially those are to prevent or treat viral infections and intracellular pathogens [Citation8,Citation9]. Therefore, it is beneficial to elicit a higher ratio of IgG2a/IgG1 antibody titres for immunization against influenza virus. The ratio of IgG2a/IgG1 antibody titres is usually used as an indicator of Th profile [Citation35]. When the IgG2a/IgG1 ratio is higher, it is in favour of Th1 immune response that is elicited by a cell-mediated response to IFN-γ [Citation36]. IFN-γ has an important role in blocking the virus spread [Citation37]. Renegar et al. [Citation38] showed that a vaccine formulation against Barratt (BAR) strain of influenza virus has significantly increased the ratio of IgG2a/IgG1 antibody titres. Also, Mahmoudi et al. investigated the immunogenicity of ISCOMs formulations loaded with a type 2 herpes simplex virus (HSV-2) antigen. Higher IgG2a antibody, IFN-γ and IL-2 levels were observed in BALBc/mice immunized subcutaneously [Citation39]. In another study, Madhum et al. [Citation40] showed a strong antibody responses and a the high frequency of multifunctional Th1 CD4 + cells after Im immunization of BALBc/mice with ISCOM-adjuvanted influenza A H5N1 virosomal vaccine than those of non-adjuvanted vaccines . In many studies, the ability of cationic ISCOM derivations (PLUSCOMs) in antigen attachment and elicitation of T cell responses was also compared to anionic ISCOMs. It has been shown that the PLUSCOMs have advantages including the incorporation of purified anionic antigen and inducing T cell responses similar to ISCOMs [Citation41,Citation42].
After In or Im administration, PR8-ISC formulation had the potential to elicit more IFN-γ levels compared with PR8-ISC-CIT formulation. However, it significantly decreased the ratio of IgG2a/IgG1 antibody titres. After nasal administration of these formulations, less immune responses than Im delivery was induced; especially when this formulation coated with CIT. Altogether, although after Im administration no significant difference was observed between anti-PR8 IgG titres of CIT-coated and non-coated formulations, both of these formulations had induced a significantly higher ratio of IgG2a/IgG1 antibody titres to those induced with non-adjuvanted PR8 antigen (p < .001). This notable achievement showed that ISCOMATRIX adjuvanted PR8 vaccines are potent inducer of Th1 immune responses, especially after Im delivery.
Conclusions
It is concluded that ISCOMATRIX NPs are efficient immunoadjuvants for immunization against PR8 whole influenza virus. This effect was proved after immunization from both nasal and Im routes. However, the chitosan coated ISCOMATRIX NPs, as cationic ISCOMATRIX adjuvant, did not show any superiority over ISCOMATRIX NPs. Chitosan-coated ISCOMATRIX NPs sowed less or comparable immune responses after In and Im administration, respectively.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kaur L, Sharma A, Yadav AK, et al. Recent advances on biodegradable polymeric carrier-based mucosal immunization: an overview. Artif Cells Nanomed Biotechnol. 2018;46:452–464.
- Morein B, Sundquist B, Höglund S, et al. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature. 1984;308:457–460.
- Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–928.
- Sharma S, Mukkur T, Benson HA, et al. Pharmaceutical aspects of intranasal delivery of vaccines using particulate systems. J Pharm Sci. 2009;98:812–843.
- Homhuan A, Prakongpan S, Poomvises P, et al. Virosome and ISCOM vaccines against Newcastle disease: preparation, characterization and immunogenicity. Eur J Pharm Sci. 2004;22:459–468.
- Sanders MT, Brown LE, Deliyannis G, et al. ISCOM-based vaccines: the second decade. Immunol Cell Biol. 2005;83:119–128.
- Sanders MT, Deliyannis G, Pearse MJ, et al. Single dose intranasal immunization with ISCOMATRIX™ vaccines to elicit antibody-mediated clearance of influenza virus requires delivery to the lower respiratory tract. Vaccine. 2009;27:2475–2482.
- Coulie PG, van Snick J. Enhancement of IgG anti-carrier responses by IgG2 anti-hapten antibodies in mice. Eur J Immunol. 1985;15:793–798.
- Coutelier J-P, Van der Logt J, Heessen F, et al. IgG2a restriction of murine antibodies elicited by viral infections. J Exp Med. 1987;165:64–69.
- D’Souza MJ, Tawde SA, Akalkotkar A, et al. Nanotechnology in vaccine delivery. Mol Vaccines. 2014;2:727–741.
- Pearse M, Drane D. ISCOMATRIX TM adjuvant: a potent inducer of humoral and cellular immune responses. Vaccine 2004;22:2391–2395.
- Sjölander A, Cox JC, Barr IG. ISCOMs: an adjuvant with multiple functions. J Leukoc Biol. 1998;64:713–723.
- Skene CD, Sutton P. Saponin-adjuvanted particulate vaccines for clinical use. Methods. 2006;40:53–59.
- Rimmelzwaan G, Baars M, Van Beek R, et al. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–765.
- Potter C, Jennings R. Effect of priming on subsequent response to inactivated influenza vaccine. Vaccine. 2003;21:(9):940–945.
- Pabreja S, Garg T, Rath G, et al. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif Cells Nanomed Biotechnol. 2016;44:532–539.
- Sambhara S, Woods S, Arpino R, et al. Heterotypic protection against influenza by immunostimulating complexes is associated with the induction of cross-reactive cytotoxic T lymphocytes. J Infect Dis. 1998;177:1266–1274.
- Coulter A, Harris R, Davis R, et al. Intranasal vaccination with ISCOMATRIX® adjuvanted influenza vaccine. Vaccine. 2003;21:946–949.
- Günbeyaz M, Faraji A, Özkul A, et al. Chitosan based delivery systems for mucosal immunization against bovine herpesvirus 1 (BHV-1). Eur J Pharm Sci. 2010;41:531–545.
- Hembram KC, Prabha S, Chandra R, et al. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif Cells Nanomed Biotechnol. 2016;44:305–314.
- Dodane V, Khan MA, Merwin JR. Effect of chitosan on epithelial permeability and structure. Int J Pharm. 1999;182:21–32.
- Kumar K, Dhawan N, Sharma H, et al. Bioadhesive polymers: novel tool for drug delivery. Artif Cells, Nanomed Biotechnol. 2014;42:274–283.
- Wen Z-S, Xu Y-L, Zou X-T, et al. Chitosan nanoparticles act as an adjuvant to promote both Th1 and Th2 immune responses induced by ovalbumin in mice. Mar Drugs. 2011;9:1038–1055.
- Johnson A, Chen L-M, Winne E, et al. Identification of influenza A/PR/8/34 donor viruses imparting high hemagglutinin yields to candidate vaccine viruses in eggs. PLoS One. 2015;10:e0128982.
- Liu H, Bungener L, ter Veer W, et al. Preclinical evaluation of the saponin derivative GPI-0100 as an immunostimulating and dose-sparing adjuvant for pandemic influenza vaccines. Vaccine. 2011;29:2037–2043.
- Mehravaran A, Jaafari MR, Jalali SA, et al. The role of ISCOMATRIX bilayer composition to induce a cell mediated immunity and protection against leishmaniasis in BALB/c mice. Iranian J Basic Med Sci. 2016;19:178.
- Amirnasr M, Sankian M, Rezaei A, et al. Immunization against HTLV-I with chitosan and tri-methylchitosan nanoparticles loaded with recombinant env23 and env13 antigens of envelope protein gp46. Microb Pathog. 2016;97:38–44.
- Tafaghodi M, Kersten G, Jiskoot W. Nano-adjuvanted polio vaccine: preparation and characterization of chitosan and trimethylchitosan (TMC) nanoparticles loaded with inactivated polio virus and coated with sodium alginate. Nanomed J. 2014;1:220–228.
- Jaafari MR, Badiee A, Khamesipour A, et al. The role of CpG ODN in enhancement of immune response and protection in BALB/c mice immunized with recombinant major surface glycoprotein of Leishmania (rgp63) encapsulated in cationic liposome. Vaccine. 2007;25:6107–6117.
- Mosafer J, Teymouri M, Abnous K, et al. Study and evaluation of nucleolin-targeted delivery of magnetic PLGA-PEG nanospheres loaded with doxorubicin to C6 glioma cells compared with low nucleolin-expressing L929 cells. Mater Sci Eng C. 2017;72:123–133.
- Badiee A, Jaafari MR, Khamesipour A. Leishmania major: immune response in BALB/c mice immunized with stress-inducible protein 1 encapsulated in liposomes. Exp Parasitol. 2007;115:127–134.
- Sjölander S, Drane D, Davis R, et al. Intranasal immunisation with influenza-ISCOM induces strong mucosal as well as systemic antibody and cytotoxic T-lymphocyte responses. Vaccine. 2001;19:4072–4080.
- Helgeby A, Robson NC, Donachie AM, et al. The combined CTA1-DD/ISCOM adjuvant vector promotes priming of mucosal and systemic immunity to incorporated antigens by specific targeting of B cells. J Immunol. 2006;176:3697–3706.
- Andersen CS, Dietrich J, Agger EM, et al. The combined CTA1-DD/ISCOMs vector is an effective intranasal adjuvant for boosting prior Mycobacterium bovis BCG immunity to Mycobacterium tuberculosis. Infect Immunol. 2007;75:408–416.
- Maruggi G, Chiarot E, Giovani C, et al. Immunogenicity and protective efficacy induced by self-amplifying mRNA vaccines encoding bacterial antigens. Vaccine. 2017;35:361–368.
- Tao W, Fu T, He Z, et al. Evaluation of immunostimulatory effects of n-(2-hydroxy) propyl-3-trimethylammonium chitosan chloride for improving live attenuated hepatitis A virus vaccine efficacy. Viral Immunol. 2017;30:120–126.
- Ito R, Ozaki YA, Yoshikawa T, et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362–2371.
- Renegar KB, Small PA, Boykins LG, et al. Role of IgA versus IgG in the control of influenza viral infection in the murine respiratory tract. J Immunol. 2004;173:1978–1986.
- Mohamedi S, Brewer J, Alexander J, et al. Antibody responses, cytokine levels and protection of mice immunised with HSV-2 antigens formulated into NISV or ISCOM delivery systems. Vaccine. 2000;18:2083–2094.
- Madhun AS, Haaheim LR, Nilsen MV, et al. Intramuscular Matrix-M-adjuvanted virosomal H5N1 vaccine induces high frequencies of multifunctional Th1 CD4+ cells and strong antibody responses in mice. Vaccine. 2009;27:7367–7376.
- McBurney WT, Lendemans DG, Myschik J, et al. In vivo activity of cationic immune stimulating complexes (PLUSCOMs). Vaccine. 2008;26:4549–4556.
- Mehravaran A, Jaafari MR, Jalali SA, et al. Cationic immune stimulating complexes containing soluble leishmania antigens: preparation, characterization and in vivo immune response evaluation. Iranian J Immunol. 2015;12:274.
