Abstract
Clinical manifestations of the elevated plasma triacylglycerol (TG) include a greater prevalence of atherosclerotic heart disease, acute pancreatitis, diabetes mellitus, hypertension, and ischemic vascular disease. Hence, these significant health troubles have attracted scientific attention for the precise detection of TG in biological samples. Numerous techniques have been employed to quantify TG over many decades, but biosensors hold the leading position owing to their superior traits such as highly specific recognition for target molecules, accuracy, minituarization, small sample requirement and rapid response. Enzyme-based electrochemical biosensors represent an instantaneous resolution for the foremost bottlenecks constraining laboratory prototypes to reach real time bedside applications. We highlight the choice of transducers and constructive strategies to design high-performance biosensor for the quantification of triglycerides in sera and early diagnosis of health problems related to it. In the present review, a small effort has been made to emphasize the significant role of enzymes, nanostructured metal oxides, graphene, conducting polypyrrole, nanoparticles, porous silicon, EISCAP and ENFET in enabling TG biosensors more proficient and taking a revolutionary step forward.
Introduction
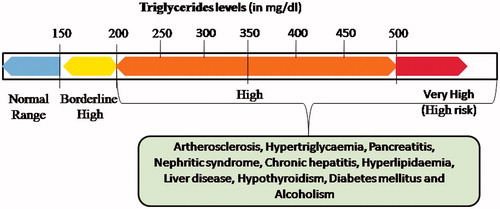
Triglycerides (TGs, triacylglycerols), also acknowledged as natural fats, an ester composed of one glycerol molecule attached to three fatty acid molecules (Unsaturated/saturated or both). The ester bond is formed between the hydroxyl groups of glycerol and the carboxyl groups of the fatty acids. Triacylglycerols are the chief components of very-low-density lipoprotein (VLDL) and chylomicrons, which are a lead source of energy in metabolism besides transporting dietary fat throughout the bloodstream [Citation1]. In the digestive tract, triglycerides are broken into monoacylglycerol and free unsaturated fats, in a procedure called lipolysis, with the discharge of bile and lipases, which in turn move to absorptive enterocytes, cells coating the digestive tract. Abnormally high rate of lipoprotein synthesis and low rate of catabolism results in accumulation of TGs. One more reason for triglyceride accumulation may be reduced activity of triacylglycerol lipase, which catalyzes the hydrolysis reaction of TG into free fatty acids and glycerol. Quantification of triglycerides (TGs) in human serum is crucial for health care diagnostics since its high levels in the bloodstream could result in hyperlipidemia. In human body, the level of TGs less than 150 mg/dl is considered to be normal. The condition is known as hyperlipoprotenemias when the level lies between 150–199 mg/dl and high risk of pancreatitis is associated with TGs level above 500 mg/dl. The reference range of triglycerides level in blood has been shown in . The presence of excessive triglycerides (above 500 mg/dl) in the blood is one of the leading causes of diabetes mellitus, liver obstruction, hypertension, atherosclerosis, Alzheimer disease [Citation2] and various cardiovascular diseases [Citation3]. In addition, the elevated amount of TGs causes reduction in nitric oxide level and raising the sum of many inflammatory compounds, which are partly responsible for vascular injury and endothelial dysfunction [Citation4]. Consequently, the determination of total triglycerides level in serum has attracted clinical significance as a marker of deviation from the normal range, which could affect the lipid metabolism in our body and early diagnosis of a range of diseases associated with it.
Figure 1 Reference range of triglycerides and various disorders associated with high level of TGs in human body.
Various methods including titrimetric method [Citation5], fluorometric method [Citation6], colorimetric method [Citation7], micro method [Citation8], spectrophotometric method [Citation9], enzymic colourimetric [Citation10], enzymatic centrifugal method [Citation11], chromatographic methods [Citation12], nuclear magnetic resonance method [Citation13] and mass fragmentographic method [Citation14] have been used for the triglyceride detection in serum or plasma. However, these conventional methods display some fine property but they are underprivileged in certain aspects such as low-sensitivity, non-specificity, instrument complexity, requirement of skilled personnel to operate, time consuming, etc. as shown in [Citation15–20]. In addition, there is requirement of pretreatment and derivatization step of sample as these methods are extremely complicated for small volume samples. Fortunately, biosensing methods overwhelms these drawbacks as they possess intrinsic advantages such as rapid sensing, broad detection range, easy operation, low-detection limit, ease of miniaturization and high sensitivity. This rare combination of properties makes biosensors suitable candidates for various promising applications in clinical diagnosis of diseases linked with high level of TGs at an early stage. Recently, the huge impact of nanomaterials as transducing element in the fabrication of improved/advanced biosensors has been reported with the progression of bionanoelectrochemistry. Many scientific articles have been published concerning the favorable alliance between the nanomaterials and biomolecules to enhance the electron transfer rate during the biochemical reaction. Nowadays, an imperative range of novel metal oxide nanostructures are being synthesized and gradually implicated in the construction of biosensors for monitoring specific biological molecules. The significance of using nanostructured modified electrodes is unquestionable as they display an unique property to augment the rate of electron transfer between the active site of enzyme and the electrode [Citation21]. In this article, we describe a comprehensive review exploring the recent trends and principal strategies utilized for improvement in the field of TG biosensors.
Table 1. Various conventional methods for triglyceride determination with their merits and demerits.
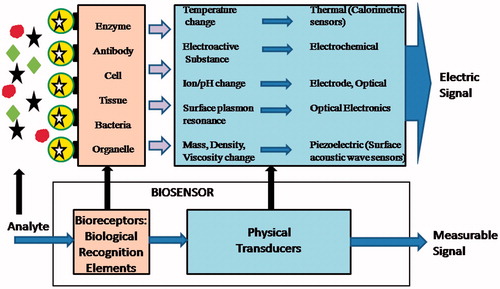
Basic strategy of a biosensor
Technology has helped us to form a biosensor, a device in which an analyte is detected by combination of a biological component with a physico-chemical detector component (). Basically, three parts constitute a biosensor – a biological element, detector element or the transducer and allied electronic or signal processors [Citation22]. Enzymes, microorganisms, tissues, organelles, antibodies, cell receptors, nucleic acids can be immobilized as a sensitive biological element for the detection of specific analyte. The transducer represents the second component of the biosensor body and works in a physico-chemical mode like electrochemical, optical, piezoelectrical or thermal that converts the resulting output signal from the interaction of the analyte with the biological element into another quantifiable signal. The continuous or discrete electric signal are generated, which are relative to the amount of single analyte or a group of analytes being sensed [Citation23].
Working of TG biosensors
For the biosensor industry, the fabrication of an ideal biosensor for the monitoring of TGs in the human sera must be a major area of concern as TG is the biomarker of various diseases such as pancreatitis, cardiovascular disease, diabetes and Alzheimer’s disease. Among various techniques and processes accessible for constructing TG biosensors, enzyme-based electrochemical biosensors have engrossed the utmost interest owing to their rapidity, simplicity, high-specificity, moderate-sensitivity and cost-effectiveness for the routine analysis [Citation24]. The interaction of three enzymes (lipase (LIP), glycerol kinase (GK) and glycerol phosphate oxidase (GPO) is involved in the working of TG biosensors. Enzymes can be immobilized on different surfaces in different modes: physical adsorption, covalent binding, entrapment and encapsulation. Mode of immobilization depends on the type of matrix or substrate and its site of action as well [Citation25]. Tributyrin and triolein have been used as substrate for enzymes. It is essential to treat both tributyrin and triolein with surfactant (Triton X-100) to convert their hydrophobic nature into hydrophilic and then it is diluted with the distilled water as per requisite. The partial solubility of serum TGs poses another constraint and consequently hampering the analysis. To combat with this problem, it is obligatory to incubate the sera samples with 0.22 mM Triton X100 in phosphate buffer (pH-6.5). This step facilitates the preparation of efficient sample for biosensing techniques. Under optimum conditions, the standard curve between the concentrations of TG and response of the biosensor deduce the level of triglyceride present in the sample.
TG biosensors: categorization
The biosensors for the determination of TG as analyte can be categorized as electrochemical biosensors, metal oxide based biosensors, flow injection based biosensors, conducting polymer based biosensors, microgel based optical biosensors and enzyme nanoparticles based biosensors as described below:
TG biosensors based on electrochemical techniques
There are different measurements principles involved in the working of various electrochemical TG biosensors and thus classified accordingly: amperometric, conductometric and potentiometric biosensors.
Amperometric TG biosensors
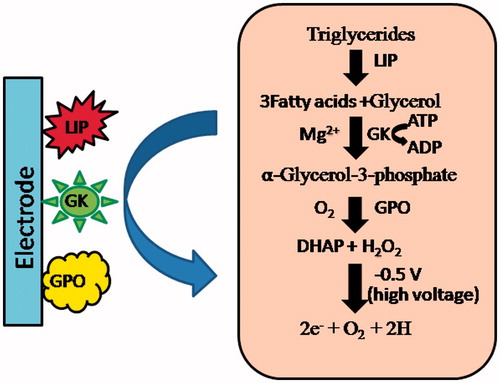
In electrochemical techniques, amperometry is insightful in which current is the signal of concern linearly depending upon the concentration of the target analyte when a steady potential is applied [Citation26]. In amperometric TG biosensors, the collective reaction of enzymes –lipase (LIP), glycerol kinase (GK) and gycerol-3-phosphate oxidase (GPO) generate H2O2 from TG, which is finally decomposed to release electrons at high voltages as shown in . In an enzymatic reaction cascade, H2O2 produced is directly proportional to the flow of electrons, i.e. current which in turn, is directly proportional to the amount of analyte (TG) in the sample [Citation27]. There are mainly two types of amperometric biosensors, which have been reported for determination of TG in past years: (1) Membrane based TG biosensors, (2) DO metric based TG biosensors
Figure 3 Schematic representation of electrochemical reactions involved in response measurement of enzyme based amperometric TG biosensor.
Membrane based TG biosensors
Amperometric TG biosensors employing different membranes such as polyvinyl acetate (PVA) [Citation17], gelatin membrane [Citation28], egg shell [Citation29], cellulose acetate (CA) [Citation16] and PVC membranes [Citation15,Citation30] have been fabricated in recent years. Various techniques such as physical adsorption, covalent binding, microencapsulation, entrapment, cross-linking were used to immobilize enzymes on these membranes. In these biosensors, the membrane bound enzymes-lipase, GK and GPO were mounted onto a Pt or glass electrode (GC) or Au electrode (in case of egg shell membrane only) to assemble a working electrode. Then, the working electrode was connected to the reference electrode (Ag/AgCl) and auxillary electrode (Pt wire) through the potentiostat. Under a potential of 0.4 V, the electrons generated from the H2O2 in the enzymatic reaction were measured by the fabricated biosensors. The measured current was directly proportional to the concentration of triolein/TG. In 1997, Schoemaker et al. constructed a sensitive three-electrode system for the determination of triglycerides [Citation31]. Collagen membrane was used to immobilize diaphorase and GIDH enzymes. In the presence of phosphate buffer containing 2, 6 dichlorophenol-indophenol (DCPIP), incubation of serum samples was done with a microbial lipase. The ensuing glycerol was then oxidized by NAD+ accompanying GIDH; the NADH generated was then oxidized by DCPIP. Serum triglycerides ranging from 10 to 500 mg/dL were simply assayed using small amount of sample in less than 15 min. Later, various TG biosensors based on membranes were fabricated, which displayed dynamic linear range along with low-detection limit in a short response time as shown in with the comparison of their analytical properties. In the near future, it is anticipated that the use of biocompatible artificial membranes would be promising as the matrix for immobilization of enzymes, which would help in enhancing the selectivity and sensitivity of biosensors for the rapid, cheap and effective detection of TGs.
Table 2. Membrane and DO metric based amperometric TG biosensors and comparison of their analytical properties.
DO metric based TG biosensors
Dissolved oxygen (DO) which is consumed in the oxidation reaction of triglycerides by membrane bound with enzymes lipase, glycerol kinase (GK), glycerol-3-phosphate oxidase (GPO) is measured in these biosensors. This dissolved O2 is directly proportional to the concentration of TG. These biosensors are very simple to handle and can be easily operated in the laboratories without employment of special trainee and expertise. The disadvantage with DO based TG biosensors is that atmospheric oxygen can interfere in their working and thus resulting in low sensitivity. Earlier in 1984, Kelly et al. developed a TG measurement system, which involved indirect monitoring of NADH using its reaction with oxygen by horseradish peroxidase (HRP) [Citation32]. The optimal response was shown at 33˚C temperature and 7.5pH within 300 s by the proposed biosensor. The biosensor showed linearity and LOD as 0.05 to 0.3 mM and 0.05 mM, respectively. provides a comparison of various DO metric based TG biosensors [Citation33,Citation34].
Conductometric/impedimetric TG biosensors
In a TG biosensor based on impedimetric technique, there is electrochemical deposition of polyaniline nanotubes (PANI-NT) onto the surface of indium-tin-oxide (ITO) coated glass plate. Then, covalent immobilization of enzyme- lipase is done by using glutaraldehyde. The change in the charge transfer resistance (RCT) of PANI-NT film as a result of production of fatty acid molecules during TG hydrolysis, detects the varying concentration of TG. The fabricated Lipase/PANI-NT/ITO bioelectrode for the detection of TG in sera displayed sensitivity of 2.59 × 10−3 K Ω−1 mg/dL and linearity ranging from 25 mg/dL to 300 mg/dL with a short response time of 20 s [Citation35]. These biosensors are easy to incorporate into microprocessor-controlled diagnostic tools and hence used as powerful method to estimate TG concentration. The demerits include the requirement of technological precision of the device along with the operating procedures [Citation36].
Potentiometric TG biosensor
Catalytic hydrolysis of triglycerides into glycerol and free fatty acid by the lipase enzyme forms the basis of potentiometric TG biosensors. The production of fatty acid causes a change in the pH of the solution containg analyte, which is then deliberated by applying an open circuit potential configuration. In these biosensors, the potential difference between the working electrode and the reference electrode is measured at zero current flow. The reference electrode generates a constant potential while a variable potential is produced by the working electrode, which rely on the analyte concentration [Citation37].
Sub-categorization of potentiometric TG biosensors
On the basis of principle followed by different potentiometric biosensors, they can be additionally classified as porous silica, electrolyte-insulator-semiconductor capacitor (EISCAP), field effect transistor (ENFET) based TG biosensors. Various potentiometric/ion selective electrode based TG biosensors and comparison of their analytical properties have been described in .
Table 3. Potentiometric /ion selective electrode based TG biosensors and comparison of their analytical properties.
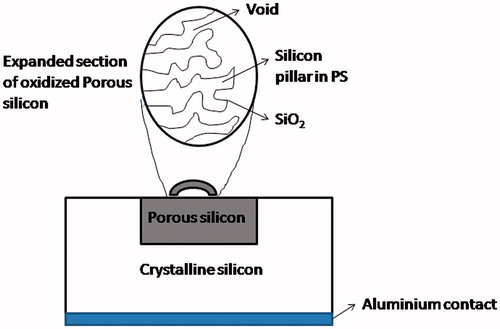
TG biosensors based on porous silicon
The advantage of easy fabrication, greater adsorption property and controllable pore size makes porous silicon a leading candidate for various applications. In addition, it is attuned with standard silicon processing technology. Porous silicon (PSi) is prepared from the p-type crystalline silicon, which was oxidized thermally. It is utilized to immobilize lipase enzyme, which hydrolyses triglycerides to fatty acids. In 2003, Reddy et al. fabricated a potentiometric biosensor based on porous silicon for the quantitative estimation of triglycerides. The schematic representation of the biosensor has been shown in . PSi was employed as an enzyme reactor. The enzyme was immobilized via physical adsorption on to the oxidized porous silicon, which was made-up from silicon. The employment of enzyme solution- oxidized porous silicon-crystalline silicon structure was done to sense the changes in pH of the solution during the hydrolysis of tributyrin as a result of shift in the capacitance-voltage (C-V) characteristics. The change in pH was proportional to the concentration of triglycerides present in the solution. The sensitivity of the sensor was high along with good reproducibility and reversibility. The LOD of the proposed biosensor for triglyceride was 1 µM [Citation38]. In 2016, Al-Hardan et al. developed a highly sensitive pH sensor, which utilized porous silicon (PSi) as an extended gate field-effect transistor (EGFET). They prepared PSi with pore sizes in the range of 500 to 750 nm and a depth of approximately 42 µm. Further when PSi was tested for sensing of hydrogen ion in different pH buffer solutions, it was revealed that the PSi had a sensitivity value of 66 mV/pH (a super Nernstian value). The sensor considers stability to be in the pH range of 2-12 was considered highly stable for the sensors based on PSi. The hysteresis values of the proposed PSi sensor were approximately 10.5 and 8.2 mV in the high and low pH loop, respectively [Citation47]. Some of the drawbacks of these PSi based sensors include less specificity, high cost and complicated operation.
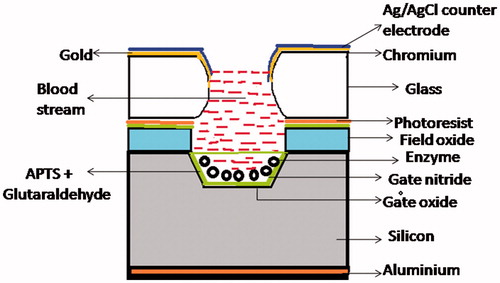
TG biosensors based on electrolyte-insulator-semiconductor capacitor (EISCAP)
Electrolyte–insulator–semiconductor capacitor (EISCAP) is mainly a pH sensor, which displays a shift in the measured capacitance-voltage (CV) characteristics with changes in the pH of the electrolyte (containing the bioanalyte) [Citation39]. Owing to its easy preparation and possessing a planar surface, EISCAP is preferred over other transducers such as NH4-ISFET and pH-FET in the biosensors [Citation40]. In EISCAP, enzyme molecules are immobilized via cross-linking, which ensures superior reproducibility. EISCAP turn into a particular biosensor because of the enzyme. When tributyrin, a short chain TG, is hydrolyzed in the presence of enzyme lipase, generates butyric acid and glycerol as a product resulting into more acidic electrolyte. The change in the pH of the solution is directly proportional to the amount of tributyrin produced. In 2008, Vemulachedu et al. developed a miniaturized TG biosensor based on the cubical pits of dimensions 1500 μm ×1500 μm × 100 μm, which have a capacity to hold 0.1 μL of an electrolyte. During the course of the reaction, the flat band voltage shift in the capacitance voltage (C-V) characteristics was monitored and the changes in pH of the solution were sensed through the EISCAP. In comparison with the sensors of bigger dimensions, these miniature cells displayed high reaction rate. The drawbacks of the proposed sensor included complexity in preparation and requirement of 30 min post processing time [Citation41]. In 2009, Fernandez et al. fabricated a biosensor for the estimation of triglycerides. For the stable measurements, the prepared biosensors imposed the existence of a buffer, which confines the sensitivity of the sensor at low amount of the analyte upto 1 mM [Citation42]. In 2011, Preetha et al. developed a modified EISCAP sensor for the determination of triglycerides in blood. The EISCAP was optimized to operate at 25 °C in 1 mg lipase in 1 mM Tris–HCl buffer (pH 7.4) and 0.25 mM phosphate buffer (pH 6) containing 1 M KCl. The sensor displayed sensitivity of 55 ± 0.5 mV per unit change of pH and correlation coefficients of 0.979 along with higher storage stability of around 180 days [Citation43]. In 2014, Veeramani et al. constructed a TG biosensor based on EISCAP as shown in . It estimated the concentration of TG within the quantifiable range of 50–150 mg/dL in short time period of less than 5 min [Citation44].
TG biosensors based on field effect transistor (ENFET)
Ion-selective field effect transistor (ISFET) is a strong platform to expand area of development in the field of biosensors. The FET devices have similar electronic properties as that of the conventional metal oxide semiconductor field-effect transistors (MOSFETs), therefore they respond quickly to the chemical environment changes, which facilitates a highly sensitive and steadfast pathway for the biomolecules detection. A variety of techniques have been utilized such as polymer entrapment or covalent bonding, to link enzymes on to the gate surface of a FET. In 1986, Nakako et al. developed an enzyme electrode based on hydrogen ion-sensitive field effect transistors (pH-FET’s) for the analysis of neutral lipid. The electrode was made up of two pH-FET’s and a platinum wire. One of the FET’s was immobilized with lipase membrane. The wide concentration ranges of triglycerides were determined by the proposed electrode within a short response time of 2 min [Citation45]. The electrode suffered few technological and fundamental problems including instability in the functional groups of the sensing layer and impurities of the semiconductor. In 2008, Vijayalakshmi et al. fabricated a FET based novel sensor in which the thermostable lipase enzyme was immobilized on the magnetic nickel ferrite nanoparticles and a permanent magnet was applied below the gate of the FET. The TG concentrations within the range of 0.1–1.5% were estimated by the method [Citation46]. The biosensor offered high-yield manufacturing, high speed and low cost, without compromising the sensitivity in diagnostics.
TG biosensors based on conducting polymer
Researchers have shown enormous interest in the electrochemical biosensors employing conducting polymer (CP) as they are cost-effective, stable, durable and easy to fabricate. In addition, these biosensors offer a direct electrical visualization of the analyte presence along with high selectivity and sensitivity when an appropriate enzyme is immobilized in the conducting polymer matrix. The utilization of conducting polymer simplifies the designs of biosensors as these materials behave both as transducers and sensing elements at the same time [Citation48]. In 2007, Speiser constructed a TG biosensor using a nanocomposite film consisting of single-walled carbon nanotubes (SWCNT) and polyaniline (PANI). Using electrophoretic technique, the film was coupled onto the surface of ITO coated glass plate. The linear sweep voltammetry was used to carry out response studies [Citation49]. In 2010, Dhand et al. fabricated a working bioelectrode represented as Lipase-GDH/PANI-SWCNT-TB/ITO, which could detect tributyrin in the concentration range of 50–400 mg/dL−1 in a short response time of 12 s. The electrode displayed high sensitivity of 4.28 × 10−4 mA mg/dL and storage stability of 91 days [Citation50]. In 2014, Jeong et al. developed a TG biosensor by immobilizing lipase, GK and GPO onto the polymer matrices via covalent binding. The detection range of the proposed biosensor was 15–20 mg/dL of micellar TG in serum of patients [Citation51]. Poor processibilty and chemical instability were critical challenge of these biosensors.
TG biosensors based on flow-injection analysis
FIA based biosensors are simple, cheap and flexible. Sample preparation and estimation is automated in these sensors along with quick start-up and shutdown times. In a flow-injection enzyme based electroanalytical system, enzymatic reactions are carried out in capillary taken after by electrochemical detection. In 2005, Wu and Cheng developed a biosensing system composed of lipase, GK and GPO, which exhibited a flow-injection analysis peak response of 2.5 min. For triglycerides, it displayed linearity range of 10−3 to 10−2 M. The storage stability was upto 2 months and 15 samples could be analyzed per hour [Citation52].
TG biosenors based on nanostructured metal oxides (NMOs)
NMOs have moved toward becoming essential resources for biosensors, as they offer a powerful surface for immobilization of biomolecules with enhanced compliance, preferred orientation and high biological activity, which results in bringing about improved detecting attributes. These fascinating NMOs are relied upon to discover appliance in an innovative generation of smart and miniaturized biosensing strategies [Citation53].
In 2007, Rejeb et al. developed an amperometric TG biosensor based on glycerol dehydrogenase/NADH oxidase, which employed a Prussian blue (PB) modified screen–printed electrode as a support for the two immobilized –enzyme systems owing to their higher operating stability. The lipase activity against triglycerols was estimated using the proposed biosensor and storage stability, response time and effect of coenzymes were evaluated [Citation54]. In 2008, Liao et al. fabricated a TG biosensor based on an iridium nano-particle modified carbon. This biosensor was evaluated using glyceryl tributyrate as a substrate. In human sera, a linear concentration range from 0 to 10 mM for glyceryl tributyrate and high sensitivity was observed. The inclusion of a selected surfactant and raising the incubation temperature resulted in better performance of the proposed sensor [Citation55]. In 2009, Solanki et al. constructed a biosensor based on nanostructured cerium oxide for the determination of TG in sera. Lipase was immobilized onto the sol-gel derived Nano-CeO2 film linked to the ITO coated glass plate. The biosensor showed minimum detection limit of 32.8 mg/dL and linearity range of 5–500 mg/dL. The shelf life of the biosensor was 12 weeks [Citation56]. Later in 2011, Narang and Pundir fabricated an amperometric TG biosensor by co-immobilizing lipase, GK and GPO enzymes onto CHIT and ZnONPs composite film electrodeposited on the surface of a Pt electrode. The proposed biosensor displayed high sensitivity, wide linear range of 50–650 mg/dL and LOD of 20 mg/dL with fast response within 6 s. At 4 °C, the biosensor showed shelf life of 210 days [Citation57]. In 2012, Narang et al. constructed TG biosensor based on the co-immobilization of the enzymes (lipase, GK and GPO) on to the AuPPy nanocomposite decked poly indole-5-caboxylic acid electrodeposited onto Au electrode. The biosensor exhibited LOD of 20 mg/dL and linear response range of 50–700 mg/dL. The response time was 4 s [Citation58]. In 2013, Chauhan et al. developed a biosensor utilizing platinum nanoparticles (PtNPs) and polypyrrole (PPY) multilayered nanocomposite films. In this amperometric TG biosensor, lipase was covalently immobilized onto the nanocomposite films electrochemically deposited on the surface of ITO coated glass plate. LOD and linearity of the biosensor was 25 mg/dL and 50–500 mg/dL, respectively. The bioelectrode exhibited shelf life of 9 weeks [Citation30]. In the same year, Narang et al. fabricated TG biosensor using nickel oxide-chitosan/zinc oxide/zinc hexacyanoferrate film. The enzymes (lipase, GK and GPO) were co-immobilized onto the hybrid film electrodeposited onto the Au electrode. The biosensor showed high sensitivity of 0.05 µA/mg/dL and shelf life of 6 months when stored at 4 °C [Citation59]. A disposable amperometric TG biosensor with high sensitivity was developed by Phongphut et al. The proposed biosensor was prepared by co-immobilizing lipase, GK and GPO onto inkjet-printed Au/PEDOT-PSS nanocomposite deposited on the surface of screen-printed carbon electrodes (SPCEs). The biosensor displayed low LOD of 7.88 mg/dL, wide linear range of 0–531 mg/dL, good reproducibility and modest response time of 30 s [Citation20]. In 2014, Gupta et al. constructed a TG biosensor utilizing sol-gel derived SiO2-CeO2 nanocomposite film decorated onto ITO coated glass substrate. Lipase was immobilized onto the surface of nano CeO2-SiO2/ITO electrode through physical adsorption. The biosensor displayed high sensitivity of 2.28 mA mg−1 dL and linearity ranging from 50 to 400 mg/dL [Citation60]. In the same year, Wu et al. [Citation61] fabricated a biosensor using lipase-nanoporous gold (NPG) biocomposite modified glassy carbon electrode (GCE) for the estimation of triglycerides. The working electrode exhibited long shelf life, high conductivity as well as anti-interference ability against uric acid, glucose and urea. The LOD of the biosensor was 2.68 mg/dL [Citation61]. Narang et al. developed TG biosensor by co-immobilizing enzymes onto a working electrode denoted as MNP-CHIT/ZnO-ZnHCF/GCE. The modified electrode showed wide dynamic range of 10–1000 mg/dL, low LOD of 10 mg/dL and excellent storage stability [Citation62]. In 2015, Bhardwaj et al. prepared a working bioelectrode made up of reduced graphene oxide (ERGO), which exhibits excellent electrochemical properties, high electron mobility as well as high surface area. Using chronoamperometric method, a homogeneous film of reduced grapheme oxide was electrodeposited on the surface of ITO electrode. The fabricated bioelectrode represented as LIP-G1DH/ERGO/ITO, displayed wide linear range of 50–300 mg/dL for estimating TG [Citation63]. In 2016, Solanki et al. fabricated a TG biosensor by covalently immobilizing lipase and G1DH onto zirconium oxide nanoparticles (nano-ZrO2)-CHIT nanocomposite electrophoretically deposited on the surface of ITO coated glass plate. The biosensor showed LOD of 155 µg ml−1 within a short response time of 45 s [Citation64].
TG biosensors based on enzyme nanoparticles (ENPs)
In TG biosensors, direct immobilization of enzymes onto nanocomposite might be able to cause their denaturation, resulting in their instability and loss of activity. Enzymes nanoparticles can be used to triumph over this problem. As ENPs are the nanosize entities of enzyme molecules, they display exceptional chemical and physical properties [Citation65]. Recently, Pundir and Aggarwal (2017) fabricated a sensitive TG bionanosensor by covalently immobilizing nanoparticles of enzymes (LIP, GK and GPO) onto the surface of gold electrode. The prepared biosensor displayed superior amperometric detection of triglycerides in blood sample. It exhibited dynamic working range of 10–500 mg/dL and LOD of 1.0 µg/mL within a short period of time (5 s) [Citation66]. Narwal and Pundir (2017) designed a TG biosensor by immobilizing nanoparticles of lipase, GK and GPO onto electrode made up of pencil graphite. This ENPs/PG working electrode showed a lower LOD of 0.1 nM within 2.5 s, long shelf life and high sensitivity.
Various TG biosensors based on conducting polymers, nanostructured metal oxides and enzyme nanoparticles have been shown and analytically compared in .
Table 4. Conducting polymer, nanostructured metal oxides (NMOs) and ENPs based TG biosensors and comparison of their analytical properties.
Conclusion and future outlook
The potential of TG biosensors is apparent to healthcare industry for the monitoring of elevated level of triglycerides in human sera, which leads to several diseases. A brief outline of the recent progress in the design of enzyme based electrochemical biosensor platforms for the triglyceride estimation has been provided. Advancement in deployment of enzymes and nanoscale electrode materials for the evaluation of TG level in the sera has risen as a promising advent having enormous potential in terms of new functionality, high specificity, miniaturization, sensitivity and robustness in solving a wide range of challenges in field of biosensors. The utilization of conducting polymers, EISCAP, ENFET, porous silicon and nanostructured metal oxides to modify the electrodes could appreciably improve the capacity of electrochemical signal for selective determination of the target molecule and the major challenges pertaining to the fabrication of point-of-care biosensors could be resolved. In near future, biosensors must be unobtrusive, versatile, economic, non-invasive, sleek and wearable that would be able to accurately quantify triglyceride concentration in the sera in real time. The use of disposable, smart and user-friendly sensing devices will be preferred to diagnose the health status of patients by the healthcare professionals. Undoubtedly, the future prospects of enzyme based electrochemical biosensors are very promising and will show an impending growth to reform the future personalized health care diagnostics. Biosensor expertise is still solicited by copious challenges to be tackled.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Skulas-Ray AC, Kris-Etherton PM, Harris WS, et al. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am J Clin Nut. 2011;93:243–252.
- Burgess BL, Mcisaac SA, Naus KE, et al. Elevated plasma triglyceride levels precede amyloid deposition in Alzheimer’s disease mouse models with abundant Aβ in plasma. Neurobiol Dis. 2006;24:114–127.
- Sarwar N, Danesh J, Eiriksdottir G, et al. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 western prospective studies. Circulation. 2007;115:450–458.
- Yoshida A, Kouwaki M, Matsutani Y, et al. Usefulness of serum total cholesterol/triglyceride ratio for predicting the presence of small, dense LDL. J Atheroscler Thromb. 2004;11:215–219.
- Klotzsch SG, McNamara JR. Triglyceride measurements: a review of methods and interferences. Clin Chem. 1990;36:1605–1613.
- Mendez AJ, Cabeza C, Hsia S. A fluorometric method for the determination of triglycerides in nanomolar quantities. Anal Biochem. 1986;156:386–389.
- Mamoru S, Tsutomu O, Kazuyuki H, et al. A simple colorimetric method for determination of serum triglycerides with lipoprotein lipase and glycerol dehydrogenase. Clin Chim Acta. 1977;81:125–130.
- Kaplan A, Lee VF. A micromethod for determination of serum triglycerides. Proc Soc Exp Biol Med. 1965;118:296–297.
- Mocho´n MCCB, Leyva JM. A new spectrophotometric method for determining triglycerides in serum. Clin Chim Acta. 1984;142:281–285.
- Kalia V, Pundir CS. Evaluation of serum triglyceride determination with lipase, glycerol kinase and glycerol-3-phosphate oxidase and peroxidase co-immobilized onto alkylamine glass beads. Indian J Biochem Biophys. 2004;41:326–328.
- Hearne C, Fraser C. Assessment of colorimetric enzymatic determination of triglyceride, by manual and centrifugal analyzer techniques, and comparison with a CDC standardized method. Clin Biochem. 1981;14:28–31.
- Brunnekreeft JW, Leijnse B. Determination of serum triglycerides by capillary on-column gas chromatography. J Clin Chem Clin Biochem. 1986;24:445–449.
- Otvos J. Measurement of triglyceride-rich lipoproteins by nuclear magnetic resonance spectroscopy. Clin Cardiol. 1999;22:1121–1127.
- Björkhem I, Sandelin K, Thore A. A simple, fully enzymic bioluminescent assay for triglycerides in serum. Clin Chem. 1982;28:1742–1744.
- Narang J, Minakshi Bhambi M, et al. Fabrication of an amperometric triglyceride biosensor based on PVC membrane. Anal Lett. 2009;43:1–11.
- Minakshi, Pundir CS. Construction of an amperometric enzymic sensor for triglyceride determination. Sens Actuators B Chem. 2008;133:251–255.
- Pundir CS, Singh BS, Narang J. Construction of an amperometric triglyceride biosensor using PVA membrane bound enzymes. Clin Biochem. 2010;43:467–472.
- Adhikari B, Majumdar S. Polymers in sensor applications. Prog Polym Sci. 2004;29:699–766.
- Liu J, Agarwal M, Varahramyan K. Glucose sensor based on organic thin film transistor using glucose oxidase and conducting polymer. Sens Actuators B. 2008;135:195–199.
- Phongphut A, Sriprachuabwong C, Wisitsoraat A, et al. A disposable amperometric biosensor based on inkjet-printed Au/PEDOT-PSS nanocomposite for triglyceride determination. Sens Actuators B Chem. 2013;178:501–507.
- Narwal V, Singh G. Application of graphene in electrochemical biosensors. Int J Basic Appl Biol. 2014;1:87–91.
- Turner AP. Biosensors: fundamentals and applications – historic book now open access. Biosens Bioelectron. 2015;65:A1.
- Evtugyn G. Biochemical components used in biosensor assemblies. Biosens Essent. 2014;84:21–97.
- Pundir CS, Narwal V, Batra B. Determination of lactic acid with special emphasis on biosensing methods: a review. Biosens Bioelectron. 2016;86:777–790.
- Palchetti I, Laschi S, Mascini M. Electrochemical biosensor technology: application to pesticide detection. Biosens Biodetect. Methods Mol Biol. 2009;504:115–126.
- Sadeghi SJ. Amperometric biosensors, In: Roberts GCK, editor. Encyclopedia of biophysics. Berlin, Heidelberg: Springer; 2013. p. 61–67.
- Das P, Das M, Chinnadayyala SR, et al. Recent advances on developing 3rd generation enzyme electrode for biosensor applications. Biosens Bioelectron. 2016;79:386–397.
- Yücel A, Özcan HM, Sağıroğlu A. A new multienzyme-type biosensor for triglyceride determination. Prep Biochem Biotechnol. 2014;46:78–84.
- Narang J, Minakshi Bhambi M, et al. Determination of serum triglyceride by enzyme electrode using covalently immobilized enzyme on egg shell membrane. Int J Biol Macromol 2010;47:691–695.
- Chauhan R, Nagar B, Solanki PR, et al. Development of triglyceride biosensor based on a platinum nano particle and polypyrrole nano composite electrode. Mater Focus. 2013;2:316–323.
- Schoemaker M, Feldbrügge R, Gründig B, et al. The lipoxygenase sensor, a new approach in essential fatty acid determination in foods. Biosens Bioelectron. 1997;12:1089–1099.
- Kelly TA, Christian GD. Amperometric determination of glycerol and triglycerides using an oxygen electrode. Analyst. 1984;109:453–456.
- Feldbrügge R, Renneberg R, Spener F. Development and practical evaluation of an amperometric triglyceride sensor. Sens Actuators B Chem. 1994;19:365–367.
- Bhambi M, Minakshi, Pundir CS. Preparation of oxygen meter based biosensor for determination of triglyceride in serum. Sens Transducer. 2006;67:561–567.
- Dhand C, Solanki PR, Sood K, et al. Polyaniline nanotubes for impedimetric triglyceride detection. Electrochem Commun. 2009;11:1482–1486.
- Rushworth JV, Hirst NA, Goode JA. Electrochemical impedance spectroscopy. In: Pike D, Ahmed A, Millner PA, editors. Impedimetric biosensors for medical applications. New York: ASME Press; 2013.
- Nakazato K. Potentiometric, amperometric, and impedimetric CMOS biosensor array. In: Rinken T, editor. State of the art in biosensors. London: IntechOpen; 2013. Available from: https://www.intechopen.com/books/state-of-the-art-in-biosensors-general-aspects/potentiometric-amperometric-and-impedimetric-cmos-biosensor-array
- Reddy RK, Chadha A, Bhattacharya E. Porous silicon based potentiometric triglyceride biosensor. Biosens Bioelectron. 2001;16:313–317.
- Salis A, Setzu S, Monduzzi M, et al. Porous silicon-based electrochemical biosensors. Intech Publishers; 2011. Chapter 17, Biosensors-emerging materials and applications; p. 333–352.
- Basu I, Subramanian R, Mathew A, et al. Solid state potentiometric sensor for the estimation of tributyrin and urea. Sens Actuators B Chem. 2005;107:418–423.
- Vemulachedu H, Fernandez RE, Bhattacharya E, et al. Miniaturization of EISCAP sensor for triglyceride detection. J Mater Sci Mater Med. 2008;20:229–234.
- Fernandez RE, Hareesh V, Bhattacharya E, et al. Comparison of a potentiometric and a micromechanical triglyceride biosensor. Biosens Bioelectron. 2009;24:1276–1280.
- Preetha R, Rani K, Veeramani MSS, et al. Potentiometric estimation of blood analytes—triglycerides and urea: comparison with clinical data and estimation of urea in milk using an electrolyte–insulator–semiconductor–capacitor (EISCAP). Sens Actuators B Chem. 2011;160:1439–1443.
- Veeramani MS, Shyam KP, Ratchagar NP, et al. Miniaturised silicon biosensors for the detection of triglyceride in blood serum. Anal Methods. 2014;6:1728.
- Nakako M, Hanazato Y, Maeda MS, et al. Neutral lipid enzyme electrode based on ion-sensitive field effect transistors. Anal Chim Acta. 1986;185:179–185.
- Vijayalakshmi A, Tarunashree Y, Baruwati B, et al. Enzyme field effect transistor (ENFET) for estimation of triglycerides using magnetic nanoparticles. Biosens Bioelectron. 2008;23:1708–1714.
- Al-Hardan N, Hamid MA, Ahmed N, et al. High sensitivity pH sensor based on porous silicon (PSi) extended gate field-effect transistor. Sensors. 2016;16:839.
- Aydemir N, Malmström J, Travas-Sejdic J. Conducting polymer based electrochemical biosensors. Phys Chem Chem Phys. 2016;18:8264–8277.
- Speiser B. Linear sweep and cyclic voltammetry. In: Bard AJ, Stratmann M, Unwin P, editors. Encyclopedia of electrochemistry. Instrumentation and electroanalytical chemistry. Vol. 3. Weinheim: Wiley-VCH; 2003. p. 81–104.
- Dhand C, Solanki PR, Datta M, et al. Polyaniline/single-walled carbon nanotubes composite based triglyceride biosensor. Electroanalysis. 2010;22:2683–2693.
- Jeong CY, Han YD, Yoon JH, et al. Bioelectrocatalytic sensor for triglycerides in human skin sebum based on enzymatic cascade reaction of lipase, glycerol kinase and glycerophosphate oxidase. J Biotechnol. 2014;175:7–14.
- Wu LC, Cheng CM. Flow-injection enzymatic analysis for glycerol and triacylglycerol. Anal Biochem. 2005;346:234–240.
- Solanki PR, Kaushik A, Aggrawal VV, et al. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011;3:17–24.
- Rejeb IB, Arduini F, Amine A, et al. Amperometric biosensor based on Prussian Blue-modified screen-printed electrode for lipase activity and triacylglycerol determination. Anal Chim Acta. 2007;594:1–8.
- Liao WY, Liu CC, Chou TC. Detection of triglyceride using an iridium nano-particle catalyst based amperometric biosensor. Analyst. 2008;133:1757–1763.
- Solanki PR, Dhand C, Kaushik A, et al. Nanostructured cerium oxide film for triglyceride sensor. Sens Actuators B Chem. 2009;141:551–556.
- Narang J, Pundir CS. Construction of a triglyceride amperometric biosensor based on chitosan–ZnO nanocomposite film. Int J Biol Macromol. 2011;49:707–715.
- Narang J, Chauhan N, Rani P, et al. Construction of an amperometric TG biosensor based on AuPPy nanocomposite and poly (indole-5-carboxylic acid) modified Au electrode. Bioprocess Biosyst Eng. 2012;36:425–432.
- Narang J, Chauhan N, Pundir CS. Construction of triglyceride biosensor based on nickel oxide-chitosan/zinc oxide/zinc hexacyanoferrate film. Int J Biol Macromol. 2013;60:45–51.
- Gupta AK, Kaushik A, Solanki PR, et al. Sol-gel derive SiO2-CeO2 nanocomposite films for riglyceride determination. J Nanosci Lett. 2014;4:1–5.
- Wu C, Liu X, Li Y, et al. Lipase-nanoporous gold biocomposite modified electrode for reliable detection of triglycerides. Biosens Bioelectron. 2014;53:26–30.
- Narang J, Chauhan N, Malhotra N, et al. Fabrication of TG biosensor based on magnetic nanoparticles/zinc oxide/zinc hexacyanoferrate film: novel matrix for electrochemical sensing. Adv Sci Lett. 2014;20:1331–1336.
- Bhardwaj SK, Mahapatro AK, Basu T. Bienzymatic triglyceride biosensor based on electrochemically reduced graphene oxide. Int J Chem Tech Res. 2015;7:858–866.
- Solanki S, Pandey CM, Soni A, et al. An amperometric bienzymatic biosensor for the triglceridetributyrin using an indium tin oxide electrode coated with electrophoreticallydeposited chitosan-wrapped nanozirconia. Microchim Acta. 2016;183:167–176.
- Pundir CS, Aggarwal V. Amperometric triglyceride bionanosensor based on nanoparticles of lipase, glycerol kinase, glycerol-3-phosphate oxidase. Anal Biochem. 2017;517:56–63.
- Narwal V, Pundir CS. An improved amperometric triglyceride biosensor based on co-immobilization of nanoparticles of lipase, glycerol kinase and glycerol 3-phosphate oxidase onto pencil graphite electrode. Enzyme Microb Technol. 2017a;100:11–16.
- Narwal V, Pundir CS. Graphene Based Lactate Biosensor. Germany: Lambert Academy Press; 2017b.