?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
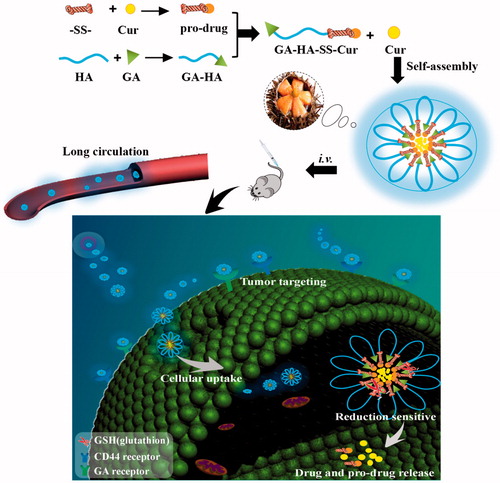
To remedy the problems resulting from the usage of anti-cancer drugs in cancer chemotherapy, such as deficient drug concentration in tumour cells, low water-solubility and non-specific distribution of antitumour drugs, a kind of reduction-sensitive polymer prodrug of curcumin (Cur) containing in the nano-echinus was synthesized and designed. The nano-echinus-like nanomedicine presented synergistic effect with glycyrrhetic acid (GA) and oligomeric hyaluronic (HA) for targeting and combating HepG2 human liver cancer cell. Firstly, a kind of small molecular prodrug of Cur, dithiodipropionic acid-Cur (-SS-Cur), was chemically conjugated onto the side chain of the conjugated glycyrrhetic acid- oligomeric hyaluronic (GA-HA) to generate an amphiphilic polymeric prodrug of Cur, GA-HA-SS-Cur. The obtained GA-HA-SS-Cur prodrug and subsidiary material mPEG-DSPE could self-assemble into a sea urchin-like micelles in aqueous media and release Cur rapidly in response to glutathion (GSH). Then, Cur was loaded into the nano-echinus with a particle size of (118.1 ± 0.2 nm) and drug-loading efficiency of (8.03 ± 2.1%). The structure of GA-HA-SS-Cur was characterized by 1H-NMR in this report. The morphology of micelles was observed with a transmission electron microscope (TEM). Subsequently, the reduction-sensitivity of the nano-echinus was confirmed by the changes in in-vitro drug release after different concentrations of GSH treatment. Besides, the cellular uptake behaviour and MTT assays of the nano-echinus were investigated, suggesting that the nano-echinus was of desirable safety and could be taken into HepG2 cells in a time-dependent manner. Later, anti-tumour efficacy in vivo revealed the effective inhibition of tumour growth.
Introduction
In cancer chemotherapy, deficient drug concentration in tumour cells, low water-solubility and non-specific distribution of antitumour drugs continues to impede the usage of anti-cancer drugs [Citation1,Citation2]. To remedy the problems resulting from the usage of anti-cancer drugs and alleviate the potential systemic toxicity, the idea of basing tumour microenvironment drug delivery was proposed [Citation2].
Aimed to improve tumour-targeting, stimuli-responsive smart drug delivery nano-system based on tumour microenvironment has received extensive attention, such as pH response, reduction response and enzyme response smart drug delivery nano-systems [Citation2,Citation3]. The reduction- sensitive structures are intensely studied drug carriers according to the tumour microenvironment [Citation3]. The existence of the disulfide bond plays an important role in the reduction sensitivity. Such a design can cause the disruption of the disulfide bond with the reduction environment leading to the destruction of the structure of the micelle and drug release from the micelle [Citation4].
It had been reported that glycyrrhetic acid (GA), one of the main effective components of a traditional Chinese medicine liquorice, can target kinases and play an important role of proteasome inhibitors [Citation5–7]. Glycyrrhetinic acid has live targeting property and can induce apoptosis of Hep G2 cells in a dose-dependent manner [Citation8]. Besides, studies have shown that GA can play a pivotal role in anticancer effect by inducing apoptosis of tumour cells, repressing cell cycle, inducing differentiation of tumour cells, inhibiting invasion of tumour cells and inhibiting multidrug resistance of tumours, and can cause synergistic anticancer effects with chemotherapy drugs [Citation9–11].
Nano-echinus, a sea urchin-like micelle, was designed based on biomimetic drug delivery systems (BDDSs), which are emerging drug delivery systems with great development potential in recent years [Citation12,Citation13]. Generally, BDDSs have good targeting and low immunogenicity [Citation14]. The polymer was designed based on the structure of echinus with mPEG as the soft “spines”, so it was called nano-echinus-like nanomedicine. But natural echinus has hard spines which are impossible to be well-embedded in the human body, so we designed the echinus structure by using mPEG as the soft “spines”, which contributed to its availability and long circulation in vivo to lead to a better antitumour efficacy [Citation15,Citation16]. The inside core of the nano-echinus was curcumin (Cur), an anti-cancer drug [Citation2]. Recent pharmaceutical studies have reported the therapeutic cancer effects of Cur, but its weak stability and bad solubility in water seriously impeded its utilization, which is an impetus for us to choose an appropriate carrier to improve its bioavailability. More interestingly, the colour of Cur was just like the colour of inside core of echinus and can be utilized as a fluorescence probe [Citation17].
In this study, as shown in , we synthesized the well-designed CD44 and liver targeting and reduction-sensitive nano-echinus-like nanomedicine. The nano-echinus-like nanomedicine was a mixed system composed of two moieties: GA-HA-SS-Cur as the functional part and mPEG-DSPE as the subsidiary material, which presented CD44 targeting property with HA and liver targeting with GA. We hypothesized that the hybrid system could self-assemble into Cur-loaded nano-echinus, resemble a sea-urchin-like structure (. Then, with the CD44 targeting ability of HA to the nano-echinus could target to CD44-receptors on the surface of tumour cells and accumulate in tumour tissues [Citation18]. Finally, with the aid of the disulfide bond as the reduction-sensitive linker, the Cur can be released in tumour environments [Citation19]. Meanwhile, with the existence of mPEG, the circulating time of drug carriers could be prolonged [Citation2].
Materials and methods
Materials
Oligosaccharide of hyaluronan (HA) (molecular weight [MW] < 10 kDa) was purchased from Shandong Freda Co. Ltd. (Shandong, China). Cur was purchased from Dengke Chemical Reagent Co., Ltd. (Tianjin, China); Glycyrrhetinic acid was purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). mPEG-DSPE was purchased from Merck (Schaffhausen, Switzerland). 3.3-Dithiodipropionic acid was obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). N-Hydroxysuccinimide and 1–(3-Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride were all of analytical grade and were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China).
For the cell culture, the HepG2 cell line (hepatoma carcinoma cell) was obtained from BeNa Culture Collection (Beijing, China); 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) was obtained from Sigma-Aldrich (St. Louis, MO); fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT). For animal studies, nude mice (weighting 18–20 g) were purchased from Beijing Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The experiments were carried out in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All animal studies were approved by the Experimental Animals Administrative Committee of Yantai University.
Polymer synthesis
The synthesis of GA-HA
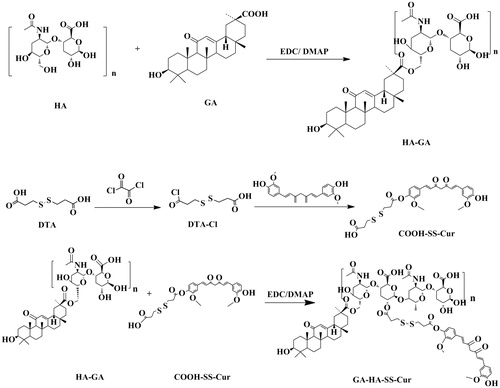
The blend of HA (77.7 mg), EDC (38.3 mg) and DMAP(24.4 mg) were dissolved in 4 ml of formamide at 60 °C. After 30 min of activation, GA (47.1 mg) was added into the mixture. After 24 h, the resulted solution was extracted by dialysis with a dialysis bag of 7000 Da (MWCO) for 48 h at room temperature. The synthetic product GA-HA can be received after freeze drying for 24 h.
The synthesis of GA-HA-SS-Cur
3,3-Dithiodipropionic acid (40 mg) was dissolved in 1 ml of THF. Then 48 μl of oxalyl chloride was added dropwise into the solution under ice bath condition. The reaction was kept at 35 °C. Two hours later, the resulted mixture was subjected to rotary evaporation to remove unreacted oxalyl chloride. Subsequently, the resulted product and Cur were redissolved in 1 ml of THF, respectively, and then mixed up. Thereafter, 34 μl of triethylamine was added dropwise into the mixture. Finally, after 5.5 h of oil bath at 45 °C, the resulted product was collected after rotary evaporation.
Following above reaction, GA-HA (12.48 mg) resolved in formamide was added into the resulted product at 50 °C. After 24 h, the resulted solution was extracted by dialysis with a dialysis bag of 7000 Da (MWCO) for 48 h at room temperature. The synthetic product GA-HA-SS-Cur can be received after freeze drying for 12 h. The synthesis is illustrated in .
Characterization of GA-HA-SS-Cur
The structure of GA-HA-SS-Cur was further confirmed by 1H-NMR spectroscopy. For the 1H-NMR measurement, 10 mg of GA-HA-SS-Cur was dissolved in 0.5 ml of D2O for the detection of chemical shifts.
Preparation of nano-echinus
The nano-echinus was obtained by using mPEG-DSPE, an amphililic material, in the way of self-assembly. GA-HA-SS-Cur (10 mg), mPEG-DSPE (10 mg) and Cur (5 mg) (at the mass ratio of 2:2:1) were dissolved with formamide. After complete dissolution of the mixture, the yellow solution was put into a dialysis bag with MWCO of 2000 Da. Following the dialysis, the obtained solution was filtrated with 0.8 μm millipore filter. From the obvious opalescence and clarified yellow solution, the formation of micelles can be preliminarily confirmed.
Characterization of nano-echinus
Particle size and zeta potential of formulations
The particle size, zeta potential of micelles were measured by Particle Analyzer Delsa Nano C (Beckman Coulter, Indianapolis, IN) with dynamic light scattering (DLS). The polydispersity index (PDI) was also shown to describe molecular weight distribution of polymers.
Entrapment efficiency (EE %) and drug loading (DL %)
HPLC analysis. High Performance Liquid Chromatography (HPLC, Agilent 1260GB12C, Santa Clara, CA) was used to determine the concentration of Cur-loaded in the nano-echinus with 0.5% glacial acetic acid and acetonitrile (40:60, v: v) as the mobile phase. The temperature of the Phenomenex C18 column (250 mm × 4.6 mm, 5 μm) was 25 °C. 425 nm was used as the detection wavelength of Cur to further detect the EE% and DL% [Citation17].
EE % and DL %. To be brief, 5 ml of micelle solution was measured precisely and put into the ultrafilter (10,000 Da) being inside the centrifuge tube, then centrifuged for 10 min at 2500 rpm. The filtered solution was measured by HPLC. The EE % and DL % were calculated using the following EquationEquations (1)(1)
(1) and Equation(2)
(2)
(2) , respectively [Citation2]:
(1)
(1)
(2)
(2)
In vitro reduction-responsive drug release study
In order to investigate the Cur-release from the nano-echinus by influence of different reductive environments in vitro, dialysis method was used. Briefly, 1 ml of micelles solution was added into a dialysis bag (MWCO 2000 Da) immersed in a 50 ml centrifuge tube and dialysed against phosphate buffer solution (PBS) (45 ml, pH 7.4) with different concentrations of glutathione (GSH, 10 mmol/l, 20 μmol/l) and tween 80 (1%, w/v) as solubiliser of Cur at 37 °C under shaking (100 r/min). At different time intervals, to maintain the total volume, removed solution was replaced with equal volume of fresh micelles solution. The concentration of Cur release was determined by HPLC in triplicate for each GSH concentration [Citation2].
In vitro cytotoxicity assays of nano-echinus
The cytotoxicity of free Cur, blank GA-HA, blank GA-HA-SS-Cur and nano-echinus were investigated in HepG2 cells by MTT assay. HepG2 cells were cultured in 96-well plates at a density of 3000 cells per well followed by being incubated at 37 °C in 5% CO2 for 24 h. Then, free Cur, different concentrations of blank GA-HA, blank GA-HA-SS-Cur and nano-echinus were diluted with culture medium, and added into different wells to replace the original solution. The concentrations were at a Cur concentration of 1, 3, 5, 10, 12 μg/ml, respectively. After respectively being incubated for 36 h, 20 μl of MTT solution (5 mg/ml) was added. Then, 100 μl of DMSO was added to resolve the purple crystal formazan after another incubation of 4 h. The microplate reader was utilized to record the absorbance at 490 nm. The following equation was used to show the percentage cell viability [Citation20,Citation21]:
(3)
(3)
where, AT was the absorbance of tested wells and Ac was the absorbance of control wells. Triplicate samples were analysed in each measurement.
In vitro cellular uptake of nano-echinus
The qualitative and reduction sensitive cellular uptake of the nano-echinus was measured by fluorescence microscrope (FM, Eclipse E400; Nikon Corporation, Tokyo, Japan) to investigate the qualitative cellular uptake with or without reduction environment (10 mM GSH). HepG2 cells were seeded in 24-well plates at a density of 2 × 104 cells per well at 37 °C for 24 h. After incubation, the culture medium was changed to that containing nano-echinus or free Cur at a Cur concentration of 5 μg/ml. The cells were incubated for 1, 2 and 4 h, respectively. Then, each well was added with 1 ml of paraformaldehyde (4%, w/v) after washing three times with PBS (pH 7.4). Washed again and cellular uptake was viewed using a fluorescence microscope [Citation22,Citation23].
In vivo antitumour efficacy in HepG2-tumour bearing nude mice
The in vivo antitumour efficacy of the nano-echinus was studied by using HepG2-tumour bearing nude mice as the animal model. HepG2-tumour bearing nude mice were injected with 0.2 ml of HepG2 cells (2 × 106) into the right flank of mice. After the volume of the tumours exceeded about 1000 mm3, all mice were tagged and grouped in four parts with four mice in every group. These four groups were administered every other day with (1) saline, (2) GA-HA-SS-Cur, (3) free Cur and (4) nano-echinus at the same time points, respectively. The weight of the mice, and the length and width of tumour were measured every time before administration. The mice were killed in a month followed by excising and weighing the tumours. The size and tumour inhibition rate were calculated by following equations:
(4)
(4)
(5)
(5)
where, L and W in EquationEquation (4)
(4)
(4) are the length and width of tumours, respectively. In EquationEquation (5)
(5)
(5) , Wcontrol was the weight of tumour in the control group, and Wtested was the weight of tumour in the tested groups.
Statistical analysis
All of the experiments were carried out at least in triplicate, and results were expressed as mean ± standard deviation (SD). Significance tests between two groups were compared by using Student’s t-test. p < .05 was set as the statistical significance.
Results
Synthesis and characterization of GA-HA-SS-Cur
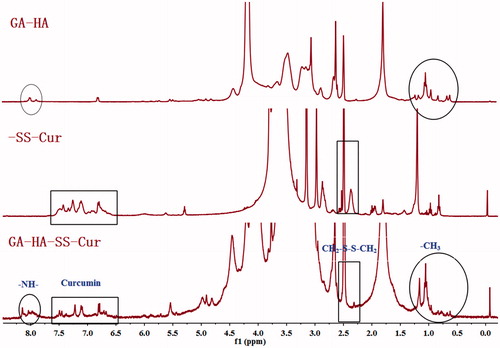
The synthesis procedure of GA-HA-SS-Cur conjugates is shown in . The structure was confirmed by 1H-NMR (. In the 1H-NMR spectrum of GA-HA-SS-Cur, the typical signals of Cur were observed at 6.5 ∼ 7.6 ppm and the peaks at 8.0 ppm was assigned to –NH– connection between GA and HA. The appearance of signal at 2.6 ppm was verified at –CH2– peak of 3,3-dithiodipropionic acid. In addition, the –CH3 of GA was seen at 0.5 ∼ 1.2 ppm. These results confirmed that the polymer GA-HA-SS-Cur was successfully obtained.
Characterization of nano-echinus
Particle size, zeta potential, morphology and EE%
Due to the fact that the synthesized amphiphilic polymer could form micelles in aqueous solution, the dialysis method was used to obtain the nano-echinus with Cur as the hydrophobic core (). The particle size, PDI, zeta potential, EE% and DL% of blank nano-echinus (the mixture of GA-HA-SS-Cur and mPEG-DSPE) and the nano-echinus are illustrated in . The particle size was (92.2 ± 0.3 nm) before Cur loading and (118.1 ± 0.2 nm) after Cur loading. TEM images presented that the nano-echinus were spherical morphology with an average size of 60 nm after freeze-drying followed by redissolving. The addition of cryoprotector and the negatively staining operation during determination process may contribute to the shrink of the nano-particles, so the particle size of TEM was smaller than the results obtained from Particle Size Analyzer (Beckman Coulter, DelsaTM Nano C, Indianapolis, IN). The zeta potential of the nano-micelles, about (–21.56 ± 1.3 mV), whose absolute value is over 20, showed the relatively stable molecular system.
Figure 4. (A) The nano-echinus with PBS; (B) The size distribution of the nano-echinus; (C) The Zeta Potential of the nano-echinus; (D) The TEM images of nano-echinus.
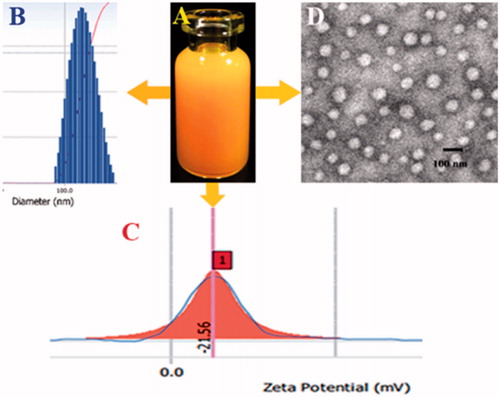
Table 1. Characterization of the nano-echinus and its drug loading capacity.
In vitro reduction-responsive drug release study
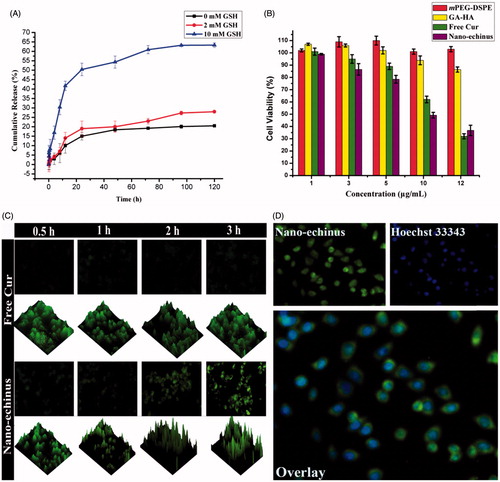
In order to simulate the physiological environment and the tumour environment, the Cur release from the nano-echinus was conducted in the condition of absence and presence of GSH to simulate the reduction environment. As demonstrated in , for the nano-echinus immersed in PBS without GSH, only <20% accelerated release of Cur was found. Addition of 2 mM GSH to the medium resulted in only approximately 25% drug increase. Dramatically accelerated release of Cur (63.3%) was found when the concentration of GSH reached 10 mM, which can be deduced that the added GSH could cleave the disulfide bond in the polymer, resulting in defects of the nano-echinus to allow easier release of Cur encapsulated in nano-echinus. In addition, no obvious difference of drug release was shown between the nano-echinus exposed 0 mM GSH and 2 mM GSH, indicating that extra-cellular environment with GSH in low concentration cannot efficiently destroy the fabrication of the nano-echinus. In contrast, intra-cellular environment of tumour with high concentration of GSH can easily lead to the disassembly of nano-echinus, which resulted in obvious Cur releasing from nano-echinus to play therapeutic effect, indicating the reduction-responsive release profile of the nano-echinus.
Figure 5. (A) In vitro release profiles of Cur from the nano-echinus in medium containing 0, 2 mM, 10 mM of GSH. (B) In vitro cytotoxicity of mPEG-DSPE, GA-HA, Free Cur, the nano-echinus. (C) 2D- and 2.5D-reconstructed FM images of cellular uptake. (D) Internalization imaging of nano-echinus in cancer cells after 3 h incubation. Hoechst 33342 was used to stain the cell nucleus which showed blue fluorescence.
In vitro cytotoxicity and cellular uptake
Cellular uptake and cytotoxicity can be utilized to evaluate the antitumour effect of the nano-echinus. The viability of HepG2 cells treated with mPEG-DSPE, GA-HA, Free Cur, the nano-echinus were determined and the results are shown in . All the formulation containing Cur exhibited cytotoxicity in a dose-dependent manner. However, only the nano-echinus showed significantly low-cell viability value, indicating that the nano-echinus had high cytotoxicity activity. The GA-HA group exhibited cytotoxicity in a dose-dependent manner, maybe due to the potential anticancer effect of GA. In contrast, the mPEG-DSPE formulation without Cur, as the blank vehicles, showed high cell viability even at a high concentration, indicating the less cytotoxicity and conceivable safety of the mPEG-DSPE. These results indicated that the nano-echinus improved the toxicity of Cur and that the desirable safety of mPEG-DSPE and GA-HA as the drug delivery carrier.
As shown in , according to our previous study showed that Cur was not only a therapeutic drug but also a fluorescence marker [Citation17], green fluorescence signals of Cur were observed in HepG2 cell lines treated with free Cur and the nano-echinus after 0.5, 1, 2, 3 h, respectively. The fluorescence of Cur significantly increased with incubation time increased from 0.5 to 3 h, which showed that the internalization of nano-echinus was a time-dependent process. demonstrated that the Cur fluorescence signal was noticeably found in nano-echinus group compared to that of the control group, free Cur group, implying the combined system of GA-HA-SS-Cur and mPEG-DSPE was fabulous drug-loaded carrier for Cur. Moreover, in 2.5D-reconstructed FM images showed that almost two-fold higher green fluorescence signal was shown in 3 h group of nano-echinus than 0.5 h and 1 h groups. As shown in , after staining nucleus with Hoechst33342, most green fluorescence was present outside of the nucleus, this result showed cellular uptake through caveolae-mediated endocytosis [Citation1]. Given all the results above, the nano-echinus, the combined system of GA-HA-SS-Cur and mPEG-DSPE, was the potentially incredible carrier to deliver anti-tumour drug into cancer cells.
In vivo antitumour efficacy in HepG2-tumour bearing nude mice
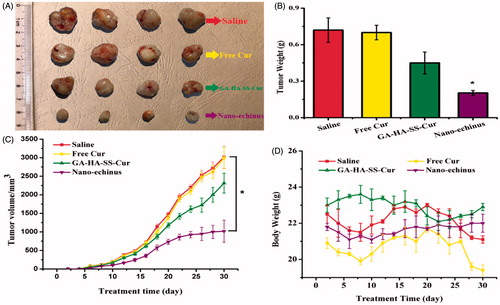
Encouraged by the superior cellular uptake imaging results and desirable safety of the nano-echinus as the drug delivery carrier, we sought to examine the potential anti-tumour therapy of the nano-echinus in vivo. As shown in , the tumour volume of the nano-echinus was kept at a significantly lower level than other groups during the treatment time, which meant that the tumour growth of the nano-echinus was almost inhibited to some extent. The antitumour efficacy of these formulations can be ranked in following order: Nano-echinus > GA-HA-SS-Cur > Free Cur > Saline, which reached a consensus with in-vitro cellular uptake and cytotoxicity. The GA-HA-SS-Cur and free Cur group showed slightly inhibiting effect, but nano-echinus group possessed the superior tumour inhibiting effect, implying that the nano-echinus is a potential carrier for antitumour delivery. In , measurements of tumour weight and body weight also approved the antitumour effect of each treatment.
Conclusions
This work reported reduction-sensitive mPEG-DSPE-modified nano-echinus-like nanomedicine, integrating the benefits of reduction-responsive property, CD44 targeting, mPEG-DSPE and polymeric nanoparticles in one nanoplatform. We hypothesized that the mixture system could self-assemble into an echinus-like structure Cur-loaded micelles, and target to CD44-receptors on the surface of tumour cells so as to accumulate in tumour tissues. Finally, due to the reduction-sensitive disulfide bond, the Cur can be released in tumour environments, which was consistent with our results.
In this study, by chemical reactions, physical methods and 1H-NMR result, we successfully synthesized the nano-echinus-like nanomedicine with reduction-sensitive property, which could be obtained as reduction-sensitive nano-echinus in aqueous solution with antitumour drug Cur in the core by spontaneously self-assembly. The drug-loaded nanoparticles possessed desirable particle size (118.1 ± 0.2 nm), favourable cellular uptake and promising antitumour efficacy. In addition, the blank nano-carriers showed almost nontoxic property, which meant that the cytotoxicity maybe obtained from drug-loaded nano-echinus instead of carriers. Noteworthy, enhanced cellular uptake was achieved compared with our previously reported Cur-loaded nano-particles. Internalisation imaging of nano-echinus in cancer cells demonstrated cellular uptake through caveolae-mediated endocytosis. Moreover, in vivo antitumour experiments confirmed that the reduction-responsive nano-echinus exhibited enhanced antitumour efficacy. Taken together, the reported Cur-loaded nano-echinus-like nanomedicine exhibited great promise to be used for delivering antitumour drugs.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- TanZou S, Zhang C, Yin W, et al. Recent developments in d-ќ±-tocopheryl polyethylene glycol-succinate-based nanomedicine for cancer therapy. Drug Deliv. 2017;24:1831–1842.
- Liu L, Yao W, Rao Y, et al. pH-Responsive carriers for oral drug delivery: challenges and opportunities of current platforms. Drug Deliv. 2017;24:569–581.
- Cai Y, Xu Y, Chan H, et al. Glycyrrhetinic acid mediated drug delivery carriers for hepatocellular carcinoma therapy. Mol Pharmaceutics. 2016;13:699–709.
- Tang Z, Zhang L, Li T, et al. Glycyrrhetinic acid induces cytoprotective autophagy via the inositol-requiring enzyme 1ќ±-c-Jun N-terminal kinase cascade in non-small cell lung cancer cells. Oncotarget. 2015;6:43911–43926.
- Jin L, Huang R, Huang X, et al. Discovery of 18beta-glycyrrhetinic acid conjugated aminobenzothiazole derivatives as Hsp90-Cdc37 interaction disruptors that inhibit cell migration and reverse drug resistance. Bioorg Med Chem. 2018;26:1759–1775.
- Li K, Ma T, Cai J, et al. Conjugates of 18beta-glycyrrhetinic acid derivatives with 3-(1H-benzo[d]imidazol-2-yl)propanoic acid as Pin1 inhibitors displaying anti-prostate cancer ability. Bioorg Med Chem. 2017;25:5441–5451.
- Liu D, Song D, Guo G, et al. The synthesis of 18beta-glycyrrhetinic acid derivatives which have increased antiproliferative and apoptotic effects in leukemia cells. Bioorg Med Chem. 2007;15:5432–5439.
- Satomi Y, Nishino H, Shibata S. Glycyrrhetinic acid and related compounds induce G1 arrest and apoptosis in human hepatocellular carcinoma HepG2. Anticancer Res. 2005;25:4043.
- Chen Q, Ding H, Zhou J, et al. Novel glycyrrhetinic acid conjugated pH-sensitive liposomes for the delivery of doxorubicin and its antitumor activities. RSC Adv. 2016;6:17782–17791.
- Cheng M, Gao X, Wang Y, et al. Synthesis of glycyrrhetinic acid-modified chitosan 5-fluorouracil nanoparticles and its inhibition of liver cancer characteristics in vitro and in vivo. Marine Drugs. 2013;11:3517–3536.
- Zeng X, Guo L, Ma L. Preparation and characterization of glycyrrhetinic acid-modified poly(ethyleneglycol)-poly(β-benzyl-L-asparate) nanoparticles as liver-targeted delivery system. Colloid Polym Sci. 2015;319–328.
- Liu H, Wu S, Yu J, et al. Reduction-sensitive micelles self-assembled from amphiphilic chondroitin sulfate A-deoxycholic acid conjugate for triggered release of doxorubicin. Mater Sci Eng C Mater Biol Appl. 2017;75:55–63.
- KangLu Y, Lan L, Ding J, et al. Redox-responsive polymeric micelles formed by conjugating gambogic acid with bioreducible poly(amido amine)s for the co-delivery of docetaxel and MMP-9 shRNA. Acta Biomater. 2018;68:137–153.
- Esfandiarpour-Boroujeni S, Bagheri-Khoulenjani S, Mirzadeh H, et al. Fabrication and study of curcumin loaded nanoparticles based on folate-chitosan for breast cancer therapy application. Carbohydr Polym. 2017;168:14–21.
- Vijayaraghavan P, Liu CH, Vankayala R, et al. Designing multi-branched gold nanoechinus for NIR light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv Mater. 2014;26:6689–6695.
- Zhu C, Xia Y. Biomimetics: reconstitution of low-density lipoprotein for targeted drug delivery and related theranostic applications. Chem Soc Rev. 2017;46:7668–7682.
- XiaHou F, Zhang W, Zhi C, et al. pH-responsive gold nanoclusters-based nanoprobes for lung cancer targeted near-infrared fluorescence imaging and chemo-photodynamic therapy. Acta Biomater. 2017;68:308–319.
- Suo A, Qian J, Xu M, et al. Folate-decorated PEGylated triblock copolymer as a pH/reduction dual-responsive nanovehicle for targeted intracellular co-delivery of doxorubicin and Bcl-2 siRNA. Mater Sci Eng C Mater Biol Appl. 2017;76:659–672.
- Dong X, Zou S, Guo C, et al. Multifunctional redox-responsive and CD44 receptor targeting polymer-drug nanomedicine based curcumin and alendronate: synthesis, characterization and in vitro evaluation. Artif Cells Nanomed Biotechnol. 2017. DOI:10.1080/21691401.2017.1416390
- Kumar S, Mahesh A, Antoniraj M, et al. Cellular imaging and folate receptor targeting delivery of gum kondagogu capped gold nanoparticles in cancer cells. Int J Biol Macromol. 2017;109:220–230.
- Almeida EAMS, Bellettini IC, Garcia FP, et al. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr Polym. 2017;171:259–266.
- Singh A, Lavkush, Kureel AK, et al. Curcumin loaded chitin-glucan quercetin conjugate: synthesis, characterization, antioxidant, in vitro release study, and anticancer activity. Int J Biol Macromol. 2017;110:234–244.
- Guo Y, Zhang P, Zhao Q, et al. Reduction-sensitive polymeric micelles based on docetaxel-polymer conjugates via disulfide linker for efficient cancer therapy. Macromol Biosci. 2016;16:420–431.