?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
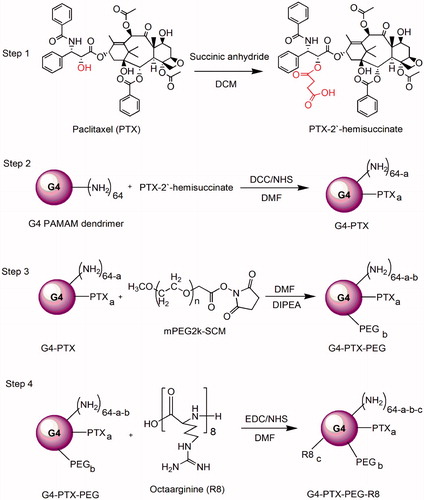
Cell penetrating peptides (CPP) have the ability to penetrate the cell membrane and have been associated with various cargos for their facile intracellular translocation. The current study involves the synthesis of a CPP, octa-arginine (R8)-modified poly(amidoamine) dendrimer of generation 4 (G4), which has additionally been PEGylated and conjugated to the poorly soluble anticancer drug, paclitaxel (PTX). The synthesized dendrimer conjugates were characterized by proton nuclear magnetic resonance (1H-NMR) Spectroscopy and zeta potential measurements and evaluated in vitro in cell monolayers and 3D spheroids. Cellular uptake study in human cervical cancer cell line (HeLa) revealed that R8 modification significantly improved the cell association of conjugates. G4-PTX- polyethylene glycol (PEG)-R8 conjugate demonstrated enhanced cytotoxic potential and higher induction of apoptosis compared to free PTX and G4-PTX-PEG. Further, the penetrability of fluorescently labeled F-G4-PTX-PEG-R8 was evaluated in 3D spheroids of HeLa at various depths by using confocal microscopy. G4-PTX-PEG-R8 induced cell death and inhibited the growth in 3D spheroids as competently as in monolayers. The enhanced intracellular translocation of R8-modified dendrimers resulted in improved anticancer efficacy of PTX. Therefore, the newly developed dendrimer system is efficient for the intracellular delivery of PTX in cancer cells and has a strong potential to be utilized as an effective chemotherapeutic agent for cancer.
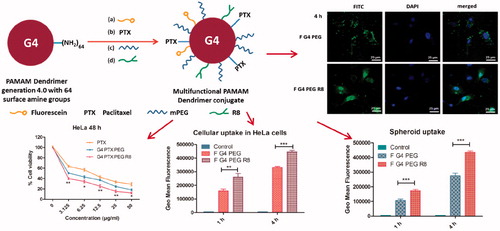
Graphical Abstract
Introduction
Cancer is considered as one of the world’s most life-threatening diseases with millions of new cases reported every year [Citation1,Citation2]. Even though the extensive research has resulted in advanced understanding of cancer, development of more sensitive detection methods and invention of chemotherapy drugs with superior efficacy compared to previous discoveries, the ability to deliver the drug molecules to the tumor tissue and specifically to the intracellular compartment of the cancer cells remains a challenge [Citation3]. The conventional cancer chemotherapy suffers disadvantages such as low solubility, poor permeability, fast clearance, non-specific toxicities, poor biodistribution and emergence of multiple drug resistance due to chronic drug exposure, which leads to meager therapeutic response and patient incompliance [Citation4]. To address the above concerns, nano drug delivery systems have attracted great interest due to the wide range of benefits they offer and significant progress has been achieved in taking these systems into clinic [Citation5]. Various nano-sized systems such as liposomes, polymeric micelles, lipid nanoparticles, nano-structured lipid carriers, drug conjugates, dendrimers and nano-suspensions have been proven to efficiently target and deliver the active moieties to cancer either passively or actively [Citation2,Citation3,Citation6–8].
Dendrimers are a class of three-dimensional tree like branched polymers in nanometer size range, monodisperse in nature and highly ordered structure with a central core branching out to a choice of functional groups at the periphery [Citation5,Citation9]. Poly(amidoamine) dendrimers (PAMAM) gained reputation from the last decade due to their unique advantages over other delivery systems and have been extensively used in delivery of small molecules, peptides and genetic material in various disease conditions [Citation10]. The empty internal cavities of the PAMAM dendrimer could physically hold the entrapped drugs whereas the surface groups (NH2, OH and COOH groups) can bind the molecules covalently [Citation5,Citation11,Citation12]. PAMAM dendrimers have been investigated for their efficiency in delivery of anti-cancer agents such as doxorubicin [Citation13,Citation14], docetaxel [Citation6], paclitaxel [Citation15], methotrexate [Citation16], 2-methoxy estradiol [Citation17] etc. effectively to tumor site either in physically entrapped or chemically conjugated form. Further, the availability of react-able functional groups on dendrimer surface allows them to be anchored by a variety of peptides/ligands/antibodies to target cancer cells [Citation18,Citation19]. In general, the nanocarrier systems passively enter the tumor tissue via leaky vasculature by a phenomenon known as enhanced permeation and retention (EPR) effect [Citation1,Citation20,Citation21]. However, following accumulation in the tumor microenvironment by EPR effect, the drug-loaded nanocarriers need to translocate through the cell membrane to reach the intracellular compartment to exert the therapeutic potential. The set back is usually the poor permeability of the nanocarriers through the cell membrane, which usually occurs via energy-dependent endocytosis [Citation22].
Invention of cell penetrating peptides (CPPs) has offered great benefits in this regard and improves the cellular internalization of a variety of molecules [Citation23–25]. CPPs are peptides consisting usually of less than 35 amino acid residues [Citation26]. They have been explored in a wide variety of theranostic applications in cancer, including the intracellular delivery of small anticancer drugs, macromolecules and nanoparticle systems [Citation27–29]. Cationic peptides which are rich in arginine residues were employed as CPPs such as TAT peptide (TATp), penetratin, low molecular weight protamine (LMWP) and oligoarginines [Citation3,Citation30,Citation31]. Octa-arginine (R8), a short chain peptide having eight arginine residues was investigated by many researchers and proved to be efficient in transporting a variety of cargoes into the cells [Citation32–34]. In the present study, we have synthesized a multifunctional PAMAM dendrimer conjugate of generation 4, which couples an anti-cancer agent Paclitaxel (PTX), polyethylene glycol (PEG) and a CPP, Octaarginine (R8). The R8 modification was expected to promote intracellular translocation of the nano-construct allowing delivery of PTX in high concentration in the intracellular compartment for enhanced cytotoxic effect. The final conjugate and the intermediates were characterized and were evaluated in the Human cervical cancer (HeLa) cell line in monolayers and 3D spheroidal system.
Experimental section
Materials
Dendrimer of generation 4 with ethylenediamine core and surface amino groups (G4 PAMAM) was purchased from Dendritech (Midland, MI). Paclitaxel (PTX) was obtained as a gift sample from Fresenius Kabi India Pvt., Ltd. (Gurgaon, India). Methoxy-polyethyleneglycol-succinimidyl carboxymethyl ester (mPEG-SCM ester, MW 2000 Da) was obtained from Jenkem Technology (Plano, TX). N-ethyldiisopropylamine (DIPEA) was obtained from Avra Chemicals, India. N-Hydroxysuccinimide (NHS)-Fluorescein was obtained from Thermo Scientific (Waltham, MA). R8 peptide was custom synthesized from USV Limited (Mumbai, India). N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC. HCl, 98%) and 98% NHS were obtained from Sigma Aldrich Chemicals (St. Louis, MO). Regenerated cellulose dialysis membrane (molecular weight-cutoff (MWCO) 2, 3.5 and 14 kDa, respectively) was obtained from Spectrum Laboratories, Inc. (Dominguez, CA). N,N′-Dicyclohexylcarbodiimide (DCC) was purchased from Spectrochem Chemicals Ltd. (India). All the other chemicals and solvents obtained commercially were of analytical grade.
Synthesis of multifunctional PAMAM dendrimer
Synthesis of fluorescent labeled dendrimer of generation 4 (G4)PAMAM dendrimer
G4 PAMAM dendrimer was fluorescent tagged as per the previously described method with a slight modification [Citation35]. Briefly, 100 mg of G4 PAMAM dendrimer was dissolved in dimethylformamide (DMF). NHS Fluorescein dissolved in DMF was added to the dendrimer solution (molar ratio 1:1) dropwise under inert atmosphere. The mixture was stirred for 8 h at room temperature in dark. DMF was removed under vacuum and the product was dialyzed for 48 h to remove the unattached NHS Fluorescein. The product was lyophilized and stored until further use.
The scheme of synthesis of the modified dendrimer has been represented in .
Synthesis of G4- Paclitaxel (PTX)
PTX has a 2′-OH group which was activated to hemisuccinate using succinic anhydride. Briefly, to 25 mg of PTX in DCM, 4.4 mg of succinic anhydride (molar ratio of 1:1.5) was added under stirring in presence of dry pyridine and this was continued for three days [Citation36]. After the reaction, product was extracted using ethyl acetate and vacuum dried to get the PTX-2′-hemisuccinate as white powder. PTX-2′-hemisuccinate was activated with DCC/NHS in DMF for 6–8 h at room temperature to make it react readily with the amine terminals of G4 PAMAM dendrimer [Citation37]. To the G4 PAMAM Dendrimer (50 mg) in DMF, activated PTX NHS ester (molar ratio of 1:4) was added drop wise under nitrogen. The reaction was continued overnight and the DMF was evaporated under vacuum. The formed product was dialyzed extensively for 48 h using 3500 Da MWCO dialysis membrane. A white fluffy solid of G4-PTX conjugate was obtained after lyophilization.
Synthesis of G4-PTX-PEG
To the synthesized G4-PTX conjugate, mPEG SCM ester of molecular weight 2 kDa was attached as described below. The mPEG SCM (molar ratio 12:1) in DMF was added under stirring to the solution of G4-PAMAM-PTX in a RBF. DIPEA (20 µl) was added as a base to the reaction mixture. The reaction was continued overnight and DMF was evaporated under vacuum. Reaction mixture was dialyzed for 48 h using 12–14 kDa MWCO dialysis membrane. Solid G4 PAMAM-PTX-PEG was obtained after lyophilization. The attachment of PEG to dendrimer was characterized and confirmed by change in zeta potential, proton nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) analysis.
Conjugation of R8 onto G4-PTX-PEG
Carboxy terminal of R8 was activated using EDC/NHS and was reacted with previously synthesized G4-PTX-PEG at a molar ratio of 3:1 in DMF. The final conjugate G4-PTX-PEG-R8 was collected as a solid after freeze drying following dialysis of the product after the reaction.
Characterization of multifunctional conjugate
All the synthesized conjugates were characterized by proton NMR (300 MHz, Bruker, Billerica, MA) and zeta potential analysis. GPC was performed to calculate the approximate number of molecules attached to each dendrimer (See Supplementary Material for the method). Zeta potential of the conjugates was determined using a Malvern Zetasizer (Nano ZS, Malvern Instruments, UK).
Hemolytic toxicity study
Hemolytic toxicity study was performed to assess the safety of synthesized multifunctional conjugate, which was executed following a previously reported procedure [Citation38]. Briefly, 5 ml of rat whole blood was collected in heparinized vials from which erythrocytes (RBCs) were separated by centrifugation at 3000 rpm for 30 min. A 5% RBC suspension was prepared by diluting the cells with phosphate buffer saline (PBS) after thorough washing. Further, 100 μL of 5% RBC solution was incubated for 1 h at 37 °C with 900 μL of multifunctional dendrimer conjugate (without PTX) dissolved in PBS at 5 and 10 mg/ml concentrations. Triton-X 100 (1% solution) and PBS were used as positive and negative controls, respectively. Following incubation, samples were centrifuged and the absorbance of hemoglobin (Hb) in the supernatant was measured by microplate reader (Spectramax™, Molecular Devices, Sunnyvale, CA). The degree of hemolysis was calculated on the basis of Hb absorbance (Abs) at 576 nm and derived from the following formula:
Cell culture
Human cervical carcinoma cells, HeLa were obtained from National Center for Cell Sciences (NCCS, Pune, India). Minimum essential medium (MEM) was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin solution was used as the growth medium. Cells were maintained in a humidified incubator at 37 °C and 5% CO2.
Confocal microscopy
To assess the internalization of multifunctional dendrimer conjugate visually, confocal microscopy was used. HeLa cells were seeded onto circular coverslips placed in 12-well culture plates at a cell population of 50,000 cells/well. Following day, F-G4-PEG and F-G4-PEG-R8 (20 µg/ml) were added to the wells and incubated at 37 °C for 1 and 4 h, respectively. After the incubation period, cells were washed three times with sterile PBS, treated with 4′,6-diamidino-2-phenylindole (DAPI;1 µg/ml) for 5 min, washed and fixed with 4% para-formaldehyde for 15 min. The coverslips were then mounted cell-side on the microscopic slides with the help of a mounting medium (Fluoromount-G). Slides were observed using confocal microscope (Leica DMi8, Leica Microsystems, Germany) and fluorescence images of cells were captured in fluorescein isothiocyanate (FITC) and DAPI filters. Picture files were processed using Image J software (NIH, Bethesda, MA).
Cellular uptake by flow cytometry
The HeLa cells were seeded in a six-well microplates at a cell population of 0.6 × 106 cells/well and allowed for attachment of cells overnight. On the following day, F-G4-PEG and F-G4-PEG-R8 were added to the cells at a concentration of 20 µg/ml and incubated for 1 and 4 h at 37 °C. Post-incubation, cells were washed with PBS and then harvested. The cells were centrifuged and were re-suspended in PBS prior to analysis by flow cytometer (Amnis, EMD Millipore, Billerica, MA). Cells without any treatment were used as control. For each sample, a minimum of 10,000 events were collected. The geo mean fluorescence of the cells was calculated from the IDEAS software V6.0 (Amnis technology, Ltd., UK).
Cytotoxicity study
The cytotoxicity of free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 was determined by MTT assay. HeLa cells were seeded at a density of 10,000 cells/well in 96-well plates on the day before treatment. Formulations equivalent to a PTX concentration ranging from 0–50 µg/ml were added to the wells and incubated at 37 °C and 5% CO2 for 24 and 48 h. After the specified time interval, formulations were discarded and treated with 50 µl of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reagent (5 mg/ml solution). The 96-well plates were incubated for another 4 h, MTT reagent was removed and dimethyl sulfoxide (150 µl) was added to each well to dissolve the purple colored formazan crystals. The absorbance at 570 nm was measured using a microplate reader (Spectramax™, Molecular Devices) keeping a wavelength of 630 nm as reference. Cells without treatment were used as control. Percentage cell viability was derived from the absorbance of sample against absorbance of control.
Apoptosis
AnnexinV FITC/PI double staining assay was carried out to determine the extent of induction of apoptosis in HeLa cells. Free PTX, G4 PTX PEG and G4 PTX PEG R8 were added to six-well plates seeded with HeLa cells at a density of 0.6 × 106 cells/well and incubated for 24 h. The concentration of PTX in each formulation was equivalent to 25 µg/ml. The study was performed as per the manufacturer’s protocol. After treatment, cells were harvested, washed with ice-cold PBS and were resuspended in 100 µl AnnexinV binding buffer. Later the cells were stained with 5 µl AnnexinV and 10 µl PI solution and incubated at room temperature for 15 min. At last, 400 µl of AnnexinV binding buffer was added to the samples and analyzed using flow cytometer (Amnis, EMD Millipore). Untreated cells were used as control for the study. Data was processed using IDEAS software version 6.0 and a scatter plot was plotted with green (FITC) channel on X-axis and red (PI) channels on Y-axis. The dot plot was divided into four quadrants representing live cells (Q1), early apoptotic (Q2), late apoptotic (Q3) and necrotic (Q4) cells, respectively.
Evaluation of multifunctional dendrimer conjugates in 3D spheroid model
Formation of HeLa spheroids
HeLa cancer cell spheroids were prepared by liquid overlay method [Citation39,Citation40]. Briefly, serum free MEM medium with 1.5% (w/v) agar was prepared and sterilized. 50 µL of the agar solution was added to the bottom of each well of the 96 well plates to prevent cell adhesion. In case of eight-well glass chamber slides, 200 µl of agar solution was added. Plates were allowed to cool down for 45 min before use. HeLa cells were harvested, counted and then seeded at a density of 8000 cells per each well. Plates were centrifuged at 1500 relative centrifugal force (RCF) for 15 min at room temperature. The formation of spheroids was continuously monitored using an inverted microscope (Leica DMi8, Leica Microsystems). Spheroids of 3–5 days old were used in the further studies.
Penetration efficiency in 3D cancer cell spheroids
The penetration of F-G4-PTX-PEG and F-G4-PEX-PEG-R8 through the spheroids was evaluated by confocal microscopy after 1 and 4 h of incubation. Following that, the tumor spheroids grown in the eight-well glass chamber slides were washed with PBS and then observed by laser scanning confocal microscope (Leica DMi8, Leica Microsystems). Z-stack images were obtained from the surface towards the tumor spheroid equatorial plane at 10 μm intervals of thickness. All images were taken using a 10X objective. Images were analyzed using Image J software.
Uptake of dendrimer conjugates in 3D spheroids by flow cytometry
Uptake of multifunctional dendrimer conjugates in 3D cultured spheroids was assessed quantitatively by flow cytometry. Five-day-old spheroids were incubated with F-G4-PEG and F-G4-PEG-R8 for 1 and 4 h, respectively. At each time point, 10 spheroids were collected as one replicate to achieve sufficient cell count. The spheroids were washed with PBS and broken down using AccutaseTM cell detachment solution (HiMedia Laboratories, Mumbai, India). The cells were dispersed as a suspension and Accutase was neutralized with FBS. Then, cells were centrifuged again and re-suspended in PBS to further analyze them using flow cytometer. A minimum of 10,000 events were collected for each sample. Same instrument conditions were maintained to study the uptake in monolayers and spheroids. Geo mean fluorescence exhibited by F-G4-PTX-PEG and F-G4-PEX-PEG-R8 treated spheroids was plotted as a bar diagram.
Inhibitory effect on the 3D spheroids
The 3D spheroids were incubated with free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 at a PTX concentration of 25 μg/mL. Growth medium was replaced after 24 h. The media was further changed on alternate days. The extent of inhibition of growth in spheroids was observed under inverted microscope (Leica DMi8, Leica Microsystems) at 10X magnification and images were captured. The data is represented as mean diameter of three spheroids with standard deviation.
In vitro cytotoxicity in spheroids
Cytotoxicity imposed by various PTX formulations in 3D spheroids was studied using Presto blue assay following the manufacturer's instructors. Visually compact spheroids (typically 3–5 days after seeding) were treated with free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 (concentration: 25 μg/mL) for 24 h. A group of 10 spheroids were included for each treatment. After the treatment period, the spheroids were washed with PBS and added with 50 μL of cell detachment solution (AccutaseTM) and incubated for 10 min at 37 °C. After that, the spheroids were gently shaken and the resulting suspension was centrifuged at 1200 rpm for 5 min. Samples for analysis were prepared by re-suspending the cell pellet in 90 μL of growth medium and adding 10 μL of Presto blue reagent. The samples were incubated at 37 °C for 2 h. The absorbance was measured at 570 nm using 600 nm as reference.
Live/dead cell assay in 3D spheroids
To assess the distribution of live and dead cell population in the 3D spheroids after various PTX treatments, calcein blue assay was performed. Briefly, the spheroids were treated with free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 at a PTX concentration of 50 μg/mL. After the incubation period of 24 h, the spheroids were washed with PBS and stained with calcein blue AM reagent and propidium iodide (PI). Calcein blue AM ester passively diffuses into the cells and gets cleaved by the intracellular esterases in viable cells. The resultant calcein blue retains in the cell and emits blue fluorescence. Further, dead cells are stained by PI can be observed using red filter of fluorescence microscope (Leica DMi8, Leica Microsystems). Images were captured and processed using Image J software.
Statistical analysis
All experiments were performed in triplicate and data are expressed as mean ± SD. The statistical significance among the groups was established using Student’s t-test using Graph Pad prism 5 (GraphPad Software Inc., San Diego, CA). Any value with p less than .05 were considered statistically significant. The representation of *, **, *** in figures corresponds to p values < .05, .01 and .001, respectively.
Results and discussion
Synthesis and characterization of multifunctional dendrimer conjugate
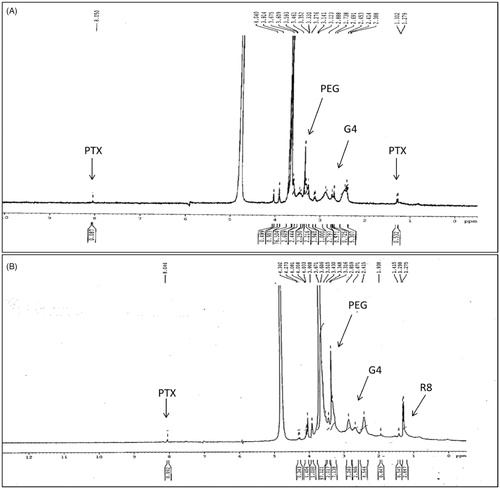
Activated PTX, which is PTX-2′-hemisuccinate was confirmed by Mass spectrometry. A Peak at 954.5 (MH+) and 976.5 (MNa+) was observed corresponding to the hemisuccinate form of PTX (Supplementary Figure S1). The proton NMR of the conjugates G4-PTX-PEG and G4-PTX-PEG-R8 was represented in . In the NMR spectrum, peaks at δ (ppm) 7–8.5 correspond to the aromatic groups of the PTX molecule. The protons of PEG chains can be seen at δ (ppm) 3.2–4.1 and dendrimeric protons from δ (ppm) 1.5–3.5. The signal at δ (ppm) 1–1.5 relates to the methylene protons of R8. These signals confirm the structure of G4-PTX-PEG-R8. The reduction in the intensity of PTX signals could be due to formation of micellar structure of dendrimer in D2O. Proton NMR spectra of intermediates are provided in Supplementary Figures S2 and S3. Further, from the GPC analysis, it was observed that approximately 2.5 molecules of PTX, 10.5 molecules of PEG and 1.8 molecules of R8 were attached to each G4 molecule (Supplementary Table S1). In addition, a change in the zeta potential value as shown in at each synthesis step supports the formation of the conjugates.
Table 1. Zeta potential values of multifunctional dendrimer conjugates (mean ± SD, n = 3).
Hemolytic toxicity study
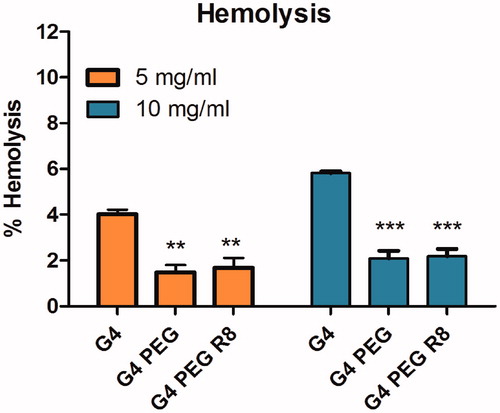
Hemolysis study was performed for the dendrimer conjugates G4-PEG and G4-PEG-R8 at 5 and 10 mg/mL concentration along with plain G4 dendrimer to measure carrier mediated toxicity, if any. Results indicated that the synthesized dendrimer conjugates pose negligible toxicity to RBCs. At a concentration of 10 mg/ml plain G4 caused a hemolysis of 5.7% whereas both the conjugates G4 PEG and G4 PEG R8 caused around 2% hemolysis only. This can be attributed to the presence of PEG chains which reduces the surface charge in comparison to plain G4 thereby giving a protective shield. Further, R8 modification on the surface of G4 dendrimer also did not pose any significant toxicity proving the safety of the conjugate for systemic administration. The hemolysis percentage of the conjgates are represented as bar diagrams in .
Cellular uptake by confocal microscopy
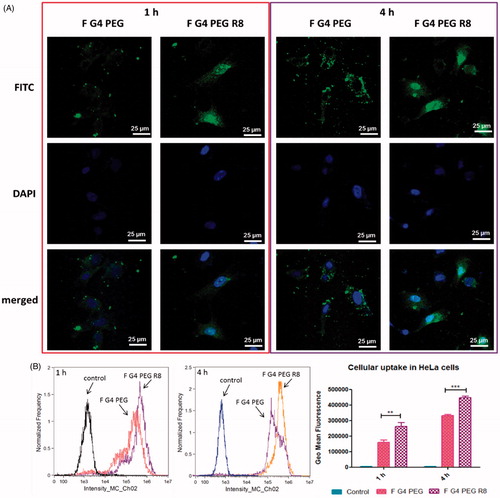
HeLa cells treated with fluorescently labeled G4-PEG and G4-PEG-R8 for 1 and 4 h were observed under confocal microscope to visualize the effect of R8 on the internalization of the conjugate. Brighter green fluorescence was detected in cytoplasm as well as nucleus with R8 attached dendrimer conjugate signifying higher cellular association relative to R8-unmodified conjugate. Confocal microscopy results are in agreement with flow cytometry data that the cells treated with F-G4-PEG-R8 demonstrated higher internalization compared to F-G4-PEG at both the time points. Photomicrographs of the cells after 1 and 4 h incubation are represented in .
Cellular uptake by flow cytometry
To measure the cellular uptake of the multifunctional carrier system and to study the effect of R8 modification on internalization of the conjugate, flow cytometry experiment was carried out. From the results, a time dependent increase in the uptake was witnessed at 1 and 4 h treatment. A 1.34- and 1.65 fold increase in the uptake was observed at 1 and 4 h, respectively for F-G4-PEG-R8 compared to F-G4-PEG. The flow cytometry data indicates that the R8 attachment promoted significantly higher cellular association of the R8-dendrimer-conjugate compared to unmodified dendrimer. Geo mean fluorescence of different treatments at 1 and 4 h are represented as bar graph along with the histograms of events captured by flow cytometer in .
Cytotoxicity study
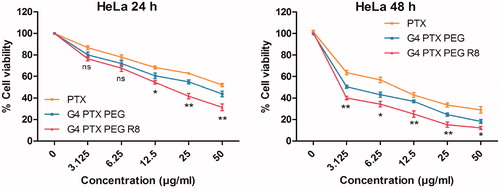
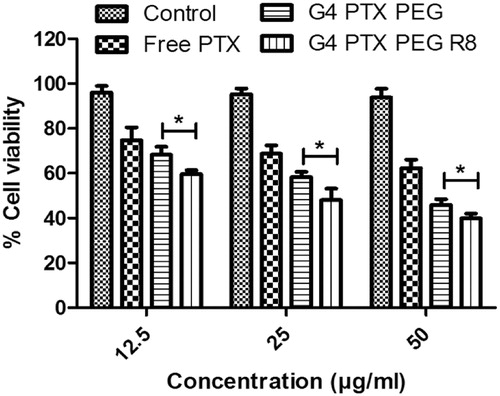
To determine the cytotoxic activity of PTX conjugates, MTT assay was executed. Cells were treated with free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 at 0–50 µg/ml concentration and incubated for 24 and 48 h. The results of the study revealed that there was a time and concentration dependent decrease in total cell viability (). At the end of 24 h, G4-PTX-PEG-R8 exhibited 31.3 ± 2.56% cell viability against 43.86 ± 2.16% of G4-PTX-PEG and 52.04 ± 1.19% of free PTX. Further, the cell viability reduced to 12.29 ± 1.22%, 18.23 ± 1.56% and 28.95 ± 2.49% for G4-PTX-PEG-R8, G4-PTX-PEG and free PTX respectively after 48 h treatment. At all the concentrations, dendrimer conjugated PTX was more active than the free PTX. In addition, G4-PTX-PEG-R8 had highest cytotoxicity which can be attributed to its ability to penetrate efficiently into the cells resulting in accumulating PTX in higher amount in the tumor cells compared to unmodified G4-PTX-PEG or free PTX.
Assessment of apoptosis inducing potential
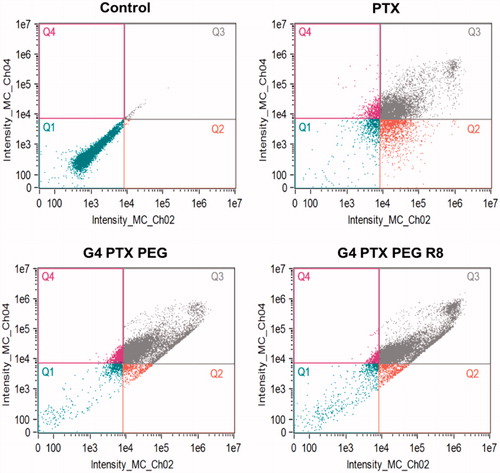
Annexin V FITC/PI double staining assay was carried out to measure the extent of apoptosis induced by free PTX and the dendrimer conjugates. The untreated cells did not show any signs of apoptosis or necrosis. As shown in , after 48 h treatment, it was observed that G4-PTX-PEG-R8 induced a total apoptosis (Q2 + Q3) of 89.5% whereas G4-PTX-PEG and free PTX induced 81.8 and 56.7% respectively. Both the dendrimer conjugates were superior compared to free PTX. The results were consistent with the MTT assay. Higher percentage of apoptosis is likely due to enhanced internalization of the R8-conjugate, more PTX was available for the cells which resulted in significant cell death.
Penetration efficiency in 3D cancer cell spheroids
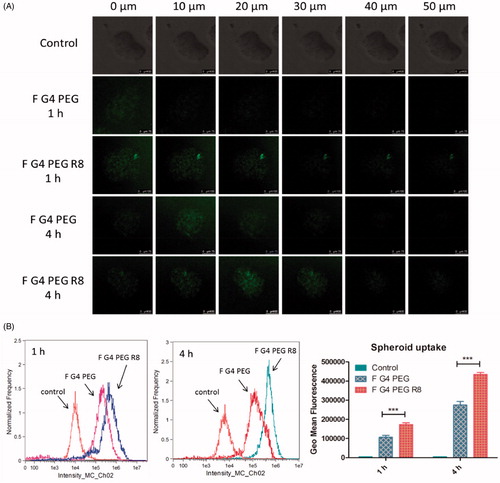
3D cell culture system, spheroids mimic the in vivo architecture and microenvironment of tumors owing to more natural morphology of cells in presence of extracellular matrix, pH and oxygen gradients. Spheroids enhance the significance of in vitro results compared to 2 D monolayers as they exhibit a drug resistance pattern similar to solid tumors [Citation41,Citation42]. These properties make it an alternative to study the transport of molecules into the tumor tissue. From this study, it was observed from Z-stack confocal microscopy images that with increase in the incubation time, F-G4-PTX-PEG-R8 moved deeper into the spheroid towards the center compared to unmodified F-G4-PEG conjugate (). R8 being a cell penetrating peptide enhanced the internalization of the dendrimer conjugate into the spheroids. The observation indicated that R8-conjugation in dendrimers overcomes the poor permeability of the dendrimers into the deeper tumor tissues.
Uptake of dendrimer conjugates in 3D spheroids by flow cytometry
The uptake of F-G4-PEG-R8 and F-G4-PEG in the 3D spheroids was quantified by breaking the spheroids and analyzing the samples using flow cytometer. Spheroids were treated with a cell detachment reagent, accutase to obtain single cell suspension. The F-G4-PEG-R8 conjugate entered the spheroids 1.58- and 1.62 folds higher in comparison to the F-G4-PEG after 1 and 4 h of treatment, respectively as calculated by the geo mean fluorescence values. This result corroborates with the confocal microscopy data, where time-dependent increase in the fluorescence intensity was observed. Fluorescence intensity observed in spheroids was relatively less in comparison to monolayers which could be due to the difference in effective surface area available for penetration in both the systems. The study in 3D spheroids suggests that R8 attachment significantly improved the cellular association of the conjugate. Data obtained after analysis by flow cytometer are represented in .
Inhibitory effect on the 3D HeLa spheroids
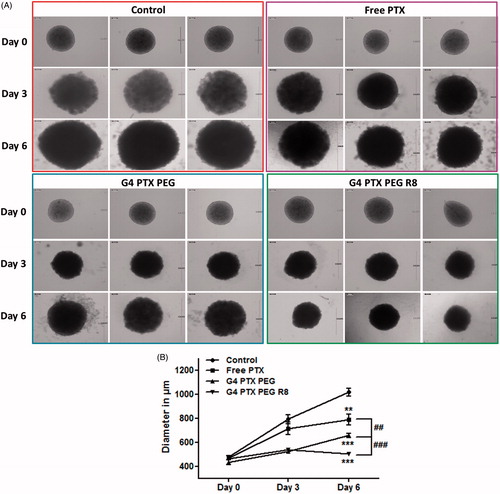
Inhibition of progress of growth of 3D tumor spheroids was studied after treating the spheroids with free PTX, G4-PTX-PEG and G4-PTX-PEG-R8 for 24 h. The spheroids were monitored for six days and brightfield images were captured on Day 0, Day 3 and Day 6. The growth progression was analyzed by measuring the diameter of the spheroids. As represented in , it was witnessed that the growth was significantly inhibited in spheroids treated with dendrimer conjugates in comparison to free PTX. Also, a drastic increase in the diameter of control spheroids was observed over the period of time in comparison to drug treated spheroids with size reaching up to 1000 µm diameter. The average diameter of spheroids at the end of six days was found to be 502 ± 8.7, 658 ± 17.3, 788 ± 43, and 1016 ± 32 µm after treatment with G4-PTX-PEG-R8, G4-PTX-PEG, Free PTX and control, respectively. The data is represented as a line graph in . R8, being a cell penetrating peptide, helps the PTX to localize in the spheroids subsequently inhibiting their growth progression. Further, the uptake data also supports that the dendrimer conjugates internalize significantly into the spheroids over time which makes the drug more available at the target site to elicit therapeutic action. As the spheroid systems are simulation of in vivo tumors, it can be extrapolated that the R8 conjugated dendrimer can significantly reduce the tumor progression in vivo.
In vitro cytotoxicity in spheroids
Presto blue assay was performed to determine the cytotoxic activity of PTX formulations in tumor spheroids. G4-PTX-PEG-R8 and G4-PTX-PEG showed higher cytotoxicity in comparison to free PTX after treatment for 24 h. The cytotoxicity was highest in case of G4-PTX-PEG-R8 with a cell viability of 39.8 ± 2.14% followed by G4-PTX-PEG and free PTX with a viability of 45.87 ± 2.5 and 62.27 ± 3.8%, respectively. The data is represented as a bar graph in The dendrimer conjugates were found to be effective in tumor spheroids as was observed in the monolayers although a slight reduction in cytotoxicity was noted. This can be explained based on the fact that the diffusion of carrier system becomes limited in tumor spheroids compared to free access to cells in monolayers.
Live/Dead cell assay in 3D spheroids
To visualize the live and dead population of cells as a response to treatment with PTX conjugates, the spheroids were incubated with calcein blue acetoxymethyl (AM) reagent. The calcein blue AM produces bright blue fluorescence due to intracellular esterase activity when internalized by live cells whereas the dead cells cannot transform the calcein blue AM reagent. On the other hand, dead cells can be identified by PI staining.
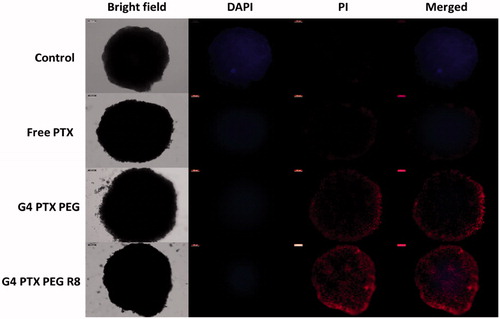
From the fluorescence micrographs of spheroids represented in , it can be noticed that G4-PTX-PEG-R8 had maximum red fluorescence in comparison to G4-PTX-PEG and free PTX. Control spheroids did not show any dead population. In spheroids treated with free PTX, dead population was present mostly on the periphery indicating its inability to reach deeper parts of spheroid. In case of G4-PTX-PEG and G4-PTX-PEG-R8, the dead population was seen evidently in the periphery as well as central parts of spheroid which can be attributed to its penetrability towards the core. R8 anchoring to the dendrimer conjugate resulted in superior penetration into the core of the spheroids and thereby higher drug accumulation and cell death.
Conclusion
Even though PAMAM dendrimers of various generations with ethylene diamine core has been utilized in delivering therapeutic cargo in cancer cells, cell membrane permeability is a challenge for this polar molecule. Therefore, a strategy needs to be undertaken to deliver the nanocarriers effectively in the intracellular compartment by facile translocation through the cell membrane. Here, we have developed an intracellularly targeted PAMAM nano-construct, G4-PTX-PEG-R8 by conjugating a potent cell penetrating peptide, R8 on the surface of the dendrimer. While PTX was conjugated on the dendrimer surface to prevent pre-mature drug release, anchorage of PEG on the surface was executed to impart the ability of long systemic circulation of the construct. GPC analysis indicated that approximately 2.5 molecules of PTX, 10.5 molecules of PEG and 1.8 molecules of R8 were attached per molecule of G4 PAMAM dendrimer. Results obtained from hemolysis study indicated that the synthesized dendrimer conjugates, G4-PTX-PEG-R8 exhibited negligible toxicity to RBCs. MTT assay performed on HeLa cells demonstrated that G4-PTX-PEG-R8 exhibited highest cytotoxicity compared to the free PTX and G4-PTX-PEG. G4-PTX-PEG-R8 demonstrated significantly higher cell membrane permeability in monolayers and spheroids of HeLa cells compared to G4-PTX-PEG, which led to the enhancement of the apoptosis inducing ability of G4-PTX-PEG-R8 as revealed by AnnexinV assay. Further, in tumor spheroids which simulate in vivo tumors, the R8 conjugated dendrimer exhibited superior cytotoxicity and inhibition of growth. In conclusion, G4-PTX-PEG-R8 could demonstrate tumor accumulation owing to long circulation property imparted by PEG chain followed by intracellular targetability by R8-conjugation. Therefore, the newly developed dendrimer-conjugate could be a promising chemotherapeutic agent for cancer treatment.
Supplemental Material
Download MS Word (484.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cui Y, Sui J, He M, et al. Reduction-degradable polymeric micelles decorated with PArg for improving anticancer drug delivery efficacy. ACS Appl Mater Interfaces. 2016;8:2193–2203.
- Estanqueiro M, Amaral MH, Conceição J, et al. Nanotechnological carriers for cancer chemotherapy: the state of the art. Colloids Surf B Biointerfaces. 2015;126:631–648.
- Shin MC, Zhang J, Min KA, et al. Cell-penetrating peptides: achievements and challenges in application for cancer treatment. J Biomed Mater Res A. 2014;102:575–587.
- Kesharwani P, Iyer AK. Recent advances in dendrimer-based nanovectors for tumor-targeted drug and gene delivery. Drug Discov Today. 2015;20:536–547.
- Ma P, Zhang X, Ni L, et al. Targeted delivery of polyamidoamine-paclitaxel conjugate functionalized with anti-human epidermal growth factor receptor 2 trastuzumab. Int J Nanomedicine. 2015;10:2173.
- Kulhari H, Pooja D, Shrivastava S, et al. Trastuzumab-grafted PAMAM dendrimers for the selective delivery of anticancer drugs to HER2-positive breast cancer. Sci Rep. 2016;6:23179.
- Akhter MH, Rizwanullah M, Ahmad J, et al. Nanocarriers in advanced drug targeting: setting novel paradigm in cancer therapeutics. Artif Cells Nanomed Biotechnol. 2018 [cited 2017 Aug 22]; [12 p.]. DOI:10.1080/21691401.2017.1366333
- Zhou B, Zhao L, Shen M, et al. A multifunctional polyethylenimine-based nanoplatform for targeted anticancer drug delivery to tumors in vivo. J Mater Chem B. 2017;5:1542–1550.
- Svenson S, Tomalia DA. Dendrimers in biomedical applications—reflections on the field. Adv Drug Deliv Rev. 2012;64:102–115.
- Kesharwani P, Jain K, Jain NK. Dendrimer as nanocarrier for drug delivery. Prog Polym Sci. 2014;39:268–307.
- He H, Li Y, Jia XR, et al. PEGylated Poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2011;32:478–487.
- Singh J, Jain K, Mehra NK, et al. Dendrimers in anticancer drug delivery: mechanism of interaction of drug and dendrimers. Artif Cells Nanomed Biotechnol. 2016;44:1626–1634.
- Fu F, Wu Y, Zhu J, et al. Multifunctional lactobionic acid-modified dendrimers for targeted drug delivery to liver cancer cells: investigating the role played by PEG spacer. ACS Appl Mater Interfaces. 2014;6:16416–16425.
- Wang Y, Cao X, Guo R, et al. Targeted delivery of doxorubicin into cancer cells using a folic acid–dendrimer conjugate. Polym Chem. 2011;2:1754–1760.
- Khandare JJ, Jayant S, Singh A, et al. Dendrimer versus linear conjugate: influence of polymeric architecture on the delivery and anticancer effect of paclitaxel. Bioconjugate Chem. 2006;17:1464–1472.
- Jiang YY, Tang GT, Zhang LH, et al. PEGylated PAMAM dendrimers as a potential drug delivery carrier: in vitro and in vivo comparative evaluation of covalently conjugated drug and noncovalent drug inclusion complex. J Drug Target. 2010;18:389–403.
- Wang Y, Guo R, Cao X, et al. Encapsulation of 2-methoxyestradiol within multifunctional poly (amidoamine) dendrimers for targeted cancer therapy. Biomaterials. 2011;32:3322–3329.
- Zhu J, Zheng L, Wen S, et al. Targeted cancer theranostics using alpha-tocopheryl succinate-conjugated multifunctional dendrimer-entrapped gold nanoparticles. Biomaterials. 2014;35:7635–7646.
- He X, Alves CS, Oliveira N, et al. RGD peptide-modified multifunctional dendrimer platform for drug encapsulation and targeted inhibition of cancer cells. Colloids Surf B Biointerfaces. 2015;125:82–89.
- Gillies ER, Frechet JM. Dendrimers and dendritic polymers in drug delivery. Drug Discov Today. 2005;10:35–43.
- Rompicharla SVK, Trivedi P, Kumari P, et al. Polymeric micelles of suberoylanilide hydroxamic acid to enhance the anticancer potential in vitro and in vivo. Nanomedicine. 2017;12:43–58.
- Biswas S, Dodwadkar NS, Deshpande PP, et al. Surface functionalization of doxorubicin-loaded liposomes with octa-arginine for enhanced anticancer activity. Eur J Pharm Biopharm. 2013;84:517–525.
- Kuwada E, Tadaki T, Kambara K, et al. Conjugation to octa‐arginine via disulfide bonds confers solubility to denatured proteins in physiological solution and enables efficient cell internalization. Biotechnol Appl Biochem. 2011;58:439–448.
- Klosz A, Henklein P, Siele D, et al. The cell-penetrating peptide octa-arginine is a potent inhibitor of proteasome activities. Eur J Pharm Biopharm. 2009;72:219–225.
- Madani F, Lindberg S, Langel U, et al. Mechanisms of cellular uptake of cell-penetrating peptides. Biophys J. 2011;2011:414729.
- Bolhassani A, Jafarzade BS, Mardani G. In vitro and in vivo delivery of therapeutic proteins using cell penetrating peptides. Peptides. 2017;87:50–63.
- Dissanayake S, Denny WA, Gamage S, et al. Recent developments in anticancer drug delivery using cell penetrating and tumor targeting peptides. J Control Release. 2017;250:62–76.
- Liu Y, Mei L, Yu Q, et al. Multifunctional tandem peptide modified paclitaxel-loaded liposomes for the treatment of vasculogenic mimicry and cancer stem cells in malignant glioma. ACS Appl Mater Interfaces. 2015;7:16792–16801.
- Li Y, Lee RJ, Yu K, et al. Delivery of siRNA using lipid nanoparticles modified with cell penetrating peptide. ACS Appl Mater Interfaces. 2016;8:26613–26621.
- Milletti F. Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today. 2012;17:850–860.
- Kang MH, Park MJ, Yoo HJ, et al. RIPL peptide (IPLVVPLRRRRRRRRC)-conjugated liposomes for enhanced intracellular drug delivery to hepsin-expressing cancer cells. Eur J Pharm Biopharm. 2014;87:489–499.
- Yamada Y, Hashida M, Harashima H. Hyaluronic acid controls the uptake pathway and intracellular trafficking of an octaarginine-modified gene vector in CD44 positive- and CD44 negative-cells. Biomaterials. 2015;52:189–198.
- Biswas S, Deshpande PP, Perche F, et al. Octa-arginine-modified pegylated liposomal doxorubicin: an effective treatment strategy for non-small cell lung cancer. Cancer Lett. 2013;335:191–200.
- Kitagishi H, Chai F, Negi S, et al. Supramolecular intracellular delivery of an anionic porphyrin by octaarginine-conjugated per-O-methyl-β-cyclodextrin. Chem Commun (Camb). 2015;51:2421–2424.
- Biswas S, Dodwadkar NS, Piroyan A, et al. Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials. 2012;33:4773–4782.
- Majoros IJ, Myc A, Thomas T, et al. PAMAM dendrimer-based multifunctional conjugate for cancer therapy: synthesis, characterization, and functionality. Biomacromolecules. 2006;7:572–579.
- Sousa-Herves A, Wurfel P, Wegner N, et al. Dendritic polyglycerol sulfate as a novel platform for paclitaxel delivery: pitfalls of ester linkage. Nanoscale. 2015;7:3923–3932.
- Kumari P, Muddineti OS, Rompicharla SVK, et al. Cholesterol-conjugated poly (D, L-lactide)-based micelles as a nanocarrier system for effective delivery of curcumin in cancer therapy. Drug Deliv. 2017;24:209–223.
- Sarisozen C, Abouzeid AH, Torchilin VP. The effect of co-delivery of paclitaxel and curcumin by transferrin-targeted PEG-PE-based mixed micelles on resistant ovarian cancer in 3-D spheroids and in vivo tumors. Eur J Pharm Biopharm. 2014;88:539–550.
- Perche F, Torchilin VP. Cancer cell spheroids as a model to evaluate chemotherapy protocols. Cancer. Biol Ther. 2012;13:1205–1213.
- van den Brand D, Massuger LF, Brock R, et al. Mimicking tumors: toward more predictive in vitro models for peptide- and protein-conjugated drugs. Bioconjugate Chem. 2017;28:846–856.
- Yang Y, Roy A, Zhao Y, et al. Comparison of tumor penetration of podophyllotoxin–carboxymethylcellulose conjugates with various chemical compositions in tumor spheroid culture and in vivo solid tumor. Bioconjugate Chem. 2017;28:1505–1518.