Abstract
Development and formulation of an efficient and safe therapeutic regimen for cancer theranostics are dynamically challenging. The use of mono-therapeutic cancer regimen is generally restricted to optimal clinical applications, on account of drug resistance and cancer heterogeneity. Combinatorial treatments can employ multi-therapeutics for synergistic anticancer efficacy whilst reducing the potency of individual moieties and diminishing the incidence of associated adverse effects. The combo-delivery of nanotherapeutics can optimize anti-tumor efficacy while reversing the incidence of drug resistance, aiming to homogenize pharmacological profile of drugs, enhance circulatory time, permit targeted drug accumulation, achieve multi-target dynamic approach, optimize target-specific drug binding and ensure sustained drug release at the target site. Numerous nanomedicines/nanotherapeutics have been developed by having dynamic physicochemical, pharmaceutical and pharmacological implications. These innovative delivery approaches have displayed specialized treatment effects, alone or in combination with conventional anticancer approaches (photodynamic therapy, radiotherapy and gene therapy), while reversing drug resistance and potential off-target effects. The current review presents a comprehensive overview of nanocarrier aided multi-drug therapies alongside recent advancements, future prospects, and the pivotal requirements for interdisciplinary research.
Introduction
The understanding of the molecular framework of cancer mechanistic, chiefly involving the origin and progression of this abnormality, has led to the development of promisingly refined cancer therapy. The optimal use of conventional chemotherapy, regarded as the most widely employed mode of cancer treatment presently, is restricted on account of the accompanied adverse effects, attributed to the non-targeted drug distribution and organ accumulation [Citation1]. Consequently, the patients tend to suffer from undesired chemo-toxicity due to an overdose of conventional regimens, as safe doses fail to administer desired therapeutic effects against tumors. Combination therapy thus aspires to amplify tumor eradication efficiency without escalating systemic toxicity, whilst concurrently controlling the issue of drug resistance [Citation2]. For this purpose, the therapeutics work synergistically on multidimensional oncogenic-signal transduction mechanisms. Hence, the combination therapies tend to optimize the therapeutic efficiency, alongside the reduction of drug resistance by targeting different mechanisms associated with distinct oncogenic pathways.
Intra-cancer heterogeneity is realized as the greatest challenge towards the successful treatment of cancer [Citation3]. Clonal heterogeneity is responsible for tumor metastasis and progression, and can substantially affect the entire cancer biology. Additionally, the diversity in tumor-genetics leads to undermined chemotherapeutic responses, allowing various resistance mechanisms. Resistance mechanisms are known to change the dependency route, permitting cancer to survive via an inceptive oncogenic pathway and involve by-pass parameters causing activation of a concurrent signaling pathway. Combination therapies offer a promising counter-measure against the resistance mechanisms via the simultaneous targeting of various oncogenic pathways involved in chemo-resistance. Hence, the simultaneous delivery of various therapeutic can lead to modulation of genetic hurdles inducing cancer mutations, alongside eradication of cancer adaptive processes.
It is noteworthy to consider various complexities associated with signaling pathways of cancer and their specific heterogeneity while fabricating the novel combination anticancer therapies. High-throughput screening has stepped up as a highly constructive technique for determining the successful combination treatments [Citation4]. Likewise, cancer cell screening for gain/loss of function throughout the genome helps in the recognition of crucial over-expression or silencing related to drug-resistance [Citation5]. An intensive consideration also needs an understanding of pharmacokinetics of anticancer drugs while optimizing the combo-drugs delivery systems. The advent of nanotechnology has served to revolutionize the domain of cancer therapy via combo delivery of therapeutic payloads, enhanced transport characteristics, improved bio-stability and distribution, and optimized release profiles of drugs [Citation6].
Improved transport vehicles governed by the advancements in nanotechnology have paved the way for revolutionizing the chemotherapy [Citation7]. Nanomedicines have facilitated to combat adverse effects associated with non-targeted drug accumulation through the tunable and controlled release of therapeutic moieties, improved pharmacological profiles, targeted delivery and simultaneously incorporating multiples compounds with varying solubility [Citation8]. Tunable release profile has served as an important hallmark in research and development of nanomedicines, as stimulus-triggered vehicles are constructed to deliver drug payload upon interaction with specific stimuli either within the cell or inside the tumor-microenvironment, thus reducing chemotherapeutics-specific systemic toxicity [Citation9]. These triggers for drug release often comprise of a sudden change in pH in the tumor microenvironment, over-expressed enzymes, hyperthermia due to an increased blood flow and enhanced glutathione levels inside the cells. The ability to control the drug release rate holds remarkable significance for co-delivery of drugs in combination therapy and to facilitate optimal synergistic effects.
A point to ponder is that whether the combination of co-formulated therapeutic regimens would be sufficient to overcome genetic mutations and associated drug resistance. On account of cancer heterogeneity and consistently developing resistance pathways, it seems inevitable that eventually, resistance will sprout against combined therapeutics as well. Nonetheless, physical eradication combined with agents may serve to achieve tumor destruction via various methods involving alternate survival pathways and enhances the chances for cancer eradication. External stimuli involving magnetic fields, near-IR light, radiation and ultrasound coupled with appropriate nanomaterials can serve as substitutive methods for cancer treatment, while concurrently releasing drug payload specifically within the localized targeting areas [Citation10]. Multi-dimensional cancer therapeutics employing chemotherapy alongside multi-functional nanomaterials has been demonstrated to substantially optimize the anticancer efficacy while offering a promising solution for overcoming the development of chemo-resistance pertaining to the conventional chemotherapeutic agents. Therefore, this review tends to overview prevalent challenges linked with cancer resistance and heterogeneity, the significance of combination treatments in battling chemo-resistance and improved anticancer outcomes, and focuses on employing advanced nanomaterials for combating the cancer menace.
Treatment of cancer
Major challenge and possible adaptations in treating cancer
The concept of personalized medicine was introduced after the inception of next-generation sequencing which enabled fabrication of targeted therapies for overcoming intrinsic and acquired drug resistance [Citation11]. Genetic diversity amongst tumors initially elicits a temporary response to chemo-therapeutics, which is ultimately followed by the development of resistance via several mechanisms. Likewise, alterations in tumor microenvironment which restrict the absorption and delivery of drugs also play a pivotal role in the development of resistance [Citation12]. Drug resistance due to intra-tumoral heterogeneity may arise via several ways including alterations in drug influx/efflux mechanisms, inactivation of the drug inside the cancer cell, activation of cell repair pathway, and mutations within specific drug targets causing the drug to be inefficacious [Citation13]. An effective approach to overcome these drug resistance mechanisms lies in the use of combination agents and simultaneously targeting of several pathways of cancer cell survival, including metabolism leading to its eradication. For instance, a recent study highlighted that the synergistic effect from the combined use of chemotherapeutic drugs can cause significant susceptibility within ovarian cancer, especially if the drug’s concentration can be adjusted according to the metabolism profile of the tumor in the patient [Citation14]. Authors demonstrated that the combined use of STAT3 and glutaminase inhibitors have shown a substantial reduction in cancer cell proliferation and growth in contrast to the use of these agents, alone.
A constructive method for terminating the adaptation process of cancer involves utilizing multiple drugs bearing variable molecular targets, which can bring about changes in the genetic hurdles responsible for eliciting mutations in cancer cells. Productive combinations may also involve the use of such a drug which serves to enhance the sensitivity of tumor cells against an ongoing therapy. For instance, productive treatment for evolved, post-menopausal estrogen-receptor overexpresses breast cancer (ER +), a subdivision responsible for 70% of all types of breast cancers, may be acquired upon simultaneous administration of tamoxifen (TAM) and hydroxyl-chloroquine (HCQ) [Citation15]. TAM is employed as an estrogen blocker; however several patients turn unresponsive and develop resistance against it. Administration of TAM along with low-dose of HCQ in athymic female mice models, having anti-estrogen/TAM cross-resistant LCC9 ER + and TAM-resistant MCF7-RR breast cancer cells, reestablished sensitivity towards anti-estrogen. Another example involving metastatic colorectal cancer includes deregulation of SET protein which in-turn results in promotion of cell proliferation and formation of colonospheres, alongside inhibition of antitumor effects exhibited by PP2A (protein phosphatase 2A) [Citation16]. Additionally, SET tends to de-sensitize the colorectal tumor cells to the drug oxaliplatin and undergoes over-expression in 24% of patient population, thus it is linked to reduce progression-free and overall survival. FTY720- a PP2A activator-induces restoration of oxaliplatin sensitivity when employed as combination treatment [Citation16].
Combo delivery of anticancer agents
For decades, chemotherapy has remained the first-line therapy against cancer, and extensive research is underway for engineering novel targeted therapies for clinical intervention [Citation11]. The concurrent administration of conventional cytotoxic agents alongside target-specific enzymes has demonstrated a promise response in clinical settings [Citation17]. Combinatorial therapies employing multiple therapeutic agents aimed against specified pathways tend to cause synergistic eradication of cancer and prevent tumor proliferation. Multifarious combination treatments are presently underway for clinical evaluation and have passed the phase III of clinical trials [Citation18–20]. Simultaneous targeting of several components within one particular pathway can lead to its optimal inhibition, and a possible shutdown of the signaling network.
Nanotechnology: rational and advantages
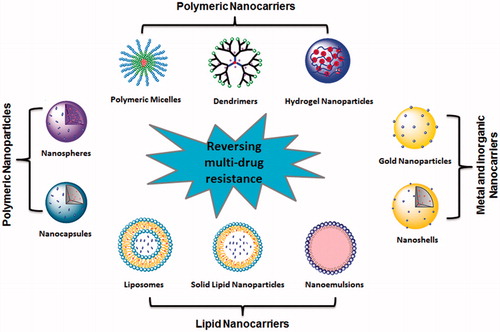
Nanotechnological applications are rapidly being inculcated in the domain of cancer-specific drug delivery, specifically in combination therapies. Nano-vehicles avert degradation of therapeutic agents via evasion of reticuloendothelial system (RES), thereby enhancing the bioavailability of drugs at the targeted area [Citation21]. As a result, systemic adverse effects are reduced, whereas the drug’s efficiency and efficacy increases. The unparalleled advantage of employing drug delivery systems is the concurrent and controlled delivery of diverse therapy agents on a common platform, concluding towards the drug’s optimal pharmacokinetic and dynamic properties. Numerous types of nanodelivery systems have been employed for the co-delivery of anticancer therapies ().
Utilization of nanocarriers for co-delivery of anti-cancer therapeutics
Nanomedicines have gained immense importance on account of their ability to deliver several chemotherapeutic agents simultaneously; thereby allowing intricate materials to achieve optimal therapeutic activity [Citation6]. Designing an appropriate vehicle for chemically contrasting agents is generally the initial step towards the efficient delivery of drug encapsulation being drastically dependent on the structural morphology and size of the delivery system. The chemotherapeutic drugs are chemically linked to peptides or polymers, and further conjugated with nanoparticles (NPs) through nanoprecipitation, soft lithography or evaporation technique [Citation22]. The nanovehicle based drug release is driven by two major processes, namely carrier-specific biodegradation and consequent diffusion, with the rate of drug release being dependent on it’s desorption and associated diffusion from the nano-matrices [Citation23]. The choice of particular materials can, therefore, regulate the drug delivery framework, for instance, ratios or release timing. Recently, a non-toxic, biodegradable, cholesterol-based NPs system was engineered for co-delivery of cisplatin (CDDP), PI103 (PI3K inhibitor) and doxorubicin (DOX) in a controlled manner via the chemical linkage [Citation24]. The NPs exhibited increased in-vitro toxicity in contrast to the concurrent application of un-conjugated free drugs in MDA-MB 231, MCF7 and HL60 cancer cells.
Similarly, a nano-polymer, fabricated via self-assembly process of metal clusters and polydentate based ligands, was employed as a promising carrier for co-delivery of gemcitabine and oxaliplatin (12 and 30 weight %, respectively) [Citation25]. The combinational therapy exhibited remarkably higher antitumor efficiency in mouse xenograft model bearing human pancreatic adenocarcinoma, with reduced systemic toxicity and prolonged half-life and blood circulation. The anticancer efficacy of co-delivered formulation was assessed by measuring the tumor volume and tumor growth (weight) [Citation25]. The results showed that the animals treated with nanomedicines carrying two drugs simultaneous (NCP-1) showed significantly higher suppression in average tumor size (1.7-fold) compared to 3.6–14.3-folds in other groups (). Tumor weight of NCP-1 group was also remarkably smaller (2.7 times than the NCP-2 and 16.6 times smaller than the NCP-3) compared with those treated with other treatments () [Citation25].
Figure 2. (A) In vivo tumor growth inhibition curves for PBS (▪), oxaliplatin and gem (♦), Zn Control (▲), NCP-1 (▶), NCP-2 (▼), and NCP-3 (◀) on subcutaneous BxPc-3 xenografts. Oxaliplatin (dose, 5 mg/kg) and gem (dose, 50 mg/kg), NCP-1 (doses, 2 mg/kg +0.8 mg/kg), NCP-2 (dose, 2 mg/kg), and NCP-3 (dose, 0.8 mg/kg) were administered on day 0, 4, and 8. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001. (B) End-point tumor weights. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001. (C) Photos of the resected BxPc-3 tumors from top to bottom: PBS, oxaliplatin and gem, Zn Control, NCP-2, NCP-3, and NCP-1. (D) In vivo tumor growth inhibition curves for PBS (▪), oxaliplatin and gem (♦), and NCP-1 (▶) on subcutaneous AsPc-1 xenografts. Oxaliplatin (dose, 5 mg/kg) and gem (dose, 50 mg/kg) and NCP-1 (doses, 2 mg/kg +0.8 mg/kg) were administered on day 0, 4, 8, 12, 16, and 20. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001 [Citation25]. Reprinted with permission from Elsevier B.V (Copyright © 2015) through Copyright Clearance Center.
![Figure 2. (A) In vivo tumor growth inhibition curves for PBS (▪), oxaliplatin and gem (♦), Zn Control (▲), NCP-1 (▶), NCP-2 (▼), and NCP-3 (◀) on subcutaneous BxPc-3 xenografts. Oxaliplatin (dose, 5 mg/kg) and gem (dose, 50 mg/kg), NCP-1 (doses, 2 mg/kg +0.8 mg/kg), NCP-2 (dose, 2 mg/kg), and NCP-3 (dose, 0.8 mg/kg) were administered on day 0, 4, and 8. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001. (B) End-point tumor weights. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001. (C) Photos of the resected BxPc-3 tumors from top to bottom: PBS, oxaliplatin and gem, Zn Control, NCP-2, NCP-3, and NCP-1. (D) In vivo tumor growth inhibition curves for PBS (▪), oxaliplatin and gem (♦), and NCP-1 (▶) on subcutaneous AsPc-1 xenografts. Oxaliplatin (dose, 5 mg/kg) and gem (dose, 50 mg/kg) and NCP-1 (doses, 2 mg/kg +0.8 mg/kg) were administered on day 0, 4, 8, 12, 16, and 20. Data are expressed as means ± SD (n = 6), *p < .05, **p < .01, ***p < .001 [Citation25]. Reprinted with permission from Elsevier B.V (Copyright © 2015) through Copyright Clearance Center.](/cms/asset/223f1a88-acd4-44fb-aba1-403b0caa136d/ianb_a_1478420_f0002_c.jpg)
Nanoencapsulation within the macromolecular framework generally leads to enhanced circulation of various drugs inside nanovehicles and permits synergistic dosing via increased therapeutic window [Citation26]. Efficient and targeted combinational treatments require controlled drug release upon stimulation with either internal or external triggers [Citation27]. Optimized NPs characterized by enhanced cancer driven selectivity, associated with target-specific drug release can serve to minimize the required therapeutic dose. For instance, formulation of “nano-cocoons” was derived from a singular self-assembled DNA strand bearing an acid-diminishable core conjugated with DNase and DOX [Citation28]. These “nano-cocoons” were specifically targeted against the folate receptor over-expressing cancer cells, followed by the controlled release of DOX upon endosomal pH exposure.
Proteins which are over-expressed in the microenvironment of tumors can also assist in selective drug release. For instance, an over-expressed matrix metallopeptidase-9 (MMP-9) within bronchial tumors was used for bortezomib and CDDP release from avidin-conjugated silica NPs [Citation29]. The proteinase was able to cleave off the avidin cap, thereby allowing the sustained release and synergistic activity in in-vitro and ex-vivo experiments. For treatment of EGFR-overexpressing metastatic KRAS bowel cancer, the anticancer drug oxaliplatin was encapsulated within cetuximab conjugated liposomes [Citation30]. Results from the in-vitro analysis showed that the final liposomal product exhibited a 3-fold enhanced level of drug delivery incorporation within EGFP overexpressing cells.
The release of multiple drugs from within a nanocarrier, either concomitantly or in a step-wise manner, is dependent upon specific kinetic properties as well as the final formulation design [Citation31]. For instance, targeted casein-PLGA (poly lactic-co-glycolic acid) NPs were conjugated with paclitaxel (PTX) and epigallo-catechingallate (EGCG) for sequence release, leading to down-regulation of NF-κB and increased PTX efficacy [Citation32]. Studies have highlighted that NF-κB induction genes impart breast cancer MDA-MB-231 resistance against PTX; however, EGCG shows profound NF-κB down-regulation thereby enhancing the therapeutic efficacy of PTX. Upon treatment with prepared drug-loaded nanoconjugates, a substantial increase in anticancer effect was observed in the PTX-resistant MDA-MB 231 cells, alongside a marked repression of P-gp expression. In addition, the NPs inhibited the activation of NF-κB and down-regulated the genes mediating metastasis, angiogenesis and overall survival of tumor.
Nanocarriers have been demonstrated to efficiently transport multiple therapeutics with varying physicochemical characteristics, simultaneously. G-camptothecin-glutamate N-carboxy anhydride (CPT(Glu)-NCA), a drug-based monomer, was conjugated with a poly-ethylene glycol (PEG) for the formation of monodispersed NPs via self-assembly approach, which for followed by loading of the anticancer drug DOX [Citation33]. Results demonstrated that these nanocarriers underwent substantial accumulation at the tumor target site thereby demonstrating enhanced anticancer efficacy in contrast to free drugs. To assess anticancer efficacy, a xenograft model was induced to generate tumor in the right dorsum [Citation33]. After the tumor volume reached to 80–100 mm3, treatment was initiated by giving multiple doses (once every four days for a total of six injections, q4d × 6) of PBS, free camptothecin (CPT) (5 mg/kg), free DOX (5 mg/kg), MB-20 (10 mg/kg, CPT equivalent) and MB-20/Dox (10 mg/kg, CPT equivalent). The anticancer efficacy was evaluated by assessing tumor sizes and body weight. Results demonstrated that the mice that have received MB-20/Cy5.5 nanocarriers displayed strong fluorescence at the tumor sites after 4 h (). The bio-distribution study revealed the significantly higher accumulation of nanocarriers at tumor site compared to heart, kidney and spleen (). Compared with the groups treated with PBS, CPT and DOX, the single-drug nanocarrier MB-20 and dual-drug nanocarrier MB-20/DOX displayed superior anticancer efficacy () [Citation33].
Figure 3. In vivo bio-distribution and anti-tumor efficacy of nanocarriers in tumor-bearing mice. (A) Near-IR optical imaging of A549 tumor-bearing nude mice at 4, 24, 48 and 72 h after intravenous injection of MB-20/Cy5.5 at Cy5.5 dose of 26 nmol/kg. The red arrow in the light phase indicated the tumor site. (B) Ex vivo imaging of tumors and other tissues of A549 tumor-bearing nude mice at 72 h post injection. (C) The quantitative analysis of fluorescence signals from tumors and other tissues. Values are means ± SD (n = 3). *p < .05 with respect to heart, spleen and kidney groups. (D) The A549 tumor growth curves after treating with either PBS, CPT, DOX, MB-20 nanocarrier or MB-20/Dox nanocarrier. Values are means ± SEM (n = 5). *p < .05 compared to PBS, CPT and Dox groups; +p < .05 compared to MB-20. (E) The body weight variation of mice after treated with different formulations [Citation33]. Reprinted with permission from Elsevier Ltd. (Copyright © 2014) through Copyright Clearance Center.
![Figure 3. In vivo bio-distribution and anti-tumor efficacy of nanocarriers in tumor-bearing mice. (A) Near-IR optical imaging of A549 tumor-bearing nude mice at 4, 24, 48 and 72 h after intravenous injection of MB-20/Cy5.5 at Cy5.5 dose of 26 nmol/kg. The red arrow in the light phase indicated the tumor site. (B) Ex vivo imaging of tumors and other tissues of A549 tumor-bearing nude mice at 72 h post injection. (C) The quantitative analysis of fluorescence signals from tumors and other tissues. Values are means ± SD (n = 3). *p < .05 with respect to heart, spleen and kidney groups. (D) The A549 tumor growth curves after treating with either PBS, CPT, DOX, MB-20 nanocarrier or MB-20/Dox nanocarrier. Values are means ± SEM (n = 5). *p < .05 compared to PBS, CPT and Dox groups; +p < .05 compared to MB-20. (E) The body weight variation of mice after treated with different formulations [Citation33]. Reprinted with permission from Elsevier Ltd. (Copyright © 2014) through Copyright Clearance Center.](/cms/asset/ce3337ca-d6f4-4f68-a1b8-e4492f3c30b8/ianb_a_1478420_f0003_c.jpg)
Utilization of nanocarriers of co-delivery of genes and chemotherapeutic agents
Quality treatment involving gene therapy ensures delivery of nucleic acids into the cancer microenvironment in order to eliminate cancer. The procedure dispenses by conveying nucleic acids to express genius apoptotic proteins or substitute changed qualities, down-control or hush oncogenic pathways, deliver tumor cytokines, and actuate the resistant framework against growth (e.g. designed T cells with chimeric antigen receptors [CARs]). One way to deal with tumour microenvironment is by utilizing joined delivery and chemotherapy against a medication resistance pathway [Citation34]. One of the difficulties related to this approach is the co-conveyance of nucleic acids and drugs with small molecular weight as a result of their altogether extraordinary physico-concoction properties. Endeavors to design nanocarriers for co-delivery of siRNA and low molecular weight drugs are most tedious challenges. Consolidated quality along with chemotherapy render nanocarriers to be inactive in nature, non-immunogenic, non-harmful, prepared specially for immobilizing nucleic acids, and embodying little particles [Citation35].
Resistance against drugs can occur through various means, and this is why it is required to devise a multi-faceted approach, for example, consolidated quality and chemotherapy. For instance, sedate resistance related with P-glycoprotein (P-gp) medicate efflux pump that is actuated fundamentally by the MDR-1 and can be switched by RNA interference (RNAi) [Citation36]. Inspite of the fact that P-gp-related medication resistance might be overcome exclusively by endocytic conveyance utilizing nanomaterials, siRNA against MDR-1 in mix with therapeutics indicates improved adequacy over nanodesgined medications, alone [Citation37]. Multifunctional mesoporous silica NPs stacked with DOX and siRNA against P-gp sedate efflux draw showed improved capacity to defeat medicate resistance in bosom malignancy models in vitro and in vivo.
For battling drug resistance insignificant to efflux pumps, RNAi against cell survival pathways, translation variables, and hostile to apoptotic proteins can be used. For instance, myeloid cell leukemia-1 (Mcl-1) and B-cell lymphoma-2 (Bcl-2) proteins, which are frequently, over-expressed in numerous malignancies, meddle with apoptosis and instigate medicate resistance, making them promising focuses of RNAi. TP53, the p53 tumor silencer quality, is regularly transformed into different diseases, and rebuilding its capacity advances antitumor impacts [Citation38].
Utilization of nanocarriers as synergistic approach in combination with conventional anticancer methods
Photo-thermally activated nanomaterials
Induction of hyperthermia is one of the effective tumor eradication methods whereby cancer cells are killed due to induction of high temperature [Citation39]. However, the clinical significance of conventional hyperthermia has been limited on account of its non-specific, non-uniform and invasive nature, thereby, requiring sophisticated expertise for effective treatment. For minimizing the adverse effects associated with photo-thermally active NPs, several advanced nanomedicines have been fabricated bearing enhanced absorptive cross-section for the conversion of externally directed energy (such as light, magnetic field, ultrasound) into thermal energy primed for inducing uniformly distributed hyperthermia [Citation40,Citation41]. Specifically, NIR irradiation-triggered hyperthermic carbon and gold nano-based materials have been extensively studied for their role in photothermal therapy. Induction of hyperthermia can cause a controlled and sustained release of drugs from the surface or interior of NPs. In addition, nan-based carriers can effectively penetrate through the tumor endothelium, followed by passive accumulation inside tumor mass on account of the leaky vasculature. The combined use of chemo and phototherapy using nanomaterials has proved to be a promising approach for effective cancer treatment [Citation40,Citation41].
Gold based nanocompounds including gold nanocages (AuNCs), nanorods (AuNRs), and nanoshells (AuNSs) have demonstrated specialized photothermal and optical properties dependent upon their morphological characteristics; mediated by NIR region governed localized SPR (surface plasmon resonance) exhibiting substantial biocompatibility [Citation42]. Gold nanoshells (AUNSs), comprising of an external gold shell and internal mesoporous silica nanocore, were explored for their ability to administer dual chemotherapy and photothermal effects through eradication of hepatic carcinomas in both in-vitro and in-vivo [Citation43]. DTX loaded with PEGylated gold NSs on mesoporous silica nano-spheres (pGSNs) were able to undergo 60% drug release within seven days. Additionally, transferin-based pGSNs exhibited efficient cancer-specific targeting. DOX-encapsulated co-polymeric PLGA-PEG micelles coated by Au shell (DOX-PLGA-PEG AuNSs) [Citation43], characterized by SPR absorption at a wavelength of 790 nm, and enhanced the intra-tumor temperature up to 60–70 °C following NIR-based radiation, causing rapid release of DOX. The treatment was followed by effective tumor elimination without any signs of recurrence of weight loss. Similarly, DOX-encapsulated, PEG-derived hollow AuNPs exhibited pH-triggered, NIR-stimulated drug release leading to enhanced anticancer efficacy with reduced systemic adverse effects in contrast to free DOX [Citation44]. Results showed that NP3-plus NIR laser displayed significantly higher suppression in tumor volume compared to other treatment groups on day 24 (). In fact, the tumors in three of the six mice treated with NP3-plus-NIR laser were reduced to scar tissue by 20 days after the initiation of treatment () [Citation44].
Figure 4: Antitumor activity in vivo against MDA-MB-231 tumor. (A). MDA-MB-231 tumor growth in mice treated with saline, free DOX, liposomal DOX, NP3, and NP3-plus-NIR laser irradiation. Arrows indicate dates of injection of each dose of 15 mg equivalent DOX/kg. In the free DOX group, the mice were injected only once on day 0. In the NP3-plus-laser group, tumor irradiation (1.5 W/cm2 for 5 min) was commenced 24 h after nanoparticles injection. (B). Photographs of MDA-MB-231 tumors after treatment with NP3-plus-laser. (C). Percentage of mean body weight change. For (A) and (C), the data are presented as mean ± standard deviation (n = 5–7) [Citation44]. Reprinted with permission from Elsevier B.V. (Copyright © 2011) through Copyright Clearance Center.
![Figure 4: Antitumor activity in vivo against MDA-MB-231 tumor. (A). MDA-MB-231 tumor growth in mice treated with saline, free DOX, liposomal DOX, NP3, and NP3-plus-NIR laser irradiation. Arrows indicate dates of injection of each dose of 15 mg equivalent DOX/kg. In the free DOX group, the mice were injected only once on day 0. In the NP3-plus-laser group, tumor irradiation (1.5 W/cm2 for 5 min) was commenced 24 h after nanoparticles injection. (B). Photographs of MDA-MB-231 tumors after treatment with NP3-plus-laser. (C). Percentage of mean body weight change. For (A) and (C), the data are presented as mean ± standard deviation (n = 5–7) [Citation44]. Reprinted with permission from Elsevier B.V. (Copyright © 2011) through Copyright Clearance Center.](/cms/asset/4044582f-e027-43cd-97fe-cf6aab70bd73/ianb_a_1478420_f0004_c.jpg)
Lipophilically modified hollow AuNPs tethered onto the surface of DOX encapsulating liposomes substantially enhanced the drug release through liposomal phase transition (LPT) and further enhanced the permeation tendency via NIR radiation exposure [Citation45]; causing marked increase in the anti-cancer efficacy in comparison to free DOX/liposomes, in in-vivo as well as in-vitro experimental models. Blockage of pores in hollow AuNCs via calcium phosphate-based metallic NPs (CaP-Fe3O4) conjugation, resulted in the prevention of premature drug release prior to complete endosomal transfer [Citation46]. The release of DOX from AuNCs was accelerated as a result of reduced fluidic viscosity following NIR radiation and demonstrated enhanced antitumor efficiency in contrast to the combined anticancer effects mediated by DOX-encapsulated AuNCs and free AuNCs following NIR treatment. The AuNCs bound Fe3O4 NPs exhibited promising targeted drug delivery and MRI and attributes [Citation46].
Carbon-based nanocompounds, specifically graphenes and nanotubes (CNTs), have been reported as potential NIR-absorbent which can be employed as dual chemotherapeutic and photothermal agents [Citation47]. Their enhanced surface area permits for high drug loading capacity through π–π stacking and covalent bonding. Biocompatible carbon-based NPs have been observed to get eliminated efficiently from the body following IV administration in animals without exhibiting substantial toxicity [Citation47].
Folic acid (FA) and polyethylenimine (PEI) functionalized carbon nanocompounds and have also exhibited profound NIR absorbing capacity with enhanced photothermal conversion efficacy on account of the graphitic morphology of the carbon NPs [Citation48]. In contrast to photothermal treatment or chemotherapy alone, DOX-encapsulated PEI-FA-CNPs exhibited substantial combined therapeutic activity in HeLa cervical cancer cells with over-expressed FAR receptors. The synergistic chemotherapeutic and photothermal activity employing DOX-conjugated PEG-linked nano-graphene oxide (NGO) showed combined therapeutic activity with minimal adverse effects and organ toxicity [Citation49].
Photo-dynamically activated nanomaterials
In recent decades, photodynamic therapy (PDT) has stepped up as an effective and non-invasive methodology for the eradication of cancer in clinical setups, whilst incurring minimal side effects [Citation50]. Following light-induced activation at an appropriate wavelength, the photosensitizers produce reactive single oxygen species via transfer of energy unto the neutral molecular oxygen, eliciting cellular death and cancerous tissue eradication. In contrast to chemotherapy alone, the synergistic effect of chemotherapy and PDT can administer optimal therapeutic effect. Thus, earnest endeavors are underway for engineering of relevant nanomaterials for compound delivery of anticancer agents and photosensitizers.
Hematoporphyrin (HP)-based, bovine serum albumin NPs (HP-BSA-NPs) conjugated with DOX were fabricated for chemo-photodynamic treatment against liver cancer [Citation51]. HP acted as a targeting ligand, directed against low-density lipoprotein (LDL) receptors over-expressed on the surface of hepatoma cells, in addition to its unique photosensitizing ability. Results from in vitro and in vivo tests demonstrated the substantial enhancement in the antitumor activity of HP-BSA-NPs directed against HepG2 hepatocellular carcinoma cell line, as revealed via changes in the instances of PDT sessions and irradiation timing [Citation51].
PTX was encapsulated into the micelle-based nanocarriers comprising of amphiphilic star-shaped, 4-sided di-block co-polymers of PCL and mPEG bearing a chlorin-based core region [Citation52]. Consequently, the prepared micelles underwent efficient PTX release within the acidic environment and enhanced the MCF-7 directed cytotoxicity in a synergistic fashion following irradiation. Likewise, chlorin-based micelles carrying the antitumor drug SN-38, elicited enhanced accumulation with the tumor as a result of prolonged residing duration within the plasma, in contrast to un-conjugated camptothecin, and demonstrated synergistic inhibition of tumor proliferation in a colorectal cancer xenograft mouse model following light-based irradiation [Citation53]. A significant reduction in tumor size was observed following administration of three doses of micelles, in addition to reduced cancer cell proliferation and microvascular density.
Synergistic radiotherapy via nanocarriers
Radiotherapy has since long been in clinical use as a conventional means of cancer therapy, employing high-frequency radiations for the eradication of tumors. Though successful in its cause, the associated side effects such as cardiac and pulmonary tissue damage, infertility and secondary cancer development due to radiation-based mutations incurred in the normal cells, undermine its optimal use. In addition, tumor cells hold higher resistance against radio-treatment on account of the intra-tumor environment’s hypoxic nature. A promising method for overcoming the said challenge is the designing of nanomedicines for combining chemotherapy with radiation-sensitizers to enhance the efficiency of radiotherapy [Citation54,Citation55].
Nano-vehicles may be used as adjuvant radio-sensitizers combined with ablation therapy. For instance, HAuNPs able to generate SPR effects in case of photothermal ablation may be conveniently conjugated with oncotherapeutics, with the AuNPs serving as potential radio-sensitizers [Citation54]. CDDP-conjugated AuNPs underwent rapid internalization within brain glioblastoma cancer cells followed by induction of caspase-based cellular apoptosis in-vitro. Pt from CDDP and Au both tend to produce ionizing Auger and photoelectrons upon irradiation, causing the generation of ROS via hydrolysis of intracellular water. The cytotoxic ROS produced is able to substantially inhibit the proliferation and growth of tumor cells. The combined therapy effect decreased the population of cells by approximately a hundred thousand folds in contrast to untreated cells, and prevented further regeneration of cancer cells [Citation54].
Magnetic nanomaterials have been utilized in combinational treatments for cancer imaging, ablation, and sustained release. The superparamagnetic iron oxide NPs (SPIONs) combined with LAMP-1 lysosomal protein-targeting antibodies caused cellular apoptosis following rotation activation via magnetic field exposure [Citation55]. Results showed that the NPs rapidly localized alongside the lysosomal membrane on account of the conjugated antibodies in human pancreatic beta cells and mouse insulinoma cancer cells. The amplified torque ensued membrane tearing, led by enhanced expression of apoptotic biomarkers, and diminished cellular growth. As the rotation of NPs is manipulated for solely affecting the penetrated tumor cells, this procedure holds superior against preceding attempts which employ magnetic field-induced heat generation for cancer ablation, resulting in possible inflammation of healthy cells and tissues [Citation55].
Conclusions
Advanced insight into the molecular framework governing cancer and tumor biology have aided in the engineering of novel therapeutic regimens employing combo-delivery of anticancer agents. Monotherapy employing single therapeutic agents scarcely meet up with the dynamic, heterogenetic and mutating cancer nature as well as its ability to develop drug resistance against specific anticancer therapy. A major issue pertaining to the use of monotherapy in cancer treatment is the achievement of two prime, but challenging goals: interaction with a particular targeted pathway and lack of multidimensional mechanistic approach against several pathogenic pathways. Consequently, combinatorial treatments with the aid of nanodelivery systems carrying multi-therapeutic moieties simultaneously for the generation of synergistic impacts is a coherent and promising way of battling cancer. These combo nanoapproaches have shown efficient, promising, safe therapies in light of the dynamically mutating nature of cancer as well as associated drug resistance.
Expert opinion and future prospects
This article overviews a thorough critical appraisal of recent developments in the synthesis, characterization and anticancer efficacy of cancer therapeutics, as well as the new advancements in drug delivery strategies of cancer thermostatic. This new approach of simultaneous delivery of cancer therapeutics opened new horizons for designing improved, efficient and rational nanotechnology-based therapies against a dynamic, stringent microenvironment of cancer. According to various search engines (SciFinder, Google, Science Direct, Willey online Library), 319 references have been identified for the pharmaceutical and clinical superiority of nanotherapeutics in the treatment various types of cancers. These studies highlighted different types of nanomedicines designed by the inclusion of polymeric nanoparticles, metal nanoparticles, liposomes, micelles and nanoemulsion. Despite of the immense promise linked with combinational therapies, it is of utmost importance to guarantee that therapies are corresponding and synergistic. Comprehensive examination of affected cancer pathways, substitutive mechanisms, feedback loops, and gene profiles ought to be taken into consideration before combining the therapeutic agents. Merely combining drugs on account of their tendency to induce cellular death will also enhance the general systemic toxicity and induce novel resistances as the evolution of cancer cells takes place. Utilizing nanotechnology in co-conveyance of various remedial agents offers substantial advantages, for instance normalized pharmacokinetic and pharmacodynamics properties, controlled bioavailability, target-specific accumulation and sustained release of drug. Nano-vehicles possess unique functional attributes which mere free drugs fail to possess, for example target-specific cellular binding, imaging signals production, and reaction to foreign triggers. It is also imperative to consider the potential side and adverse effects whilst preparing combinational therapies. Some nano-compounds have been shown to exhibit oxidative stress, undesired inflammation, as well as geno-toxicity. In-depth assessment is essential for determining whether potential benefits overweigh the possible adverse effects. Additionally, the combinational nano-based therapy ought not to require high levels of intricacy in formulation and characterization. A transition from laboratory-based research to the clinical implementation is challenging and the cost/benefit analysis needs to be critically chalked out. The combinational nanomedicines have allowed for the combined use of immunotherapy, gene therapy, chemotherapy, photodynamic therapy and photo-thermal therapy, which may present as the solution for overcoming multiple types of resistances in cancers. Thus, it is of prime significance that shared endeavors are carried out for fabricating such nano-based treatments by consolidating the diverse fields of nanotechnology, cancer biology and pharmaceutical drug development.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Krishnan V, Rajasekaran AK. Clinical nanomedicine: a solution to the chemotherapy conundrum in pediatric leukemia therapy. Clin Pharmacol Ther. 2014;95:168–178.
- Morton SW, Lee MJ, Deng ZJ, et al. A nanoparticle-based combination chemotherapy delivery system for enhanced tumor killing by dynamic rewiring of signaling pathways. Sci Signal. 2014;7:ra44.
- Burrell RA, McGranahan N, Bartek J, et al. The causes and consequences of genetic heterogeneity in cancer evolution. Nature. 2013;501:338–345.
- Griner LAM, Guha R, Shinn P, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci USA. 2014;111:2349–2354.
- Berns K, Bernards R. Understanding resistance to targeted cancer drugs through loss of function genetic screens. Drug Resist Updat. 2012;15:268–275.
- Gao Z, Zhang L, Sun Y. Nanotechnology applied to overcome tumor drug resistance. J Control Release. 2012;162:45–55.
- Schroeder A, Heller DA, Winslow MM, et al. Treating metastatic cancer with nanotechnology. Nat Rev Cancer. 2012;12:39–50.
- Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73:2412–2417.
- Peiris PM, Bauer L, Toy R, et al. Enhanced delivery of chemotherapy to tumors using a multicomponent nanochain with radio-frequency-tunable drug release. ACS Nano. 2012;6:4157–4168.
- Zhang Z, Wang L, Wang J, et al. Mesoporous silica-coated gold nanorods as a light-mediated multifunctional theranostic platform for cancer treatment. Adv Mater. 2012;24:1418–1423.
- Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–692.
- Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–354.
- Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–316.
- Yang L, Moss T, Mangala LS, et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol. Syst. Biol. 2014;10:728.
- Cook KL, Wärri A, Soto-Pantoja DR, et al. Hydroxychloroquine inhibits autophagy to potentiate antiestrogen responsiveness in ER + breast cancer. Clin Cancer Res. 2014;20:3222–3232.
- Cristóbal I, Rincón R, Manso R, et al. Deregulation of the PP2A inhibitor SET shows promising therapeutic implications and determines poor clinical outcome in patients with metastatic colorectal cancer. Clin Cancer Res. 2015;21:347–356.
- Robert NJ, Diéras V, Glaspy J, et al. RIBBON-1: randomized, double-blind, placebo-controlled, phase III trial of chemotherapy with or without bevacizumab for first-line treatment of human epidermal growth factor receptor 2-negative, locally recurrent or metastatic breast cancer. J Clin Oncol. 2011;29:1252–1260.
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 study. Jco. 2012;30:2585–2592.
- Herbst RS, Sun Y, Eberhardt WEE, et al. Vandetanib plus docetaxel versus docetaxel as second-line treatment for patients with advanced non-small-cell lung cancer (ZODIAC): a double-blind, randomised, phase 3 trial. Lancet Oncol. 2010;11:619–626.
- Stupp R, Hegi ME, Gorlia T, et al. European Organisation for Research and Treatment of Cancer (EORTC); and CENTRIC study team, Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylatedMGMT promoter (CENTRIC EORTC 26071-22072 study): amulticentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1100–1108.
- Ortac I, Simberg D, Yeh YS, et al. Dual-porosity hollow nanoparticles for the immunoprotection and delivery of nonhuman enzymes. Nano Lett. 2014;14:3023–3032.
- Chu KS, Schorzman AN, Finniss MC, et al. Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials. 2013;34:8424–8429.
- Soppimath KS, Aminabhavi TM, Kulkarni AR, et al. Biodegradable polymeric nanoparticles as drug delivery devices. J Control Release. 2001;70:1–20.
- Palvai S, More P, Mapara N, et al. Chimeric nanoparticle: a platform for simultaneous targeting of phosphatidylinositol-3-kinase signaling and damaging DNA in cancer cells. ACS Appl Mater Interfaces. 2015;7:18327–18335.
- Poon C, He C, Liu D, et al. Self-assembled nanoscale coordination polymers carrying oxaliplatin and gemcitabine for synergistic combination therapy of pancreatic cancer. J Control Release. 2015;201:90–99.
- McRae Page S, Henchey E, Chen X, et al. Efficacy of polyMPC-DOX prodrugs in 4T1 tumor-bearing mice. Mol Pharm. 2014;11:1715–1720.
- Kwon HJ, Byeon Y, Jeon HN, et al. Gold cluster-labeled thermosensitive liposmes enhance triggered drug release in the tumor microenvironment by a photothermal effect. J Control Release. 2015;216:132–139.
- Sun W, Jiang T, Lu Y, et al. Cocoon-Like self-degradable DNA nanoclew for anticancer drug Delivery. J Am Chem Soc. 2014;136:14722–14725.
- van Rijt SH, Bölükbas DA, Argyo C, et al. Protease-mediated release of chemotherapeutics from mesoporous silica nanoparticles to ex vivo human and mouse lung tumors. ACS Nano. 2015;9:2377–2389.
- Zalba S, Contreras AM, Haeri A, et al. Cetuximab–oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J. Control. Release. 2015;210:26–38.
- Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer–drug conjugates as anticancer nanomedicines. Adv. Drug Deliv. Rev. 2009;61:1203–1213.
- NarayananMony SU, Vijaykumar D, Koyakutty K, Paul-Prasanth M, Menon BD. Sequential release of epigallocatechin gallate and paclitaxel from PLGA-casein core/shell nanoparticles sensitizes drug-resistant breast cancer cells. Nanomed. 2015;11(6):1399–1406.
- Tai W, Mo R, Lu Y, et al. Folding graft copolymer with pendant drug segments for co-delivery of anticancer drugs. Biomaterials. 2014;35:7194–7203.
- Yamamoto Y, Lin PJ, Beraldi E, et al. siRNA lipid nanoparticle potently silence clusterin and delay progression when combined with androgen receptor co-targeting in enzalutamide resistant prostate cancer. Clin Cancer Res. 2015;21:4845–4855.
- Zheng C, Zheng M, Gong P, et al. Polypeptide cationic micelles mediated co-delivery of docetaxel and siRNA for synergistic tumor therapy. Biomaterials. 2013;34:3431–3438.
- Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515–1519.
- Meng H, Mai WX, Zhang H, et al. Codelivery of an optimal drug/siRNA combination using mesoporous silica nanoparticles to overcome drug resistance in breast cancer in vitro and in vivo. ACS Nano. 2013;7:994–1005.
- Wang W, El-Deiry WS. Restoration of p53 to limit tumor growth. Curr Opin Oncol. 2008;20:90–96.
- May JP, Li SD. Hyperthermia-induced drug targeting. Expert Opin Drug Deliv. 2013;10:511–527.
- Yi X, Yang K, Liang C, et al. Imaging-guided combined photothermal and radiotherapy to treat subcutaneous and metastatic tumors using iodine-131-doped copper sulfide nanoparticles. Adv Funct Mater. 2015;25:4689–4699.
- Park J, Park J, Ju EJ, et al. Multifunctional hollow gold nanoparticles designed for triple combination therapy and CT imaging. J Control Release. 2015;207:77–85.
- Cobley CM, Chen J, Cho EC, et al. Gold nanostructures: a class of multifunctional materials for biomedical applications. Chem Soc Rev. 2011;40:44–56.
- Lee SM, Park H, Yoo KH. Synergistic cancer therapeutic effects of locally delivered drug and heat using multifunctional nanoparticles. Adv Mater. 2010;22:4049–4053.
- You J, Zhang R, Zhang G, et al. Photothermal–chemotherapy with doxorubicin-loaded hollow gold nanospheres: a platform for near-infrared light-trigged drug release. J Control Release. 2012;158:319–328.
- You J, Zhang P, Hu F, et al. Near-infrared light-sensitive liposomes for the enhanced photothermal tumor treatment by the combination with chemotherapy. Pharm Res. 2014;31:554–565.
- Shi P, Qu K, Wang J, et al. pH-responsive NIR enhanced drug release from gold nanocages possesses high potency against cancer cells. Chem Commun (Camb). 2012;48:7640–7642.
- Gong H, Peng R, Liu Z. Carbon nanotubes for biomedical imaging: the recent advances. Adv Drug Deliv Rev. 2013;65:1951–1963.
- Xu G, Liu S, Niu H, et al. Functionalized mesoporous carbon nanoparticles for targeted chemo-photothermal therapy of cancer cells under near-infrared irradiation. RSC Adv. 2014;4:33986–33997.
- Zhang W, Guo Z, Huang D, et al. Synergistic effect of chemophotothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32:8555–8561.
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380–387.
- Chang JE, Yoon IS, Sun PL, et al. Anticancer efficacy of photodynamic therapy with hematoporphyrin-modified, doxorubicin-loaded nanoparticles in liver cancer. J Photochem Photobiol B Biol. 2014;140:49–56.
- Peng CL, Shieh MJ, Tsai MH, et al. Self-assembled star-shaped chlorin-core poly(ɛ-caprolactone)-poly(ethylene glycol) diblock copolymer micelles for dual chemo-photodynamic therapies. Biomaterials. 2008;29:3599–3608.
- Peng CL, Lai PS, Lin FH, et al. Dual chemotherapy and photodynamic therapy in an HT-29 human colon cancer xenograft model using SN-38- loaded chlorin-core star block copolymer micelles. Biomaterials. 2009;30:3614–3625.
- Setua S, Ouberai M, Piccirillo SG, et al. Cisplatin-tethered gold nanospheres for multimodal chemo-radiotherapy of glioblastoma. Nanoscale 2014;6:10865–10873.
- Di Corato R, Béalle G, Kolosnjaj-Tabi J, et al. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano. 2015;9:2904–2916.