Abstract
Background: The human cervical carcinoma oncogenic mechanisms still remain elusive. Thus, we proposed to understand the biological role of a newly discovered therapeutic miRNA.
Methods: MiR-1297 related to human cervical carcinoma was selected for this study. TaqMan qRT- PCR assay was used to profile miRNA, phosphatase and tensin homolog (PTEN) expression in randomly chosen tumour with non-tumour tissues, and the apoptosis factors expression. Cell proliferation was monitored by CCK-8 assay and colony formation assay. Apoptosis was determined by flow cytometry. Protein level was determined by western blotting. 3’UTR was performed to validate the direct binding sites of miR-1297 on PTEN. SPSS was used for statistical analyses.
Results: MiR-1297 is repressed and PTEN activated in human cervical cancer tissues. After miR-1297 overexpression, HeLa cells had an increase in cell proliferation and decrease in apoptosis. PTEN expression is negatively correlation with miR-1297. PTEN silencing display the similar pattern as miRNA-1297 overexpression to inhibit HeLa cell growth and apoptosis in vitro.
Conclusions: Our data indicate that miR-1297 contribute to the human cervical carcinoma through PTEN. miR-1297 could be a reasonable miRNA for future studies.
Keywords:
Introduction
Human cervical cancer is the 4th leading cause of cancer death in women worldwide. It is also one of the main causes of cancer-related death in the developing countries [Citation1]. It remains the second malignancy in women [Citation2]. Despite extensive basic as well as clinical research efforts, very little is known regarding the molecular mechanisms in the cervical cancer. Therefore, it is important to disclose the molecular mechanisms responsible for the cervical cancer for the prognosis, diagnosis and treatment.
MicroRNAs (miRNAs) are a class of evolutionary conserved endogenous non-coding small RNAs that regulates gene expression at the post-transcriptional level by binding to specific complementary sites at the 3’UTR of target mRNAs [Citation3]. Abnormal expression of miRNAs has been shown to be associated with cell proliferation, differentiation and apoptosis in multiple type of cancers [Citation4]. Currently, the small size and low molecular weight of miRNAs made them as attractive options for therapeutic targets for cancer molecular therapy. In the past decade, hundreds of miRNAs have been well studied including miR-21, miR-26b and miR-let-7 family. More new miRNAs have been found in recent studies, including miR-1297. However, the biological roles of miR-1297 and its underlying regulatory network have still poorly understood in the human cervical carcinoma.
Phosphatase and tensin homolog (PTEN) is a well-known tumour suppressor gene containing a tensin-like domain and a catalytic domain. The dysfunction of PTEN have been found to contribute to tumour genesis, metastasis, proliferation and apoptosis [Citation5]. Recently, PTEN has been observed to involved in the cell progression in Laryngeal squamous cell carcinoma (LSCC) by direct miR-1297 regulation [Citation6]. In the A549 cells, PTEN inhibits cell proliferation, promotes cell apoptosis by downregulating the PI3K/AKT/hTERT pathway [Citation7]. However, the correlation of miR-1297 and PTEN has not been disclosed in the human cervical carcinoma.
In this study, we identified the expression of miR-1297 in human cervical carcinoma, as well as investigated the effects of miR-1297 on HeLa cell growth. We also discussed that miR-1297 contributed to the HeLa cell proliferation and apoptosis through regulating PTEN. Our results indicate that miR-1297 may be a new therapeutic target in human cervical carcinoma and add another layer of complexity in miRNA regulation.
Materials and methods
Cell culture
The human cervical carcinoma cell line, HeLa, is commercially available at American Type Cell Collection (Manassas, VA). The cells were cultured in RPMI 1640 medium (Gibco) supplemented with 10% foetal bovine serum (FBS) (Atlanta Biologicals, Atlanta, GA), penicillin and streptomycin (100 U/mL, and 100 μg/mL) (Sigma-Aldrich, St Louis, MO). The cells were cultured in the humidified chamber with 5% CO2 at 37 °C.
RNA isolation and quantitative real-time PCR (qRT-PCR)
The total RNA was extracted from cells and human tissues by TRI (Molecular Research Center, Cincinnati, OH). RNA quality and quantity were determined by Nanodrop 1000 (Nanodrop; Thermos Fisher Scientific, Waltham, MA) by spectrophotometric analysis. After removal of residual DNA, the complement DNA (cDNA) was synthesized by Moloney murine leukemia virus reverse transcriptase (Invitrogen, Carlsbad, CA) for gene expression Taqman qRT-PCR. miRNA expression quantification was performed by the Taqman advanced miRNA cDNA synthesis kit (Applied Biosystems). The miR-1297 expression was normalized to the U6 snRNA internal reference. The U6 cDNA was generated by a TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems). Quantitative real-time PCR (qRT- PCR) was performed in triplicate using the ABI 7500 system (Applied Biosystems, Foster City, CA). All the gene and miRNA Taqman qRT-PCR specific probes are commercial available from Applied Biosystems [Citation8].
MiR-1297 overexpression
Overexpression of miR-1297 was achieved by the lentiviral system in vitro (Applied Biological Materials Inc, Richmond, BC) according to the manufacturer’s instruction [Citation9]. The empty lentiviral vector (Lenti-NC) was used as vector control.
3’ UTR luciferase assay
HeLa cells were seeded in a 96-well plate (2 × 104 cells per well). The cells were cotransfected with 0.3 μg firefly luciferase reporter plasmid, 0.15 μg β-galactosidase expression vector (VC) (Ambion, Austin, TX) and same amounts of miRNA vector control (miR-Ctrl) or miR-1297 mimic by lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions [Citation10]. The cells were analysed with luciferase assay kit (Promega) after 48h. β-galactosidase expression vector was used as a transfection control. The miR-1297 and miR-Ctrl was commercially available from life technologies (Carlsbad, CA).
PTEN silencing
Silencing of PTEN was achieved by transfecting cells with si-PTEN with lipofectamine 2000 (Life technologies, Carlsbad, CA). The matched negative control vector (si-Ctrl) was also commercially available from System Biosciences (Mountain View, CA) [Citation11].
Western blotting
The proteins were harvested after the cells were lysed with RIPA lysis buffer. 15 μg of total protein extracts were separated on 4–15% precast gels (Bio-Rad, Richmond, CA) and transferred to nitrocellulose membranes (Bio-Rad) following 5% non-fat dry milk blocking for 1 h in room temperature. membranes were incubated with PTEN primary antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. After being washed with TBST for 3 times, the membranes were incubated with the secondary antibody (Bio-Rad) for 1h. Band signals were visualized by the enhanced chemiluminescence kit (Pierce, Minneapolis, MN). β-actin (Santa Cruz, CA) was used as internal control [Citation12].
Quantification of cell viability
The cell viability was qualified with cell counting kit-8 (CCK-8) according to the manufacture's protocol (Promega, Madison, WI). HeLa cells were seeded in 96-well plates in 100 μl medium at the density of 5000 per well. Cells overexpressed with miR-1297 or miR-NC were cultured with 100 μl culture medium and CCK-8 dye was added at indicated time points [Citation13].
Colony formation assay
HeLa cells with miR-1297 or miR-NC expression were seeded in the 6-well plates (1000 cells per well). After 2 weeks culture, clones were fixed with methanol and stained with 2% Giemsa solution (Merck, Darmstadt, German) for 10 min [Citation14].
Apoptosis by flow cytometry
The cell viability of the untreated HeLa cells was compared against their treated miR-1297 overexpression or PTEN loss and correlated control counterparts. The cells (0.5M/condition) were harvest after trypsin treatment, and apoptotic or dead cells were detected by the annexin V-FITC kit according to the manufacturer’s protocol (BD Biosciences) during flow cytometry analysis [Citation15].
Statistics
SPSS software (version 16.0.1, SPSS Inc., Chicago, IL) was performed for the statistical analyses. Data are expressed as mean ± standard deviation (n = 3). Unpaired Student t-test was used to compare the difference in different treatment. p (probability) less than 0.05 was considered as significant difference.
Results
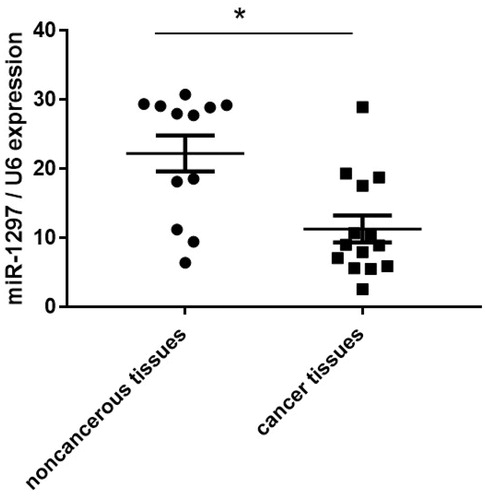
MiR-1297 is down-regulated in tumour tissues
We first determined the expression patterns of miR-1297 in human cervical carcinoma tissues. MiR-1297 expression was observed to be significantly down-regulated with about 2-fold in the cancer tissues compared with non-cancerous tissues (. We randomly used 15 cervical cancer tissues and 12 non-cancerous tissues.
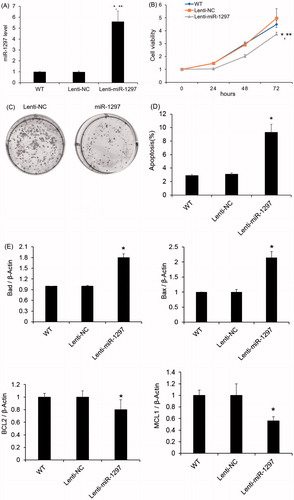
miR-1297 overexpression inhibits HeLa cell proliferation and promotes apoptosis
To decipher the biological role of miR-96 in the progression of cervical carcinoma, we next overexpressed has-miR-1297 in the HeLa cells to determine the effect on cellular proliferation. HeLa cells produced high level of miR-1297 after transfection with lentiviral vector containing miR-1297 (). The miR-1297 overexpressing cells were observed to inhibit the cell proliferation rate versus the Lenti-NC overexpressing and non-treated cells (). In addition, compared with the Lenti-NC transfected cells, HeLa cells transfected with miR-1297 formed less colonies (). Furthermore, Hela cells overexpressing has-miR-1297 exhibits significant higher apoptosis amount compared to the parental cells (p < .05) (). The mRNA levels of anti-apoptotic factors (BCL2 and MCL1) increased while the pro-apoptotic factors (BAD and BAX) decreased in Hela cells overexpressing has-miR-1297 ().
Figure 2. Effects of miR-1297 on HeLa cell proliferation. HeLa cells were transfected with lentiviral vector control (lenti-NC) and miR-1297. (A) The expression level of miR-1297 in HeLa cells after miR-1297 overexpression; (B) CCK-8 cell proliferation assay was used to monitor the cell proliferation of HeLa cells or HeLa cells expressing either Lenti-NC or miR-1297; (C) Representative colony formation assays of HeLa cells expressing either Lenti-NC or miR-1297. Data represent the mean ± SE (n = 3). *p < .05, versus WT; **p < .05, versus Lenti-NC. (D) Effect of has-miR-1297 overexpression on HeLa cell apoptosis as measured by Annexin V flow cytometry. The cell viability was presented as mean ± SE (n = 3). *p < .05 versus WT; (E) mRNA expression analysis of pro-apoptotic (Bad and Bax) or anti-apoptotic (Bcl-2 and Mcl1) factors by taqman qRT-PCR in the HeLa cells with or without has-miR-1297 overexpression. β-Actin was used as an internal control. The results were presented as mean ± SE (n = 3). *p < .05 versus WT.
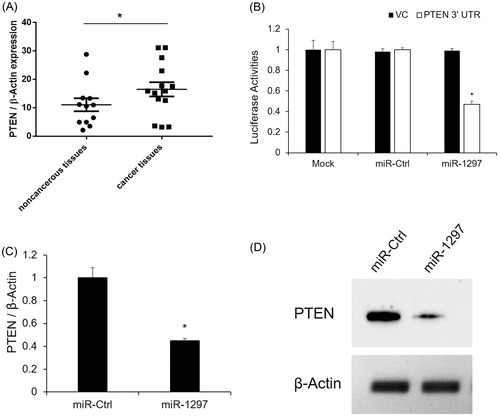
PTEN is up-regulated in tumour tissues and suppressed by miR-1297
To further explore the molecular mechanisms involved in the miR-1297 mediated cell proliferation, we predicted the target gene of miR-96 using the miRbase Target [Citation1]. Among these potential regulatory genes, PTEN has been well known for its regulatory function in cancer progression [Citation16]. In this study, we found PTEN was up-regulated in the tumour tissues than non-tumor tissues by qRT- PCR (). Subsequently, we verified whether PTEN is a direct target of miR-1297 by 3’UTR luciferase assay. As seen in , miR-1297 significantly decreased the luciferase activity of PTEN 3’ UTR as compared to the untransfected cells (Mock). MiR-Ctrl also had no effect on the luciferase activity of PTEN 3’ UTR (). Next, we investigated the mRNA and protein levels of PTEN after miR-1297 overexpression. Both the mRNA and protein levels of PTEN were significantly decreased after miR-1297 overexpression (). These results suggest that PTEN is repressed by miR-1297 and involved in the tumour progression as well.
Figure 3. PTEN expression is negatively correlated with miR-1297 (A) PTEN mRNA expression level was determined by qRT-PCR in human cervical carcinoma tissues. Data represents the mean ± SE, *p < .05, versus noncancerous-tumour tissues; (B) PTEN is validated as the direct target of miR-1297 by 3’ UTR luciferase assay; (C) PTEN mRNA expression with HeLa cells after overexpression of miR-NC or miR-1297. Results are mean ± SE. *p < .05 versus WT; **p < .05 versus miR-Ctrl; (C) PTEN protein level was determined by western blotting. β-actin was used as an internal control.
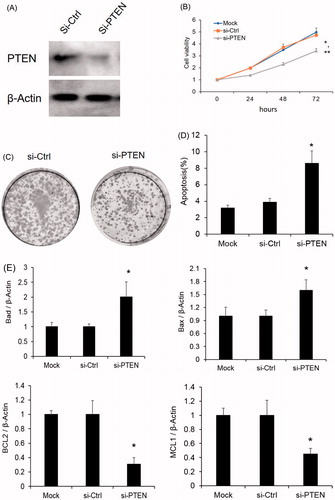
Down-regulation of PTEN represses HeLa cell proliferation and enhances apoptosis
To clarify the role of PTEN involved in the miR-1297 regulatory network, we silenced PTEN and monitored the cell proliferation. As shown in , si-PTEN dramatically reduced the PTEN protein expression in HeLa cells. After PTEN knockdown, HeLa cell proliferation was found to be increased which is similar to miR-1297 overexpression (). Additionally, the colony numbers were also increased after PTEN silencing (). At the same time, HeLa cells with PTEN silencing also exhibits significant higher apoptosis amount compared to the parental cells (p < .05) (). The mRNA levels of anti-apoptotic factors increased while the pro-apoptotic factors decreased in Hela cells with PTEN loss (). These data provide further evidence that miR-1297 could inhibit human cervical carcinoma progression through regulation of PTEN.
Figure 4. PTEN silencing effects the HeLa cell proliferation (A) PTEN protein expression level was determined by western blotting after PTEN knock-down. β-actin was used as an internal control. (B) Cell proliferation was monitored by CCK8 assay after PTEN silencing. *p < .05 versus Mock; **p < .05 versus si-Ctrl; (C) PTEN mRNA expression with HeLa cells after overexpression of miR-NC or miR-1297. Results are mean ± SE. *p < .05 versus WT; **p < .05 versus miR-Ctrl; (C) Representative colony formation assays of HeLa cells expressing either si-Ctrl or si-PTEN. (D) Effect of PTEN silencing on HeLa cell apoptosis as measured by Annexin V flow cytometry. The cell viability was presented as mean ± SE (n = 3). *p < .05 versus WT; (E) mRNA expression analysis of pro-apoptotic (Bad and Bax) or anti-apoptotic (Bcl-2 and Mcl1) factors by taqman qRT-PCR in the HeLa cells with or without PTEN silencing. β-Actin was used as an internal control. The results were presented as mean ± SE (n = 3). *p < .05 versus WT.
Discussion
Accumulating evidences showed that dysfunctions of miRNAs were frequently observed in multiple types of cancers [Citation17,Citation18]. Since the dysregulation of miRNAs is also commonly associated with the initial and developmental stages of human cancers, the correction of miRNA expression may emerge as a promising therapeutic strategy. In this study, miR-1297 expression was significantly down-regulated in the tumour tissues than non-tumour tissues, which is consistent with a previous observation [Citation19]. Subsequently, we found miR-1297 can suppress HeLa cell proliferation and promotes cell apoptosis. Additionally, miR-1297 down-regulation is negatively correlated with PTEN expression in tissue samples. Next, PTEN was found as a direct target of miR-1297. PTEN silencing suppresses HeLa cell proliferation and enhances apoptosis, which is similar to miR-1297 overexpression. Our results indicate that miR-1297 contributes to the cell proliferation via PTEN.
MiRNAs are evolutionary conserved non-coding small RNAs which have been found to regulate cancer phenotypes through targeting genes. MiR-1297 is one of the poorly understood miRNAs. The expression and functions of miR-1297 are cell and tissue specific. Recently, miR-1297 has been found to be found to promotes cell proliferation by inhibiting RB1 in liver cancer [Citation20]. In the non-small cell lung cancer cells, miR-1297 was also found to promote cell proliferation that involved in PTEN/Akt/Skp2 signalling pathway [Citation21]. In addition, miR-1297 contributed to the cell progression in Laryngeal squamous cell carcinoma (LSCC) through PTEN [Citation19]. MiR-1297 has also been reported to promote apoptosis and inhibit the proliferation and invasion of hepatocellular carcinoma cells by targeting HMGA2 (PMID: 26398017). In this study, we found that miR-1297 was significantly down-regulated in human cervical tumours compared with non-tumour tissues. Moreover, miR-1297 overexpression has been found to contribute to HeLa cell proliferation and apoptosis in vitro.
PTEN is a dual lipid and a protein phosphatase, as well as a tumour suppressor gene down-regulated in multiple types of cancers [Citation22–31]. The PTEN gene plays important roles in a wide range of advanced human malignancies, such as gastrointestinal stromal tumour, hepatoma, breast cancer, lung cancer, thyroid cancer, head and neck cancers, malignant melanoma, and lymphoma [Citation24–27,Citation29,Citation30]. Recently, PTEN was found to be down-regulated by miR-1297 in Hep-2 cells [Citation32]. In our study, we also found PTEN expression is negatively correlated with miR-1297 expression. Furthermore, PTEN has been reported to inhibit cell proliferation and induce apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells [Citation33]. In this study, we found PTEN silencing can suppress the HeLa cell proliferation and promote cell apoptosis, which exhibit the similar effects as miR-1297 overexpression. Our study adds a new layer to the complexity of miR-1297 functions in cancers.
Collectively, our study demonstrated that miR-1297 was down-regulated in the human cervical carcinoma tissues and the overexpression of miR-1297 inhibited HeLa cell proliferation and promoted cell apoptosis. Additionally, PTEN is negatively correlated with miR-1297 expression. Moreover, PTEN silencing can suppress the HeLa cell proliferation and promote cell apoptosis, which exhibit the similar effects as miR-1297 overexpression. The identified link of miR-1297/PTEN may be useful in the understanding of human cervical carcinoma progression and provide a new candidate for the therapeutic target for treating human cervical carcinoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Griffiths-Jones S. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–D144.
- Bosch FX, de Sanjose S. Chapter 1: human papillomavirus and cervical cancer–burden and assessment of causality. J Natl Cancer Inst Monogr. 2003;2003:3–13.
- Rana TM. Illuminating the silence: understanding the structure and function of small RNAs. Nat Rev Mol Cell Biol. 2007;8:23–36.
- El-Daly SM, Abba ML, Gamal-Eldeen AM. The role of microRNAs in photodynamic therapy of cancer. Eur J Med Chem. 2017;142:550–555.
- Lu X-X, Cao L-Y, Chen X, et al. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:1.
- Georgescu MM. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1:1170–1177.
- McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249–279.
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008.
- Li F, Xu JW, Wang L, et al. MicroRNA-221-3p is up-regulated and serves as a potential biomarker in pancreatic cancer. Artif Cells Nanomed Biotechnol. 2018;46:482–487.
- Wu L-P, Wu J, Shang A, et al. miR-124 inhibits progression of hepatocarcinoma by targeting KLF4 and promises a novel diagnostic marker. Artif Cells Nanomed Biotechnol. 2017. DOI: 10.1080/21691401.2017.1415918
- Liang L, Feng L, Wei B. microRNA-1297 involves in the progression of oral squamous cell carcinoma through PTEN. Saudi J Biol Sci. 2018. DOI:10.1016/j.sjbs.2018.01.013
- Fu H, Tan J, Yin Q. Effects of recombinant adeno-associated virus-mediated CD151 gene transfer on the expression of rat vascular endothelial growth factor in ischemic myocardium. Exp Ther Med. 2015;9:187–190.
- Le Pape F, Richard G, Porchet E, et al. Adhesion, proliferation and osteogenic differentiation of human MSCs cultured under perfusion with a marine oxygen carrier on an allogenic bone substitute. Artif Cells Nanomed Biotechnol. 2018;46:95–107.
- Ma C, Huang T, Ding YC, et al. microRNA-200c overexpression inhibits chemoresistance, invasion and colony formation of human pancreatic cancer stem cells. Int J Clin Exp Pathol. 2015;8:6533–6539.
- Cho H, Herzka T, Stahlhut C, et al. Rapid in vivo validation of candidate drivers derived from the PTEN-mutant prostate metastasis genome. Methods. 2015;77–78:197–204.
- Ortega-Molina A, Serrano M. PTEN in cancer, metabolism, and aging. Trends Endocrinol Metab. 2013;24:184–189.
- Hata A, Lieberman J. Dysregulation of microRNA biogenesis and gene silencing in cancer. Sci Signal. 2015;8:re3.
- Yates LA, Norbury CJ, Gilbert RJ. The long and short of microRNA. Cell. 2013;153:516–519.
- Chen P, Wang B-L, Pan B-S, et al. MiR-1297 regulates the growth, migration and invasion of colorectal cancer cells by targeting cyclo-oxygenase-2. Asian Pac J Cancer Prev. 2014;15:9185–9190.
- Liu C, Wang C, Wang J, et al. miR-1297 promotes cell proliferation by inhibiting RB1 in liver cancer. Oncol Lett. 2016;12:5177–5182.
- Bu W, Luo T. miR-1297 promotes cell proliferation of non-small cell lung cancer cells: involving in PTEN/Akt/Skp2 signaling pathway. DNA Cell Biol. 2017;36:976–982.
- Akca H, Demiray A, Aslan M, et al. Tumour suppressor PTEN enhanced enzyme activity of GPx, SOD and catalase by suppression of PI3K/AKT pathway in non-small cell lung cancer cell lines. J Enzyme Inhib Med Chem. 2013;28:539–544.
- Kong L, Schäfer G, Bu H, et al. Lamin A/C protein is overexpressed in tissue-invading prostate cancer and promotes prostate cancer cell growth, migration and invasion through the PI3K/AKT/PTEN pathway. Carcinogenesis. 2012;33:751–759.
- Levine RA, Forest T, Smith C. Tumor suppressor PTEN is mutated in canine osteosarcoma cell lines and tumors. Vet Pathol. 2002;39:372–378.
- Mehrian-Shai R, Chen CD, Shi T, et al. Insulin growth factor-binding protein 2 is a candidate biomarker for PTEN status and PI3K/Akt pathway activation in glioblastoma and prostate cancer. Proc Natl Acad Sci USA. 2007;104:5563–5568.
- Yang Y, Shao N, Luo G, et al. Mutations of PTEN gene in gliomas correlate to tumor differentiation and short-term survival rate. Anticancer Res. 2010;30:981–985.
- Zhang Y, Yu D, Li X, et al. Reduced expression of PTEN protein and its prognostic significance in the gastrointestinal stromal tumor. J Huazhong Univ Sci Technol [Med Sci]. 2010;30:165–169.
- Bian Y, Hall B, Sun Z-J, et al. Loss of TGF-beta signaling and PTEN promotes head and neck squamous cell carcinoma through cellular senescence evasion and cancer-related inflammation. Oncogene 2012;31:3322–3332.
- Ghosh-Choudhury T, Mandal CC, Woodruff K, et al. Fish oil targets PTEN to regulate NFkappaB for downregulation of anti-apoptotic genes in breast tumor growth. Breast Cancer Res Treat. 2009;118:213–228.
- Nagy R, Ganapathi S, Comeras I, et al. Frequency of germline PTEN mutations in differentiated thyroid cancer. Thyroid. 2011;21:505–510.
- Vinciguerra M, Carrozzino F, Peyrou M, et al. Unsaturated fatty acids promote hepatoma proliferation and progression through downregulation of the tumor suppressor PTEN. J Hepatol. 2009;50:1132–1141.
- Li X, Wang H-l, Peng X, et al. miR-1297 mediates PTEN expression and contributes to cell progression in LSCC. Biochem Biophys Res Commun. 2012;427:254–260.
- Zhao H, Dupont J, Yakar S, et al. PTEN inhibits cell proliferation and induces apoptosis by downregulating cell surface IGF-IR expression in prostate cancer cells. Oncogene 2004;23:786–794.