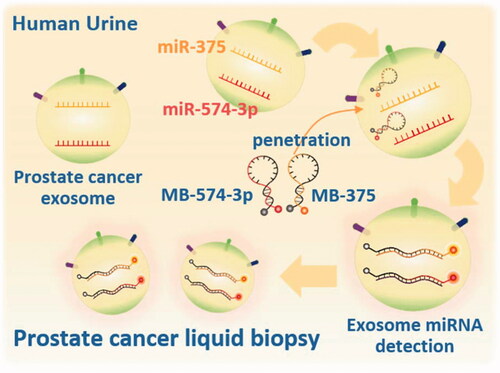
Abstract
Prostate cancer is the fifth leading cause of cancer-related deaths among males worldwide. However, the biomarker for diagnosing prostate cancer that is used currently has limitations that must be overcome. Recently, several studies have demonstrated that the cancer liquid biopsy can be implemented by using exosome miRNAs. However, the current methods for the detection of exosome miRNAs are time-consuming, expensive, and laborious. Thus, we investigated a novel method for diagnosing prostate cancer that involves the use of molecular beacons for the in situ detection of miRNAs in exosomes from prostate cancer cells. We chose miRNA-375 and miRNA-574-3p as the target miRNAs for prostate cancer, and these markers in exosomes produced by prostate cancer cells including DU145 and PC-3 were successfully detected using molecular beacons. High fluorescent signals were obtained from MB and miRNA hybridization in exosomes in a concentration-dependent manner. In addition, exosome miRNAs can be detected even in the presence of human urine, so this method can be applied directly using human urine to perform liquid biopsies for prostate cancer. Overall, the in situ detection of exosome miRNAs using molecular beacons can be developed as a simple, cost effective, and non-invasive liquid biopsy for diagnosing prostate cancer.
Introduction
Prostate cancer is the second most commonly diagnosed cancer in males worldwide, and, despite the significant advances in early diagnosis and management, it still remains a leading cause of cancer-related death among males [Citation1–3]. Currently, the conventional diagnostic methods for the diagnosis and monitoring of prostate cancer are the digital rectal examination (DRE), the prostate-specific antigen (PSA) level in the blood, and transrectal ultrasonography (TRUS) [Citation4,Citation5]. However, these methods have been criticized because they cause patients some discomfort, and they have both low specificity and accuracy [Citation6–9]. Thus, the development of specific prostate cancer markers and methods for detecting these markers is very important.
Exosomes are extracellular vesicles with diameters that range from 30 to 150 nm, and they are secreted constantly by most cells [Citation10–13]. High concentrations of exosomes are found in a variety of body fluids, including blood, urine, semen, saliva, breast milk, and amniotic fluid [Citation14–18]. Recent studies have proven that exosomes enable intercellular communication due to their ability to transfer molecules, such as DNAs, mRNAs, microRNAs (miRNAs), and the proteins that originate from their parental cells, to other cells and tissues in the human body [Citation19–22]. Especially, miRNAs can be encapsulated in exosomes and secreted for cell-to-cell communication. This exosome-mediated transfer of miRNAs is a novel mechanism of genetic exchange among cells that affects many physiological and pathological conditions. In addition, miRNAs are very stable in the exosomes where they are protected from a harsh environment [Citation23,Citation24]. These findings provide evidence that exosome miRNAs can be used as biomarkers for diagnosing various diseases, including cancer. However, the methods that currently are used for the detection of exosome miRNA require a series of steps, all of which are time-consuming and laborious. Therefore, it is necessary to develop an alternative method for the detection of exosome miRNA.
The molecular beacon (MB) is a nano-sized oligonucleotide probe and a bi-labelled hairpin molecule with a fluorescent dye at one end and a quencher at the other end. Since the loop of the MB is designed to include a complementary sequence to target miRNA, it spontaneously hybridizes to its specific target and the hairpin-loop structure opens, resulting in fluorescence. Thus, MB can be used as an efficient probe for detecting specific exosome miRNA. Recently, we demonstrated an in situ detection method for the detection of specific miRNAs in exosomes using MBs [Citation25,Citation26]. This report proposed that the exosome miRNA can be detected directly using MB targeting miRNA and will open great opportunities for the diagnosis of various diseases, including cancer.
Based on this method, we investigated the detection of miRNAs in exosomes secreted by prostate cancer cells using MBs for the development of a novel method for diagnosing prostate cancer (. To achieve this goal, we isolated exosome from the non-cancerous prostate RWPE-1 cell line as a control and from the DU145 and PC-3 prostate cancer cell lines. We selected exosome miR-375 and miR-574–3p as target miRNAs since these two miRNAs are known as biomarkers of prostate cancer in cells and exosomes [Citation27–30]. We also explored the detection of exosome miRNA in urine to develop a liquid biopsy for prostate cancer using exosomes in fluids taken from the human body.
Materials and methods
Molecular beacon design
lists the sequences of MBs and synthetic miRNAs used in this study. Cy3-labelled MB-375 and Cy5-labelled MB-574–3p were designed and synthesized to hybridize with mature miR-375 and miR-574–3p, respectively. FAM-, Cy3-, and Cy5-labelled MBs w/o quencher also were synthesized to observe the fluorescent properties of human urine. Synthetic target miRNAs were synthesized to confirm the specificity of MBs. Also, FAM- and Cy3-labelled MBs w/o a quencher, Cy3-labelled MB-375, and Cy5-labelled MB-574–3p were synthesized by Integrated DNA Technologies (IDT, San Diego, CA), and other MBs were synthesized by Bio Basic Inc. (Markham, Canada). Synthetic target miRNAs, including miR-375, miR-574–3p, and random target (Random T) were synthesized by Cosmoegenetech, Inc. (Seoul, Korea) [Citation25,Citation26].
Table 1. Design of MBs and synthetic miRNAs.
Solution assay of MB-target hybridization
All solution assays of MB-target hybridization were conducted in PBS. For the detection of a single miRNA, 100 nM of Cy3-labelled MB-375 or Cy5-labelled MB-574–3p were incubated with their synthetic miRNA targets, respectively, in a black, 384-well microplate for 1 h at 37 °C. The fluorescence signals from the MB and target were analyzed using a Varioskan™ Flash Multimode Reader (Thermo Scientific, Waltham, MA) for the calculation of the signal-to-background ratio (S/B ratio). Excitation wavelengths of 550 and 650 nm and emission wavelengths of 570 and 670 nm were used for Cy3 and Cy5, respectively. For dual detection of miRNAs, 100 nM of Cy3-labelled MB-375 and Cy5-labelled MB-574–3p were co-incubated together with their synthetic target miRNAs for 1 h at 37 °C for hybridization reaction. The S/B ratio was measured using a VarioskanTM Flash Multimode Reader (Thermo Scientific, Waltham, MA).
Cell culture and exosome production
The human prostate, non-cancer, epithelial cell line, RWPE-1, and the human prostate cancer epithelial cell lines, DU145 and PC-3, were used in this study. DU145 and PC-3 cells were cultured at the Roswell Park Memorial Institute 1640 (RPMI-1640, Gibco, Carlsbad, CA). Both media were supplemented with 10% (v/v) fetal bovine serum (FBS, RDT, USA) and 1% (v/v) penicillin and streptomycin (Life Technologies, Carlsbad, CA). To exclude the contamination of prostate cell-derived exosomes from bovine exosomes in FBS, the FBS was ultracentrifuged for 10 h, 120,000 ×g at 4 °C using a TLA-100.3 fixed angle rotor in an Optima TL-100 centrifuge (Beckman Coulter, Brea, CA), and only the supernatant was used for cell culture. The RWPE-1 cells were cultured in a keratinocyte serum-free medium (K-SFM, Gibco, Carlsbad, CA) supplemented with bovine pituitary extract at the concentration of 0.05 mg/mL (BPE, Gibco, Carlsbad, CA) and human recombinant epidermal growth factor at the concentration of 5 ng/mL (EGF, Gibco, Carlsbad, CA). K-SFM in the absence of BPE was prepared to isolate the exosome from the RWPE-1 cell line. All cell lines were cultured in a humidified atmosphere of 5% CO2 at 37 °C.
Isolation of exosomes from cell culture media
For the isolation of exosomes from cell culture media, the polymer-precipitation method using ExoQuick-TC™ (System Biosciences, Palo Alto, CA ) was used according to the manufacturer’s instruction. Briefly, cultured media were collected and then centrifuged at 3000 × g for 15 min to remove cells and cellular debris, and the supernatant was incubated at 4 °C overnight with ExoQuick-TC™ reagent. Then, the mixture of ExoQuick and media was centrifuged at 1500 × g for 30 min. After removal of the supernatant, the pellets were centrifuged again at 1500 × g for 5 min. The pellets that contained the exosomes were resuspended in nuclease-free PBS.
Quantification and characterization of the exosomes
The concentration of the exosomes was determined using an Exosome Quantitation Kit (EXOCET, System Biosciences, Palo Alto, CA ), which measures the activity of acetyl-CoA acetylcholinesterase (AChE) enriched in exosomes [Citation31]. EXOCET was used according to the manufacturer’s instructions. Briefly, exosomes were isolated using ExoQuick-TCTM and then they were lysed and centrifuged at 1500 × g for 5 min. Then, the supernatant was incubated at 37 °C for 20 min with reaction buffer and measured using a VarioskanTM Flash Multimode Reader (Thermo Scientific, Waltham, MA) at 405 nm. The size distribution and the concentration of the exosomes were measured by nanoparticle tracking analysis [Citation32] using NanoSight NS300 (Malvern Instruments, Worcestershire, UK) that tracks the Brownian motion of nanoparticles. The morphology of the exosomes was determined by transmission electron microscopy (TEM) using a JEM-1010 electron microscope (JEOL Ltd., Tokyo, Japan). The exosomes were absorbed onto a formvar/carbon-coated grid for 10 min and washed with distilled water. Then, the exosomes were fixed in 2% paraformaldehyde and washed twice with distilled water. The samples were negatively stained with 2% uranyl acetate for 10 min, dried for 15 min, and observed with a TEM operated at 60 kV.
Exosome RNA isolation, cDNA synthesis, and real-time PCR analysis
Total RNA was extracted from the exosomes using the Isol-RNA Lysis Reagent (ISOL; 5PRIME) according to the protocol provided by the manufacturer. The concentrations and purities of the RNA samples were quantified using a NanoDrop Lite Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Each RNA sample was transcribed reversely to cDNA using a miScript RT II kit (Qiagen, Hilden, Germany). The expression levels of mature miR-375 and miR-574–3p from the exosomes were measured by quantitative, real-time PCR using an ABI PRISM 7300 (ABI, Vernon, CA) with a miScript SYBR green PCR kit (Qiagen, Hilden, Germany).
In situ detection of single or dual miRNAs in exosomes from prostate cells using MBs
For the detection of single exosome miRNAs, the exosomes that were isolated from prostate cancer cells or prostate non-cancer cells were incubated with 100 nM of MB for 1 h at 37 °C. For dual exosome miRNA detection, 100 nM of each MB were incubated together with the exosomes for 1 h at 37 °C. The S/B ratio was measured using a Varioskan™ Flash Multimode Reader (Thermo Scientific, Waltham, MA). The background signal indicated the intensity of the fluorescence from the MB without exosomes. The S/B ratio of the prostate cancer cells was compared with that of the non-cancer prostate cells. For dual and simultaneous detection of miR-375 and miR-574–3p, a concentration of 12 × 107 exosomes/μL was used in the hybridization reaction with both MBs for detection. After 1 h of incubation, the S/B ratio was assessed with an excitation at 550 nm and an emission at 570 nm for MB-375 with Cy3 and with an excitation at 650 nm and an emission at 670 nm for MB-574–3p with Cy5. To evaluate the presence of miRNA outside the exosomes, the exosomes derived from PC-3 were incubated with a high concentration of RNase (1 μg/μL) for 1 h at 37 °C before the addition of MB-375 or MB-574–3p [Citation33].
Collection and preparation of human urine
The sample of human urine was collected from four healthy male volunteers. All urine samples were obtained in the morning prior to any eating, drinking, or smoking by the volunteers [Citation34]. The urine samples were collected in sterile containers, anonymously labelled, and processed within 3 h [Citation35]. For the development of a liquid biopsy for prostate cancer using exosomes in human body fluid, ultracentrifugation was used to remove the exosomes from the urine of the healthy volunteers. Briefly, the human urine samples that were collected were cleared by both centrifugation (300 ×g at 4 °C for 10 min and 17,000 ×g at 4 °C for 20 min) and filtration (0.2 μm) to remove cells and cellular debris. Then, the exosomes were removed by ultracentrifugation steps (200,000 ×g at 4 °C for 75 min) [Citation36]. The supernatant was exosome-free urine, and it was stored at −80 °C before use [Citation34,Citation37]. The exosome-free urine was used at concentrations of 0%, 20%, 40%, and 60% (v/v).
In situ detection of single miRNAs in exosomes spiked in exosome-free urine using MB
Before the detection of exosome miRNA in urine, the inherent fluorescence from exosome-free urine was measured at different excitations and emission wavelengths used for FAM (ex. 494 nm/em. 518 nm), Cy3 (ex. 550 nm/em. 570 nm), and Cy5 (ex. 650 nm/em. 670 nm) dye without MB and target. The fluorescence signals from exosome-free urine were analyzed using a Varioskan™ Flash Multimode Reader (Thermo Scientific, Waltham, MA). The background signal indicated the intensity of the fluorescence from the PBS without exosome-free urine. The effect of urine on the fluorophores also was observed using MBs without a quencher. One-hundred nM of FAM-labelled MB-21 w/o quencher, Cy3-labelled MB w/o quencher, and Cy5-labelled MB w/o quencher were incubated without target in exosome-free urine for 1 h at 37 °C. The fluorescence signals from MB in the exosome-free urine were analyzed using a Varioskan™ Flash Multimode Reader (Thermo Scientific, Waltham, MA). For the detection of exosome miRNA in urine, different concentrations of exosomes derived from PC-3 prostate cancer cells were spiked into the exosome-free urine and incubated with 100 nM of MB for 1 h at 37 °C. The fluorescence signals from the MB and target were analyzed using a Varioskan™ Flash Multimode Reader (Thermo Scientific, Waltham, MA).
Statistical analysis
Statistical analyses were performed using a paired t-test of the SigmaPlot version 8.0 (Systat Software Inc., San Jose, CA) to assess differences between the two groups.
Ethics statement
This study was approved by the Institutional Review Board of Incheon National University (IRB approval number: 7007971-201612-001). All of the healthy volunteers received an explanation of the study and signed a consent form. All methods in this study were conducted in accordance with the guidelines and regulations.
Results
Detection of miRNAs in solution using MBs
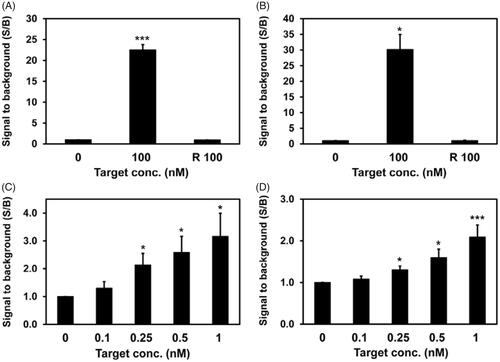
To evaluate the specificity of MBs for targeting miR-375 or miR-574–3p, each MB was incubated with its synthetic target miRNA, and the signal-to-background ratio (S/B ratio) was measured. shows that the S/B ratio between MB-375 and its synthetic target increased drastically to 22.5 after 1 h of the hybridization reaction. However, in the case of a random target that did not have a complementary sequence to MB-375 (Random T), there was no increase in the S/B ratio despite the high concentration of the target (100 nM). Similar results were obtained from MB-574–3p in that the S/B ratio against its target increased to 30.2, but the S/B ratio did not change when a random target was used (). This indicated that MB-375 and MB-574–3p are highly specific to targets miR-375 and miR-574–3p, respectively. In addition, each of MBs had an increased S/B ratio even with the low concentrations (below 1 nM) of synthetic targets miR-375 and miR-574–3p in a concentration-dependent manner, respectively (). Thus, the results indicated that the miRNA targeting MB had high specificity as well as high sensitivity in detecting miRNA. Based on the results showing the detection of miRNA in solution, MB-375 and MB-574–3p were used for the in situ detection of miRNAs in exosomes.
Figure 2. Detection of miR-375 and miR-574–3p using MBs in solution. MBs targeting miR-375 or miR-574–3p were incubated with different concentrations of synthetic miR-375 (A, C) and miR-574–3p (B, D), respectively: (A, B) 100 nM MB-375 and MB-574–3p were used for the detection of high concentration of miR-375 (A) and miR-574–3p (B), respectively, incubated for 1 h. R 100 indicates 100 nM of random target (Random T) that does not have complementary sequence to MBs. No signal increase was detected even with the high concentration of R 100 that was used; (C, D) 5 nM each MBs were used for the detection of lower concentrations of miR-375 (C) and miR-574–3p (D), respectively; all values are mean ± SD (*p < .05, **p < .01, ***p < .001; n = 3).
Characterization and analysis of exosomes from cancerous and non-cancerous prostate cells
The size distribution and the concentration of the exosomes from each cell were analyzed by NTA, and the average size of the exosomes for RWPE-1, DU145, and PC-3 cells was 144.5, 109, and 133.5 nm, respectively (). TEM analysis of the isolated exosomes showed a round morphology with sizes ranging from 30 to 100 nm, as shown in , indicating that the exosomes isolated from each of the types of cells were intact. The size of the exosomes from TEM was smaller than the size from the NTA results because the exosomes shrunk during the preparation process for TEM [Citation38]. No notable differences were observed among the exosomes from different cells.
Figure 3. Characterization of exosomes from prostate cells and the quantification of their miRNA levels in cells and exosomes: (A, B) size distribution and concentration of exosomes isolated from RWPE-1 (left), DU145 (middle), and PC-3 (right) analyzed by NTA (A) and TEM (B); scale bar =100 nm; (C, D) real-time PCR analysis was conducted for cellular (C) and exosome (D) miR-375 and miR-574–3p, respectively. CT values for miR-375 and miR-574–3p in cells and exosomes were significantly lower in DU145 and PC-3 than in RWPE-1, and the results indicate higher expression of miR-375 and miR-574–3p in the order of PC-3, DU145, and RWPE-1 cells and exosomes. All values are represented as mean ± SD (*p < .05, **p < .01, ***p < .01; n = 3).
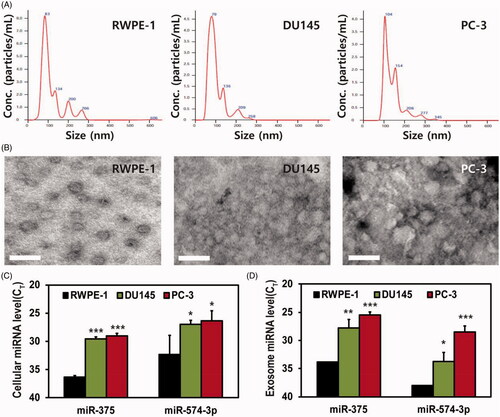
To estimate the exosomal levels of miR-375 and miR-574–3p, miRNAs were extracted from exosomes derived from their parental cells. Then, relative miR-375 and miR-574–3p levels in cells and exosomes were compared by measuring the threshold cycle (Ct) value using real-time PCR analysis (). First, as shown in , both miR-375 and miR-574–3p were highly expressed in the DU145 and PC-3 cells compared with those in RWPE-1 cells; this was consistent with a previous report that indicated that miR-375 and miR-574–3p can be potential biomarkers for the diagnosis of prostate cancer [Citation39]. Also, miRNA levels were measured in the exosomes derived from each type of cells (). As expected, the Ct values of both miR-375 and miR-574–3p were lower in the exosomes derived from the DU145 and PC-3 cells than the Ct value in the exosomes from RWPE-1 cells. This indicated that both miRNAs were highly expressed. The miR-375 and miR-574–3p levels were higher in the PC-3 exosomes than in the DU145 exosomes, which could be inferred from a previous report that PC-3 cells have high metastatic potential compared whereas DU145 cells have moderate metastatic potential [Citation40].
In situ detection of miRNAs in exosomes from prostate cancer cells using MBs
For the detection of miR-375 and miR-574–3p in exosomes from prostate cancer cells using molecular beacons, 100 nM of each of the MBs targeting miRNAs were used in the hybridization reaction with various concentrations of exosomes (0–6 × 107 particles/μL) produced from different cell lines. For the in situ detection of miR-375 in exosomes, MB-375 was delivered into different concentrations of exosomes from RWPE-1, DU145, and PC-3 exosomes (). The S/B ratio from MB-375 and exosome miR-375 hybridization increased gradually as the concentration of exosomes increased, irrespective of the types of cells. The S/B ratios from DU145 and PC-3 exosomes were significantly higher than the ratio from the RWPE-1 exosomes when the same concentrations of exosomes were used. For instance, the S/B ratio was 4.06 for exosomes from RWPE-1 cells, but the S/B ratios were 6.57 and 10.9 for exosomes from the DU145 and PC-3 cells, respectively, when the concentration of exosomes was 6 × 107 particles/μL. Also, the S/B ratio from the PC-3 exosomes was higher than the ratio from the DU145 exosomes, and this result was consistent with the result of the real-time PCR analysis shown in . A similar pattern was observed for the detection of miR-574–3p in the exosomes from cancer cells (). The hybridization fluorescent intensity increased as the exosome concentration increased, and the S/B ratio for the PC-3 exosomes was the highest, and the ratio for the RWPE-1 exosomes was the lowest.
Figure 4. In situ detection of miRNAs using molecular beacons in exosomes. The indicated amounts of exosomes were incubated with MBs for 1 h for the detection of exosome miR-375 (A, E) and miR-574–3p (C, F); (A, C) exosomes from RWPE-1 (left), DU145 (middle), and PC-3 (right) cells used for the intra-exosomal hybridization reaction between MBs and miR-375 (A) and miR-574–3p (C). The background signal indicates the fluorescence intensity from MB without exosomes, and the S/B ratio of each reaction was normalized to control (without exosomes); (B, D) 6 × 107 particles/μL of exosomes from PC-3 cells were incubated with or without RNase followed by adding MB-375 (B) or MB-574–3p (D). (E, F) The S/B ratios from (A) and (C) are integrated for direct comparison among exosomes derived from prostate cells and prostate cancer cells. All values are mean ± SD (*p < .05, **p < .01, ***p < .001; n = 3).
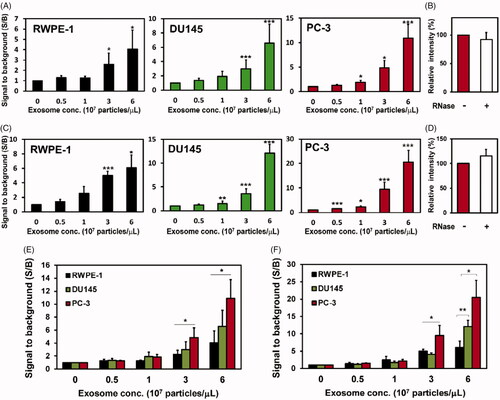
To rule out the possibility of detecting miRNA outside the exosomes and confirm that MB hybridized with exosome miRNA, exosomes produced from PC-3 were incubated with 1 μg/μL of RNase before the detection by MB-375 () or MB-574–3p (), respectively. There was no significant change in fluorescent intensity from MB and miRNA hybridization regardless of the RNase treatment. This indicated that the fluorescence signals shown here originated from MB hybridized with miRNAs in the exosomes. Thus, we can infer from the results that MB can detect and distinguish the different levels of the prostate cancer biomarker miRNA in intact exosomes. In addition, this indicates that the in situ detection of exosome miRNA using MB can be used as a novel, effective method for the diagnosis of prostate cancer.
The use of MBs for the simultaneous detection of miR-375 and miR-574–3p in solution
The clinical application of individual biomarkers has limitations since a single biomarker cannot reflect the exact symptoms of a disease or even the stages of a disease in many cases. We believe that multiplexed detection is the key advantage of using exosomes for the diagnosis since various biomarkers are embedded in a single exosome. Therefore, the development of simultaneous exosome biomarker detection is required to achieve the diagnosis of the disease with high accuracy and specificity. Thus, we investigated the dual detection of miR-375 and miR-574–3p in the same exosomes from prostate cancer cells. To eliminate the possibility of non-specific cross hybridization between MB and non-target miRNA, the MB-375 was incubated with synthetic miR-375 or miR-574–3p, and the fluorescent signals were compared. As expected, the S/B ratio between MB-375 and its target, miR-375, was 20.9, while the ratio between MB-375 and miR-574–3p was 1.11, which was almost the same as that from the MB-375 alone. This indicated that there was no non-specific binding between MB-375 and miR-574–3p (). Similarly, MB-574–3p was incubated with synthetic miR-574–3p or miR-375, and the S/B ratio from MB-574–3p and its complementary target, miR-574–3p, was 28.1. However, the S/B ratio from MB-574–3p and miR-375 was only 1.22, which was almost the same as background signal (). Therefore, the results demonstrated that two MBs only hybridize with their complementary target miRNAs and that there was no cross hybridization.
Figure 5. Dual detection of miRNAs using MBs in solution and exosomes: (A, B) single miRNA detection in solution. (A) 100 nM of Cy3-labelled MB-375 were incubated with 100 nM of synthetic miR-375 or miR-574–3p. (B) 100 nM of Cy5-labelled MB-574–3p were incubated with 100 nM of synthetic miR-375 or miR-574–3p in solution. Each MB also was cross incubated with synthetic miRNAs. (C) Dual miRNA detection using MBs in solution. MB-375 and MB-574–3p were co-incubated with both miR-375 and miR-574–3p and incubated for 1 h before analysis. (D) Detection of single (S) or dual (D) miRNAs in exosomes from prostate cancer cells. Exosomes from PC-3 cells were isolated and 12 × 107 particles/µL of exosomes were co-incubated with MB-375 and MB-574–3p for 1 h before analysis; then, the fluorescent signals were assessed for each of the MBs. All values are mean ± SD (**p < .01, ***p < .001; n = 3).
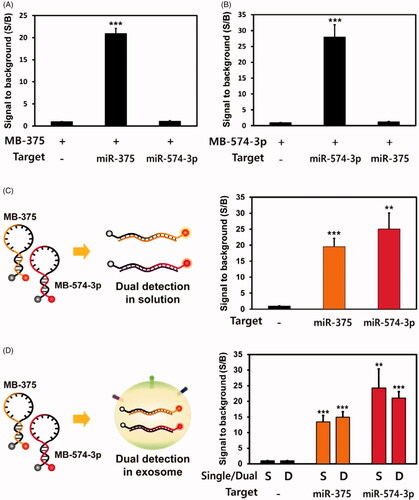
To test the dual detection of miRNAs in solution, MB-375 and MB-574–3p were incubated simultaneously with 100 nM miR-375 and 100 nM miR-574–3p in solution. One hundred nM of Cy3-labelled MB-375 and Cy5-labelled MB-574–3p were incubated together with 100 nM of each of the synthetic target miRNAs (). After 1 h of incubation, the hybridizations of both MBs with their own target miRNAs in the reactant were analyzed, and the results were compared with those of single miRNA detection. The S/B ratio from MB-375 and miR-375 hybridization was 19.6 and that from MB-574–3p and miR-574–3p was 25.1 in dual miRNAs detection in solution. These values were almost the same as those in the single miRNA detection (). Based on these results, the dual detection of miRNAs was conducted successfully using their specific MBs in solution.
The use of MBs for the simultaneous detection of miRNAs in exosomes from prostate cancer cells
To investigate the dual detection of miRNAs in exosomes, 100 nM of Cy3-labelled MB-375 and 100 nM of Cy5-labelled MB-574–3p were incubated together with 12 × 107 particles/µL of exosomes from PC-3 prostate cancer cells. One hour after delivery, the hybridization of both MBs was analyzed, and the results were compared with those of single miRNA detection. The S/B ratios from MB-375 and MB-574–3p in single miRNA detection were 13.5 and 24.2, respectively. And the S/B ratios in dual miRNA detection were 14.9 and 21.1, respectively (). This provided evidence for the successful detection of dual miRNAs in prostate cancer exosomes because there were no significant differences between single and dual exosome miRNA detection. To achieve a highly accurate method for diagnosing prostate cancer, the multiplexed detection of biomarkers is vitally important because it provides more information about the pathological status of the patient. Therefore, the results shown here demonstrate that the dual detection of miRNAs in exosomes using MBs will open new possibilities for the diagnosis of prostate cancer.
Characterization of human urine for the detection of exosome miRNA in urine
Urine contains many components that may interfere substantially with the detection of miRNA in exosomes [Citation41,Citation42]. Thus, those components must be removed entirely from urine before the diagnosis is made, and this imposes additional resources and processes on the method. Since the goal of the method is to eventually develop a liquid biopsy for prostate cancer using exosome miRNA derived from urine, we spiked the exosomes derived from prostate cancer cells into human urine to determine whether MB is capable of detecting exosome miRNAs in the presence of different concentrations of human urine, i.e. 0%, 20%, 40%, and 60%. Before this, the inherent fluorescence from urine was measured since urine contains many intrinsic fluorophores that may adversely affect the sensitivity of detection by providing high background fluorescence [Citation43,Citation44]. Fluorescent intensity in PBS without urine was used as the background signal. shows that there was a huge increase in fluorescence from urine when the fluorescence was measured at ex. 488 nm/em. 519 nm, and higher urine concentrations showed higher S/B ratios. The result indicates that there is large number of components in urine that produce fluorescence in this optical setting. However, there was only a small increase in the fluorescence signal produced in the urine when the fluorescence was measured at ex. 550 nm/em. 570 nm. Interestingly, there was little increase in the fluorescence signal from the urine when the fluorescence was measured at the Cy5 detection condition. As a consequence, fluorophores that have optical properties similar to those of the FAM dye can be avoided in performing liquid biopsies for exosome miRNA detection using a molecular beacon if the MB detection fluorescent signal is not sufficiently higher than the background signals from urine.
Figure 6. Detection of exosome miRNA in human urine using MB: to develop a liquid biopsy method for prostate cancer by detecting exosome miRNA in urine, the exosomes from PC-3 prostate cancer cells were spiked into different concentrations of urine from healthy males (0%, 20%, 40%, and 60%). (A) The inherent fluorescence values from different concentrations of urine were assessed at different excitation and emission wavelengths, especially those used for FAM (ex. 494 nm/em. 518 nm), Cy3 (ex. 550 nm/em. 570 nm), and Cy5 (ex. 650 nm/em. 670 nm); (B) the effect of urine and its concentration on fluorophores conjugated to a molecular beacon that does not have a quencher. One hundred nM of FAM-, Cy3-, and Cy5-labelled MBs without quenchers were incubated with different concentrations of human urine, and the fluorescent intensity was measured; (C) different concentrations (0, 3 × 107, and 6 × 107 particles/μL) of exosomes from PC-3 cells were isolated and added to exosome-free male urine. Different concentrations of urine solutions (0%, 20%, 40%, and 60%) were prepared, and 100 nM of Cy5-labelled MB-574–3p were added for the detection of exosome miR-574–3p. All values are mean ± SD (*p < .05, **p < .01, ***p < .001; n = 3).
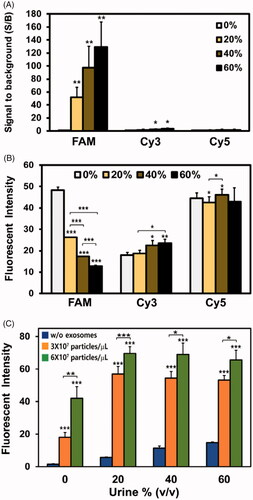
Next, we estimated the effect of urine and its concentration on fluorophores conjugated to molecular beacon without a quencher. These MBs always emit fluorescence regardless of their target miRNAs because they do not have a quencher. One hundred nM of FAM-, Cy3-, and Cy5-labelled MBs without quencher were incubated with different concentrations of human urine, and the fluorescent intensity was measured. Since the high concentration of MB did not have a quencher, the fluorescent intensity from MB was much higher than that from urine, as shown in . Then, the fluorescence from FAM-labelled MB without a quencher diminished drastically during incubation with urine in a concentration-dependent manner (). The fluorescent intensity decreased from 48.4 (100%) to 26.2 (54.1%), 17.3 (35.7%), and 12.8 (26.4%) in 20%, 40%, and 60% urine solution, respectively. This indicated that the FAM dye was severely affected in the presence of urine and, thus, is not suitable for the detection of exosome miRNA in urine. For Cy3-labelled MB without a quencher, the signals increased slightly to 104.3%, 125.6%, and 131.1% in 20%, 40%, and 60% urine solution as compared with the signals in the PBS solution. Interestingly, the Cy5-labelled MB without a quencher was the oligonucleotide that was the least affected by urine. There were just small differences in fluorescence among different concentrations of urine. Overall, we have elucidated that Cy5 dye has the most relevant optical properties for the in situ detection of exosome miRNA in urine.
Use of molecular beacon for the in situ detection of exosome miRNA in human urine
To develop a liquid biopsy method for prostate cancer by detecting exosome miRNA in urine, we extracted the exosomes from PC-3 prostate cancer cells and spiked healthy male urine with them. To rule out the possibility of detecting innate exosome miRNAs in urine and to prepare exosome-free urine, urine was ultracentrifuged to eliminate any exosomes that might have existed in the urine. Figure S1 shows that the concentration of urinary exosomes decreased drastically, and this means that most of the exosomes were removed from the urine that was analyzed by NTA. Then, the different concentrations of PC-3 exosomes (0, 3 × 107, and 6 × 107 particles/μL) were added to the different concentrations of exosome-free human urine. After the addition of the PC-3 exosomes, 100 nM of Cy5-labelled MB-574–3p were added for the detection of exosome miR-574–3p in the urine. Since we found there were substantial differences in the background signals (without exosomes) for different concentrations of urine, the fluorescent intensity rather than the S/B ratio was used to analyze the data. As expected, the fluorescent intensity increased as the concentration of exosomes increased (). In urine, the fluorescent intensities without exosomes (background signal) increased in accordance with increases in the concentration of the urine. In comparing the fluorescent intensities of the PBS solution without urine and the PBS solution with 20% urine, the latter solution showed a 381% increase in intensity. For the PBS solutions with 40% and 60% urine, the increases in the fluorescent intensities were 761% and 990%, respectively. shows that there was no difference in the fluorescent intensities from the Cy5-labelled MB without a quencher for the different concentrations of urine in the solution.
In addition, it is unlikely that this increase in the background fluorescence was due to the hybridization of MB-574–3p with the circulating free miR-574–3p that exists in urine. We treated exosome-free urine with RNase to degrade the circulating miRNAs, and then we added MB574–3p. If there were circulating miRNAs in the urine solution, the background fluorescence signals should decrease compared to the signals from untreated urine. However, Figure S2 shows that there was no significant decrease in the fluorescent intensities from the RNase-treated urine solution. The results indicated that there was a negligible amount of miR-574–3p in the urine. Thus, the increase in background fluorescent intensity shown in probably was due to the structural change of MB caused by the low pH of the urine, which creates increased fluorescent intensity by partially opening the MB stem region. Then, the fluorescent intensity increased drastically when 3 × 107 particles/μL of exosomes were added to the urine, and it increased further when 6 × 107 particles/μL of exosomes were added, irrespective of the urine concentration. Thus, the results demonstrated that the in situ detection of exosome miRNAs using MB can be accomplished successfully without any additional treatment of the urine. Further study is required to determine the exact reason for the effect of urine on fluorophores, MB structure, MB delivery into exosomes, and miRNA hybridization in exosomes.
Discussion
Prostate cancer is one of the leading causes of cancer-related death among males, so the development of a diagnostic method with high efficiency, accuracy, and sensitivity is a high priority. However, the methods that are used currently for diagnosing prostate cancer have many limitations. For instance, diagnosing cancer by means of a tissue biopsy is a severely invasive method, which makes it difficult to use as an early diagnosis tool, and it also has limitations in identifying high-risk disease [Citation45]. In addition, liquid biopsy using current prostate cancer biomarkers, including PSA, have been criticized due to their inability to discriminate between various prostate diseases, e.g. benign prostatic hyperplasia and infection, resulting in low specificity, low accuracy, and many false positives [Citation6]. Thus, it is essential to develop an alternative method for the effective and accurate diagnosis of prostate cancer. Exosomes contain vitally important physiological and pathological information originating from their mother cells, and they circulate throughout the circulatory system in the human body. Prostate cancer cells also produce substantial amount of exosomes, and they also circulate in the human body, resulting in their enrichment in the blood and urine [Citation46,Citation47]. Thus, exosome biomolecules, including miRNAs, are attractive biomarkers that can enable minimally invasive or even non-invasive liquid biopsies for the diagnosis of prostate cancer. The circulation of miRNAs in body fluids recently have been highlighted as biomarkers for diagnosis, but there are several defects that must be overcome before they can be used for this purpose. For instance, circulating miRNAs can be degraded by exposure to nucleases in body fluids, resulting in inaccurate diagnoses [Citation48,Citation49]. After miRNAs are secreted and leave the cells, they are highly diluted in body fluids, and the low concentrations of miRNAs can only be detected accurately by extremely high detection sensitivity. Also, miRNAs from different cells in every tissue are mixed in the body fluids, making it impossible to track their origins [Citation49]. However, the use of miRNAs in exosomes as biomarkers for diagnosis provides several significant benefits. Just like cells, exosomes are encompassed within a lipid layer that protects biomarkers, including miRNAs, against nucleases and shear stresses [Citation50]. Since exosomes are composed of various biomolecules from cells, there can be multiple types of markers, such as mRNAs, miRNAs, lipids, and proteins, which present various bits of information concerning where the exosomes originate and the status of their parental cells [Citation51]. The protein on the surfaces of exosomes also can be used as exosome capturing targets using antibodies, and the exosomes can be enriched effectively, enabling high detection specificity as well as sensitivity. Thus, in this article, we have presented the results of our investigation of a novel method for diagnosing prostate cancer that uses MB for the detection of miRNAs in exosomes from prostate cancer cells.
Urine is an ideal, non-invasive source of biomarkers in liquid biopsy for diagnosing prostate cancer. The discovery of new urinary biomarkers and the assessment of their related detection techniques definitely will facilitate the development of techniques for the early, cost-effective, and accurate diagnosis of prostate cancer as well as the clinical development of drugs designed to treat prostate cancer. High concentrations of exosomes are known to be excreted in human urine [Citation52]. Tumour-derived exosomes also can be detected readily in urine [Citation53]. Thus, the aberrant expression of miRNAs related to prostate cancer is enveloped in urinary exosomes. In addition, urinary exosomes have been demonstrated to be intact and stable, even after long-term storage. Recently, effective methods for the collection and preservation of exosomes from urine have been studied extensively [Citation34]. It was reported that despite the repeated freeze-thaw cycles, miRNAs in the exosomes from urine were intact and stable. Also, nucleic acids and proteins inside the exosomes are protected and stable in urine despite its low pH [Citation54]. Considering all of these factors, urinary exosomes and their miRNAs support their potential as non-invasive biomarkers for prostate cancer. Thus, it is very promising that MB successfully detected exosome miRNAs in urine without significant loss of the fluorescence signal, especially for Cy5-labelled MB.
We also demonstrated the importance of the choice of fluorophore for use in MB for the detection of exosome miRNA. Unlike Cy3- and Cy5-labelled MB, a noticeable, concentration-dependent decrease was observed in the fluorescent intensity of FAM-labelled MB in urine, and this may have affected the background signals and hybridization signals. This can be explained by the quenching of fluorophore due to the effect of oligonucleotide at low pH values. It was reported previously that the fluorescence from FAM-labelled oligonucleotide was diminished significantly in an acidic solution. Conversely, Cy3- and Cy5 dye-labelled oligonucleotides generally showed stable fluorescence signals in low pH conditions [Citation55,Citation56]. The average pH of urine is about 6.2, and the range is approximately 5.5–7.0 [Citation57]. We determined the pH of the different concentrations of the urine solutions, i.e. 20%, 40%, 60%, and 100%, used in this study. The average pH of the urine used in this study was 5.83, and the range was from 5.53 to 6.26. The pH decreased as the concentration of urine increased (Figure S3); the average pH values for 20%, 40%, 60%, and 100% urine were 6.73, 6.37, 6.13, and 5.83, respectively. In addition, the innate fluorescence from urine also may interfere with the fluorescent signal from exosome miRNA, which makes it difficult to analyze, especially when the target concentration is low. Thus, we recommend using alternative fluorophores, including Cy3 and Cy5, to overcome this issue. Cy5 had better performance for the detection of exosome miRNA in urine because its fluorescent signals were not affected by the urine and because minimal innate fluorescence was observed from urine in the Cy5 detection condition. Overall, the proposed method we have described is suitable for conducting liquid biopsies for prostate cancer, and, with minimal modifications, it is a new approach for the diagnosis of other types of diseases, including various cancers, infectious diseases, and neurodegenerative diseases.
Conclusions
In this study, we demonstrated the in situ detection of miRNAs in prostate cancer cell-derived exosomes using MBs. Both miR-375 and miR-574–3p, which are two candidate miRNA biomarkers for prostate cancer, were detected successfully in the prostate cancer cell-derived exosomes in a concentration-dependent manner. The fluorescent intensities from the hybridization between MB and its target miRNA in exosomes were consistent with the exosome miRNA level analyzed by real-time PCR. Dual and simultaneous detections of miRNAs in the same exosomes from prostate cancer cells also were demonstrated, which means that the method can be developed for the multiplexed diagnosis of prostate cancer with enhanced accuracy. In addition, the method was also applied for liquid biopsies for prostate cancer in that the prostate cancer cell-derived exosome miRNA was detected successfully, even in the presence of human urine. The results demonstrated that the method can be developed as a novel and efficient diagnosis for prostate cancer. The in situ exosome miRNA detection technique provides great opportunities for simple, time- and labour-saving, and non-invasive liquid biopsies for diagnosing prostate cancer. The method is not restricted to the diagnosis of prostate cancer; it also was proven to be viable as a novel platform technique for the diagnosis of other urological cancers which have been difficult to diagnose, such as colorectal, kidney, and bladder cancers.
Supporting_Information__Revised_.docx
Download MS Word (423.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
- Minciacchi VR, Zijlstra A, Rubin MA, et al. Extracellular vesicles for liquid biopsy in prostate cancer: where are we and where are we headed? Prostate Cancer Prostatic Dis. 2017;20:251–258.
- Artibani W. Landmarks in prostate cancer diagnosis: the biomarkers. BJU Int. 2012;110:8–13.
- Dijkstra S, Mulders PF, Schalken JA. Clinical use of novel urine and blood based prostate cancer biomarkers: a review. Clin Biochem. 2014;47:889–896.
- GL A, III GR, SS B. Mortality results from a randomized prostate-cancer screening trial. Eur Assoc Urol. 2009;55:1481–1489.
- Lilja H, Ulmert D, Vickers AJ. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat Rev Cancer. 2008;8:268–278.
- Heijnsdijk EA, der Kinderen A, Wever EM, et al. Overdetection, overtreatment and costs in prostate-specific antigen screening for prostate cancer. Br J Cancer. 2009;101:1833–1838.
- Roobol MJ. Is prostate cancer screening bad or good? Summary of a debate at the innovation in urology meeting, September 17–19, 2010, Milan, Italy. Eur Urol. 2011;59:359–362.
- Conde-Vancells J, Rodriguez-Suarez E, Embade N, et al. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J Proteome Res. 2008;7:5157–5166.
- Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579.
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383.
- Peterson MF, Otoc N, Sethi JK, et al. Integrated systems for exosome investigation. Methods 2015;87:31–45.
- Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci USA. 2004;101:13368–13373.
- Caby MP, Lankar D, Vincendeau-Scherrer C, et al. Exosomal-like vesicles are present in human blood plasma. Int Immunol. 2005;17:879–887.
- Poliakov A, Spilman M, Dokland T, et al. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–167.
- Ogawa Y, Kanai-Azuma M, Akimoto Y, et al. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31:1059–1062.
- Admyre C, Johansson SM, Qazi KR, et al. Exosomes with immune modulatory features are present in human breast milk. J Immunol. 2007;179:1969–1978.
- Valadi H, Ekstrom K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659.
- Xiao D, Ohlendorf J, Chen Y, et al. Identifying mRNA, microRNA and protein profiles of melanoma exosomes. PLoS One. 2012;7:e46874.
- Thakur BK, Zhang H, Becker A, et al. Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res. 2014;24:766–769.
- Takahashi A, Okada R, Nagao K, et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat Commun. 2017;8:15287.
- Png KJ, Halberg N, Yoshida M, et al. A microRNA regulon that mediates endothelial recruitment and metastasis by cancer cells. Nature. 2012;481:190–194.
- Zhang J, Li S, Li L, et al. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24.
- Lee JH, Kim JA, Kwon MH, et al. In situ single step detection of exosome microRNA using molecular beacon. Biomaterials. 2015;54:116–125.
- Lee JH, Kim JA, Jeong S, et al. Simultaneous and multiplexed detection of exosome microRNAs using molecular beacons. Biosens Bioelectron. 2016;86:202–210.
- Hessvik NP, Sandvig K, Llorente A. Exosomal miRNAs as biomarkers for prostate cancer. Front Genet. 2013;4:36.
- Soekmadji C, Russell PJ, Nelson CC. Exosomes in prostate cancer: putting together the pieces of a puzzle. Cancers (Basel). 2013;5:1522–1544.
- Hessvik NP, Phuyal S, Brech A, et al. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim Biophys Acta. 2012;1819:1154–1163.
- Valentino A, Reclusa P, Sirera R, et al. Exosomal microRNAs in liquid biopsies: future biomarkers for prostate cancer. Clin Transl Oncol. 2017;19:651–657.
- Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–1851.
- Rhee WJ, Santangelo PJ, Jo H, et al. Target accessibility and signal specificity in live-cell detection of BMP-4 mRNA using molecular beacons. Nucleic Acids Res. 2008;36:e30.
- Royo F, Diwan I, Tackett MR, et al. Comparative miRNA analysis of urine extracellular vesicles isolated through five different methods. Cancers (Basel). 2016;8:112.
- Zhou H, Yuen PST, Pisitkun T, et al. Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int. 2006;69:1471–1476.
- Musante L, Saraswat M, Ravida A, et al. Recovery of urinary nanovesicles from ultracentrifugation supernatants. Nephrol Dial Transplant. 2013;28:1425–1433.
- Barutta F, Tricarico M, Corbelli A, et al. Urinary exosomal microRNAs in incipient diabetic nephropathy. PLoS One. 2013;8:e73798.
- Cheruvanky A, Zhou H, Pisitkun T, et al. Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol. 2007;292:F1657–F1661.
- Zhang W, Peng P, Kuang Y, et al. Characterization of exosomes derived from ovarian cancer cells and normal ovarian epithelial cells by nanoparticle tracking analysis. Tumor Biol. 2016;37:4213–4221.
- Brase JC, Johannes M, Schlomm T, et al. Circulating miRNAs are correlated with tumor progression in prostate cancer. Int J Cancer. 2011;128:608–616.
- Pulukuri SMK, Rao JS. Activation of p53/p21Wafl/Cipl pathway by 5-aza-2'-deoxycytidine inhibits cell proliferation, induces pro-apoptotic genes and mitogen-activated protein kinases in human prostate cancer cells. Int J Oncol. 2005;26:863–871.
- Wachalska M, Koppers-Lalic D, van Eijndhoven M, et al. Protein complexes in urine interfere with extracellular vesicle biomarker studies. J Circ Biomark. 2016;5:4.
- Fernandez-Llama P, Khositseth S, Gonzales PA, et al. Tamm-Horsfall protein and urinary exosome isolation. Kidney Int. 2010;77:736–742.
- Leiner MJP, Hubmann MR, Wolfbeis OS. The total fluorescence of human urine. Anal Chim Acta. 1987;198:13.
- Kušnír J, Dubayová K, Lešková L, et al. Concentration matrices—solutions for fluorescence definition of urine. Anal Lett. 2005;38:1559–1567.
- D’Elia C, Cerruto M, Cioffi A, et al. Upgrading and upstaging in prostate cancer: from prostate biopsy to radical prostatectomy. Mol Clin Oncol. 2014;2:1145–1149.
- Mitchell PJ, Welton J, Staffurth J, et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med. 2009;7:4.
- Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Prot Clin Appl. 2015;9:358–367.
- Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA. 2011;108:5003–5008.
- Turchinovich A, Weiz L, Langheinz A, et al. Characterization of extracellular circulating microRNA. Nucleic Acids Res. 2011;39:7223–7233.
- Cheng L, Sharples RA, Scicluna BJ, et al. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3:23743.
- Christianson HC, Svensson KJ, van Kuppevelt TH, et al. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. PNAS. 2013;110:17380–17385.
- Taylor DD, Zacharias W, Gercel-Taylor C. Exosome isolation for proteomic analyses and RNA profiling. Methods Mol Biol. 2011;728:235–246.
- Zoller M. Pancreatic cancer diagnosis by free and exosomal miRNA. Wjgp. 2013;4:74–90.
- Lv LL, Cao Y, Liu D, et al. Isolation and quantification of microRNAs from urinary exosomes/microvesicles for biomarker discovery. Int J Biol Sci. 2013;9:1021–1031.
- You Y, Tataurov AV, Owczarzy R. Measuring thermodynamic details of DNA hybridization using fluorescence. Biopolymers. 2011;95:472–486.
- Torimura M, Kurata S, Yamada K, et al. Fluorescence-quenching phenomenon by photoinduced electron transfer between a fluorescent dye and a nucleotide base. Anal Sci. 2001;17:155–160.
- Rose C, Parker A, Jefferson B, et al. The characterization of feces and urine: a review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol. 2015;45:1827–1879.