?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Iron oxide nanoparticles (IONs) have been extensively applied in cancer therapy and theranostics due to their admissible magnetic properties, excellent chemical stability and biocompatibility. Herein, a novel stimuli-responsive magnetic nanocomposite was synthesized for cancer therapy; thereby, the triblock copolymer of poly[(2-succinyloxyethylmethacrylate)-b-(N-isopropylacrylamide)-b-dimethylaminoethylmethacrylate) [poly(SEMA-b-NIPAM-b-DMAEMA)] was prepared by reversible addition of fragmentation chain transfer (RAFT) polymerization. This triblock copolymer with carboxylic groups of succinyloxyethylmethacrylate was adsorbed onto the surface of Fe3O4 nanoparticles. The morphology, nanocomposite properties and stimuli-responsive behaviours were investigated by field emission scanning electron microscopy, X-ray diffraction, dynamic light scattering, vibrating sample magnetometer (VSM) and thermogravimetric analysis. Doxorubicin (DOX) encapsulation efficacy was 94.3%. Release behaviours of DOX from the magnetic nanocomposite exhibited that the rate of DOX release could be efficiently controlled through temperature and pH. The cytotoxicity of the drug was investigated in vitro against breast cancer cell line (MCF7) using (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assays, 4′,6-diamidino-2-phenylindole (DAPI) staining and cellular uptake. In conclusion, the synthesized DOX@nanocomposite can be applied in theranostic applications and anticancer drug delivery owing to admissible properties.
Introduction
Application of inorganic nanoparticles as contrast agents and therapeutic actuators is one of the most promising methods for cancer therapy and theranostics [Citation1–3]. The low cytotoxicity of nanomaterials makes them ideal candidates for drug delivery [Citation4,Citation5], MRI contrast enhancement [Citation6], magnetic separation and hyperthermia [Citation7]. The magnetic targeting offers some major advantages for drug delivery, in particular, an ability to consider a specific site such as a tumour [Citation8], the systemic distribution of cytotoxic compounds [Citation9] and enhancing uptake at the target site resulting in effective treatment at lower doses [Citation10]. Iron nanoparticles (NPs) with stimuli-responsive polymeric shells have been appropriately reputed as a smart bio-platform in therapeutic applications [Citation11]. The several stimuli-responsive polymers targeted to release the encapsulated therapeutics in respond to several endogenous (pH value, redox and enzyme) and exogenous (temperature, light, etc.) stimuli have been developed by incorporating different responsive building blocks into polymers [Citation12–16]. Among all stimuli, pH sensitivity is the most investigated one, owing to the pH value gradients found within the physiological environment.
The pH-responsive block copolymers have weak acid or base moieties such as carboxylic acid and tertiary amines as core-forming blocks [Citation17,Citation18]. The polymers that bear tertiary amino groups, such as poly[2-(dimethylamino) ethyl methacrylate] (PDMAEMA) and poly[2-(diethylamino) ethyl methacrylate] (PDEAEMA), have been widely exploited in various applications as pH-responsive materials [Citation19]. As a result of the protonation and deprotonation of the tertiary amino groups, these polymer chains can swell and shrink upon a change in pH across the pKa [Citation20]. In addition, the stimuli-responsive block copolymers of poly(N-isopropylacrylamide) (PNIPAM) were utilized as a thermo-responsive polymer This polymer has a cloud point (or lower critical solution temperature (LCST)) of 32 °C [Citation21,Citation22]. The major drawback of PNIPAM block copolymers for developing the polymeric nanoparticles for pharmaceutical applications is nano-degradability. Moreover, the local hyperthermia is necessary for nanomaterial destabilization and subsequent release of the encapsulated drug [Citation23,Citation24]. Doxorubicin (DOX) is a well-known anti-cancer drug that has been used for the effective treatment of many types of cancers [Citation25]. However, its clinical application is limited by side effects and drug resistance. DOX is introduced as a chemotherapeutic drug, due to its short- and long-term cardiac toxicity. Hence, it is interesting to improve the anticancer activity and reduce the systemic toxicity of DOX with the development of a drug delivery system [Citation26–28].
The demanded polymers were prepared using the reversible deactivation radical polymerization or living radical polymerization including nitroxide-mediated polymerization (NMP) [Citation29], atom transfer radical polymerization (ATRP) [Citation30] and reversible addition fragmentation chain transfer (RAFT) [Citation31]. The commonly employed technique to produce copolymers with narrow molecular weights is the RAFT technique.
The main purpose of this study was the introduction of a facile strategy to synthesize the stimuli-responsive magnetic nanocomposites for simultaneous cancer diagnosis and therapy. The copolymers of poly(2-hydroxyethylmethacrylate-b-N-isopropylacrylamide-b-N,N-dimethylaminoethylmethacrylate (poly(HEMA-b-NIPAM-b-DMAEMA)) were synthesized via RAFT approach. The esterification of the hydroxyl groups on poly(HEMA) blocks with excess amount of succinic anhydride resulted in poly(SEMA-b-NIPAM-b-DMAEMA). These copolymers with carboxylic functional groups were then adsorbed onto the surface of Fe3O4 nanoparticles through the interaction with hydroxyl groups on the nanoparticles surface. The doxorubicin hydrochloride (DOX) was interacted with nanocomposite via ionic interaction and hydrogen bonding. The tumour assistant release behaviour and antiproliferative efficacy of novel developed nanocomposites were investigated on breast cancer cell line (MCF7) by the (3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT) assay, 4′,6-diamidino-2-phenylindole (DAPI) and cellular uptake.
Experimental
Materials
The RAFT agent (4-cyano-4-[(phenylcarbothioyl)sulfanyl] pentanoic acid) was synthesized in our research centre [Citation32]. The 2-hydroxyethyl methacrylate (HEMA), (N,N-dimethylamino)ethyl methacrylate (DMAEMA) and N-isopropylacrylamide (NIPAM, 99%) were purchased from Sigma-Aldrich, USA. The ferrous chloride tetrahydrate (FeCl2. 4H2O, 99%) and ferric chloride hexanhydrate (FeCl3. 6H2O, 98%) were also prepared from Merck, USA and employed as received. Doxorubicin hydrochloride (DOX) was purchased from Sobhan Pharmaceutical Co., 109 Ltd., Tehran, Iran.
Synthesis of poly(HEMA)
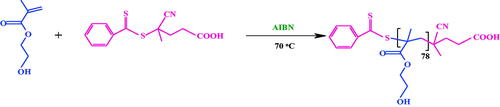
Poly(2-hydroxyethyl methacrylate) was synthesized based on Scheme 1, and further details are reported in the literature [Citation32] (Scheme 1).
Synthesis of poly(HEMA-b-NIPAM) copolymer
A two-neck reactor was charged with macro-RAFT agent (poly(HEMA), 0.5 g, 0.048 mmol), NIPAM monomer (0.7 g, 6.18 mmol), AIBN (2 mg, 0.012 mmol) and DMF (15 mL). The reaction mixture was degassed with three freeze-pump-thaw cycles and transferred in an oil bath at 85 °C for about 48 h. At the end of reaction time, the flask was transferred in cooled ice/water bath to quench the polymerization. The reaction mixture was precipitated in cold diethyl ether (150 mL), and subsequently, the product was filtrated and dried under vacuum at 25 °C temperature (Scheme 2).
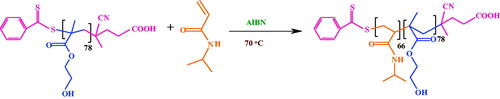
Synthesis of poly(HEMA-b-NIPAM-b-DMAEMA) triblock copolymer
The macro-RAFT agent of poly(HEMA-b-NIPAM) (1.8 g, 0.1 mmol), DMAEMA monomer (2 g, 12.7 mmol), AIBN (2.5 mg, 0.015 mmol) and DMF (12 mL) were dissolved in a two-necked flask. The reaction mixture was degassed with four freeze-pump-thaw cycles and refluxed at 80 °C for 24 h. After completion of the reaction, the flask was transferred in ice bath to quench the polymerization. The reaction was precipitated in cold diethyl ether (100 mL). The product was then filtrated and dried under vacuum at room temperature (Scheme 3).
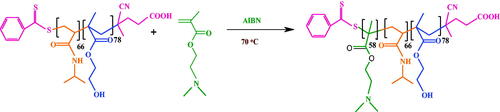
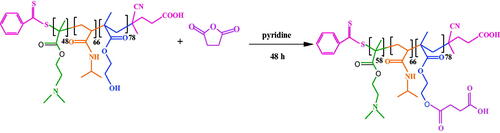
Synthesis of poly(SEMA-b-NIPAM-b-DMAEMA)
Briefly, the poly(HEMA-b-NIPAM-b-DMAEMA) triblock copolymer (0.5 g, 0.02 mmol) was added into 20 mL of anhydrous pyridine and stirred until the solution was dissolved completely. Then, the succinic anhydride (0.5 g, 5 mmol) was added to the solution under a stream of nitrogen and stirred for 48 h under argon atmosphere. The soluble product was precipitated into 100 mL cold diethyl ether and dried overnight at 60 °C in a vacuum oven (Scheme 4).
Synthesis of superparamagnetic iron oxide nanoparticles (SPIONs)
The SPIONs were prepared by coprecipitation method as described in the literature [Citation16].
Synthesis of poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite
Poly(SEMA-b-NIPAM-b-DMAEMA) terpolymers (100 mg) were dispersed in 2 mL dimethyl sulfoxide (DMSO) and SPIONs (100 mg) was added to the stirring solution for two day. The mixture was irradiated with ultrasonic vibrations for 30 min. The solution was set into a dialysis bag and subjected to dialysis against deionized water for about 48 h with vigorous stirring. At the end of this period, the un-modified SPIONs were precipitated by centrifugation at 7000 rpm for 10 min, dried and weighted. In addition, poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 was further purified through external magnetic field and decantation process for several times in order to remove the un-adsorbed polymeric chains. This product was denoted as magnetic nanocomposite.
Preparation of DOX@magnetic nanocomposite
A 25-mL dark flask was charged with 50 mg nanocomposite in 2 mL deionized water and DOX (5 mg). The content of reactor was sonicated for 10 min and then stirred for about 48 h at 25 °C to achieve the high loading content. Finally, DOX@magnetic nanocomposite was collected by centrifugation at 11000 rpm for about 20 min. The encapsulation efficiency of DOX@magnetic nanocomposite was characterized by analysing the unloaded drug concentration using UV–Vis spectroscopy at 480 nm. The DOX encapsulation efficiency (%) was calculated using following equation.
In vitro release investigation
The DOX-loaded nanocomposite (30 mg) was dispersed in 2 mL of desired buffer solution at pHs of 5.4 and 7.4 as a release medium. The release solution was kept at 41 and 37 °C with stirring at a rate of 220 rpm for 30 h. The sample was transferred to 2 mL of fresh buffer solution, and the released DOX content was determined. Then, the released DOX was collected in different times and evaluated with UV–Vis spectrophotometer at 480 nm.
In vitro cytotoxicity test
The cytotoxicity of the DOX@nanocomposite was investigated against MCF7 cells using MTT assay. The MCF7 cell line (human breast adenocarcinoma cell line) was purchased from NCBI (National Cell Bank of Iran, Pasteur Institute). In brief, the MCF-7 cells were seeded in 96 well plates and after overnight incubation (37 °C, 5% CO2), the MCF7 cells were treated with different concentrations of nanocomposite (50, 100, 200, 400, 800 and 1600 µg mL−1), DOX@nanocomposite (0.05, 0.5, 5, 50 and 500 µg mL−1) and free DOX for 24 and 48 h. The MTT solution (5 mg/mL) was then added, and the cells were incubated for another 4 h. The MTT formazan was dissolved in 200 μL of DMSO and measured using a spectrophotometric plate reader (Elx808, Biotek, USA) at a wavelength of 570 nm. The results were compared with control cells containing only cell-cultured medium.
DAPI staining test
The 4′,6-diamidino-2-phenylindole (DAPI) is a fluorescent stain that binds strongly to adenine thymine-rich regions in DNA. It is used vastly in fluorescence microscopy. The effect of DOX@nanocomposite on was investigated on MCF-7 cell. The cells were seeded and after incubation for 24 h at 37 °C, the MCF7 cells were treated with different doses of DOX@nanocomposite and free DOX within 24 h. After 48-h incubation, the MCF7 cells were washed three times with PBS and fixed with fresh 4% paraformaldehyde for 50 min at 25 °C. The MCF7 cells were subsequently stained with DAPI solution (Sigma, USA) for 540 min, and the stained cells were observed by a fluorescence microscopy (Bh2-RFCA, Olympus, Japan).
Cellular uptake
A 20 mg/mL solution of nanocomposite was dispersed in PBS by ultrasonic at a room temperature. Afterwards, the nanocomposite was collected by centrifuge at 10000 rpm for 10 min. The MCF7 cells were seeded in 6 well plates and incubated for 24 h and then treated with DOX@nanocomposite and free DOX. The cells were collected and washed three times with PBS after 0.5 and 3 h. Rhodamine uptake was measured using a FACScalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
Characterization
Fourier transform infrared (FT-IR) spectra of the samples were obtained by a Shimadzu 8101 M FT-IR (Shimadzu, Kyoto, Japan) at the wavenumber ranges of 4000 to 400 cm−1. Proton nuclear magnetic resonance (1HNMR) spectra were obtained at 25 °C using an FT-NMR (400 mHz) Bruker spectrometer (Bruker, Ettlingen, Germany). The samples were prepared in the deuterated dimethyl sulfoxide solvent (DMSO-d6) or water (D2O). The size exclusion analyses were carried out using a Waters 1515 (USA) gel permeation chromatography (GPC) instrument equipped with Breeze 1515 isocratic pump and 7725 manual injector. Ultraviolet-visible (UV–Vis) spectroscopy was taken in a Shimadzu 1650 PC UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan). The magnetic properties of Fe3O4/poly(SEMA-b-NIPAM-b-DMAEMA) nanocomposites were evaluated by vibrating sample magnetometer (VSM, AGFM, Iran) at 25 °C temperature. The field emission scanning electron microscope (FESEM) type 1430 VP (LEO Electron Microscopy Ltd, Cambridge, UK) was applied to determine the morphologies of the synthesized samples. The structural properties were probed via using X-ray powder diffraction (XRD) with a X’pert-PRO advanced diffractometer by Cu (Kα) radiation (wavelength: 1.5406 Å), operated at 40 kV and 40 mA in the 2θ range of 20–70° at a room temperature. The particle size of nanocomposite was measured by a laser scattering technique (Zetasizer Nano ZS90; Malvern Instruments, Malvern, UK) at pH =4, 9 and 25, 40 and 50 °C.
Results and discussion
The magnetic nanocomposites have demonstrated a great potential in cancer therapy because of improved drug accumulation inside solid tumour models. Most core shell nanoparticles(a magnetic core made of magnetite) (Fe3O4) have shown promising results in vitro, yet only some of them have demonstrated improved tumour accumulation and anticancer pharmacological efficacy in various in vivo models [Citation33,Citation34]. The aim of this study was the synthesis of stimuli-responsive poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 nanocomposite and study of its application as a nano-carrier for the anticancer drug delivery.
Characterization of poly(SEMA-b-NIPAM-b-DMAEMA) triblock copolymer
FT-IR spectra of poly(HEMA), poly(HEMA-b-NIPAM), poly(HEMA-b-NIPAM-b-DMAEMA), and poly(SEMA-b-NIPAM-b-DMAEMA) triblock copolymers are shown in . FT-IR spectrum of poly(HEMA) revealed the characteristic absorption bands of C–O–C stretching vibration at 1271 cm−1, the stretching vibration of C–O group at 1374 cm−1, C–H bending vibration at 1465 cm−1, the stretching vibration of carbonyl group at 1712 cm−1, aliphatic C–H stretching vibrations at 2918 and 2958 cm−1 and the stretching vibration of hydroxyl group as a strong broad band centred at 3444 cm−1. FT-IR spectrum of poly(HEMA-b-NIPAM) diblock copolymers demonstrated the typical bands corresponding to both poly(HEMA) and poly(NIPAM) segments. The main absorption bands in this sample consisted of the stretching vibrations of ester and amid carbonyl groups at 1726 and 1660 cm−1, the aliphatic –CH stretching and –CH bending vibrations at 2918 to 2852 cm−1 and 1488 and 1390 cm−1, the absorption bands of –NH secondary amid and hydroxyl groups at 3550 cm−1. The main absorption bands in FT-IR spectrum of poly(HEMA-b-NIPAM-b-DMAEMA) were the stretching vibrations of ester carbonyl groups at 1734 cm−1, C–H bending vibration at 1471 cm−1, the stretching vibration of C–O group at 1392 cm−1, C–O–C stretching vibration at 1177 cm−1 and the stretching vibration of hydroxyl group as a strong broad band centred at 3502 cm−1. After esterification of diblock copolymers with succinic anhydride, no conspicuous alteration was detected in FT-IR spectra ().
Figure 1. FT-IR spectra of poly(HEMA), poly(HEMA-b-NIPAM), poly(HEMA-b-NIPAM-b-DMAEMA) and poly(SEMA-b-NIPAM-b-DMAEMA) (a); nanocomposite and Fe3O4 (b).
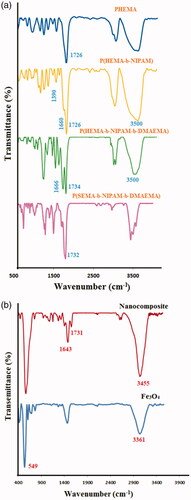
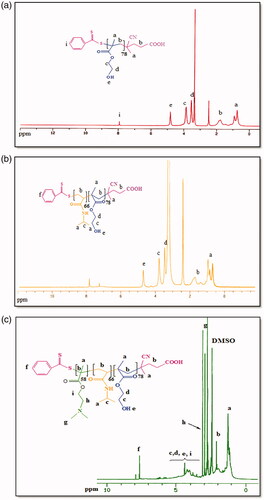
FT-IR spectrum of Fe3O4 nanoparticles demonstrated the absorption bands of 567 cm−1 for oxygen-metal stretching vibration. The bands around 1592 and 3367 cm−1were also ascribed to the bending and stretching vibrations of the hydroxyl groups on the surface of Fe3O4 nanoparticles, respectively. In FT-IR spectrum of Fe3O4/poly(SEMA-b-NIPAM-b-DMAEMA) nanocomposite, after adsorption of polymer onto Fe3O4 nanoparticles, the absorption bands of C–H at 2833 cm−1, carbonyl groups at 1731 cm−1, C–H bending vibration at 1500 cm−1, C–O–C stretching vibration at 1181 cm−1, the stretching vibration of hydroxyl group as a strong broad and centred at 3455 cm−1 and oxygen–metal stretching vibration band at 567 cm−1 were detected (). The successful synthesis of poly(HEMA), poly(HEMA-b-NIPAM) and poly(HEMA-b-NIPAM-b-DMAEMA) samples were further described by means of 1HNMR spectroscopy. 1HNMR spectra of the remarked compounds are exhibited in . 1HNMR spectrum of poly(HEMA) depicted the chemical shifts at 0.76−0.93 (a) and 1.1−2.1 (b) ppm related to the methyl group of poly(HEMA) backbone and methylene protons of RAFT backbone, respectively. The chemical shifts at 3.56 (d) and 4.07 (c) ppm were related to –CH2OH, and –OCH2 protons, respectively. The chemical shift at 4.83 (e) ppm was attributed to the hydroxyl group of poly(HEMA). In addition, the chemical shift at 7.94 ppm was correlated with the aromatic protons of the RAFT agent (). As seen in 1HNMR spectrum of poly(HEMA-b-NIPAM)copolymers, the chemical shifts at 0.67−2.1 (a–b) ppm were related to the methyl and methylene of poly(HEMA-b-NIPAM) backbone. The new chemical shift at 1.15 (a) ppm was also related to the methylene (–CH-CO) group of poly(NIPAM) backbone. The chemical shift at 4.42 (e) ppm was corresponded to –OCH2 and –CH-NH protons, respectively (). The synthesis of poly(HEMA-b-NIPAM-b-DMAEMA) triblock copolymers was approved by the appearance of new chemical shifts at 2.93, 3.22 and 4.1 ppm, which were related to N–(CH3)2 protons, N–CH2 and O–CH2 of poly(DMAEMA) segments. All other chemical shifts are labelled on 1HNMR spectrum of the triblock copolymer ().
Figure 2. 1HNMR spectra of poly(HEMA) (a); poly(HEMA-b-NIPAM) copolymer (b); poly(HEMA-b-NIPAM-b-DMAEMA) copolymer (c).
The GPC chromatograms of poly(HEMA), poly(HEMA-b-NIPAM) and poly(HEMA-b-NIPAM-b-DMAEMA) samples are shown in . The polydispersity index (PDI) values of poly(HEMA) (PDI = 1.14), poly(HEMA-b-NIPAM) (PDI = 1.19) and poly(HEMA-b-NIPAM-b-DMAEMA) (PDI = 1.26) synthesized via RAFT polymerization were relatively low, suggesting a good control over the RAFT technique. The molecular weights obtained from GPC and 1HNMR are compared with each other in .
Figure 3. GPC traces of poly(HEMA), poly(HEMA-b-NIPAM) and poly(HEMA-b-NIPAM-b-DMAEMA) triblock copolymers.
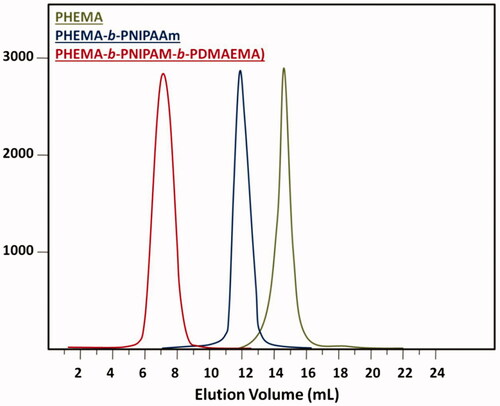
Table 1. Data analyses of poly(HEMA), poly(HEMA-b-NIPAM) and poly(HEMA-b-NIPAM-b-DMAEMA) by GPC and 1HNMR.
Characterization of the magnetic nanocomposites
The Fe3O4 nanoparticles had spherical morphologies with an average diameter of 22 ± 3 nm (. Ploy(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite exhibited a uniform and well-dispersed spherical morphology with the average diameter about 35 ± 5 nm. The X-ray diffraction (XRD) patterns collected from the Fe3O4 and poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite are shown in . As illustrated in , all samples revealed only one diffraction peak at about 2θ = 30.24, 35.68, 43.16, 53.4, 56.92 and 62.5 corresponding to (221), (312), (400), (421), (512) and (440) prisms of Fe3O4 crystalline structure, respectively. Therefore, the characteristic peaks of Fe3O4 magneticnanoparticles were observed in poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4magnetic nanocomposite, confirming that no phase change occurred.
Figure 4. FESEM image of the triblock copolymer (a), Fe3O4 (b) and poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic composite (c).

Figure 5. X-ray diffraction patterns (a), magnetization curve (b) and thermogravimetry (TG) (c) of poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite and Fe3O4 nanoparticles. The blue spectra stood for Fe3O4 and red spectra was for poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite.
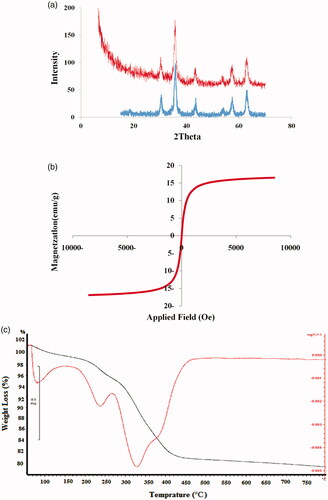
The magnetic properties of the prepared Fe3O4 nanoparticles and poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite as a nano-carrier were analysed by the vibrating sample magnetometer (VSM) at a room temperature. The superparamagnetic behaviour of the nano-carrier is shown in . The saturation magnetization (δs) of p(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 nanomagnetic was about 17 emug−1, which meant that the synthesized magnetic composite was superparamagnetic and it represented a good magnetic character for biomedical applications. The saturated magnetization values of Fe3O4 and polymer/Fe3O4 samples were 63.2 and 17 emug−1, respectively. The gradual decrease in the saturated magnetization values by surface modification of core material (Fe3O4) can be attributed to a decrease in the magnetic interaction with diamagnetic coating. Thermogravimetry (TG) (black line) and derivative TG (DTG) (red line) curves of poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite are also reported in . Nanocomposite demonstrated several stages of weight loss with heating. The first step of mass loss about 2.2% was attributed to the removal of the absorbed physical and chemical water between 50 and 160 °C. The second step of mass loss at 170 − 280 °C was about 3.9% and attributed to the decomposition of acidic segments of the copolymer. The third step of massive weight loss about 14.9% showed a sharp mass reduction step at 328 °C. The fourth step mass loss about 2.2% at 400−450 °C was ascribed to the poly(NIPAM) segments.
Stimuli-responsive of the magnetic nanocomposite
Thermo-responsive drug delivery is among the most investigated stimuli-responsive strategies and has been widely explored in oncology. Thermo-responsiveness is usually governed by a nonlinear sharp change in the properties of at least one component of the nano-carrier material with temperature. The poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite showed LCST at 42 °C. Poly(SEMA-b-NIPAM-b-DMAEMA) also displayed a pH responsive behaviour. The poly(SEMA) precipitated from aqueous solution at acidic pH (=4) and thereby formed the core [Citation32,Citation35].
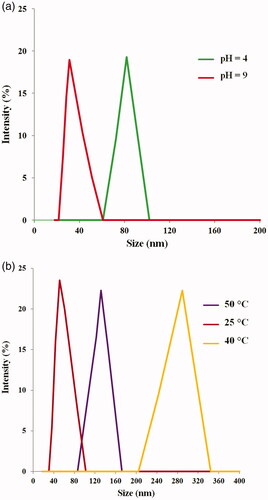
The dynamic light scattering (DLS) studies also confirmed the pH and thermosensitivity of magnetic nanocomposite. The corresponding DLS intensity distributions of the magnetic nanocomposite are shown in . At pH =4, the sizes of the magnetic nanocomposite increased due to strong hydrogen bonding between poly(SEMA) and poly(NIPAM) segments. In contrast, the sizes of the magnetic nanocomposite significantly decreased with increase of pH (=9) because of ionization of poly(SEMA) carboxylic acid group. The average sizes of poly(SEMA-b-NIPAM-b-DMAEMA) magnetic nanocomposite at pHs of 4.0 and 9.0 were 81.9 and 31.4 nm, respectively. On the other hand, the size of the magnetic nanocomposite increased with increasing the temperature above the LCST (42 °C), which was attributed to the dehydration of poly(NIPAM) segments [Citation36,Citation37]. The average diameters of poly(SEMA-b-NIPAM-b-DMAEMA) magnetic nanocomposite at temperatures of 25, 40 and 50 were obtained to be 53, 289 and 137 nm, respectively ().
In vitro DOX release studies
To investigate the controlled release of poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite, DOX was selected as model anticancer drug for convenient detection and in vitro experiments. The core-shell structures were synthesized with the cores of magnetic iron oxide (Fe3O4) and the shells of triblock copolymers, so that drugs could be loaded in poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite (). The drug-loading capacity of nanocomposite was analysed by UV-Vis spectrophotometry at 480 nm. Drug loading content and DOX encapsulation efficiency were calculated to be 8.69 and 94.3%, respectively. The release performances of DOX@poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite in different PBS buffers (pH =5.4 and 7.4) are displayed in .
Figure 7. The photographs (a) and cumulative in vitro release profiles (b) of DOX@poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic nanocomposite.
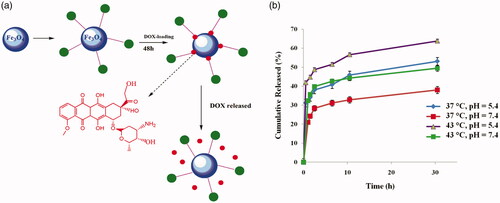
The release rate of DOX in a buffer solution at pH 5.4 (T = 37 °C) was accelerated and 53.13 wt% was released after 30 h. At pH of 5.4 and temperature of 37 °C, due to the protonation of carboxyl groups of poly(SEMA) and poly(DMAEMA) nanocomposite, the ionic interaction between DOX and nanocomposite was disappeared. The release rate in a buffer solution at pH of 7.4 (T = 37 °C) was quite low and about 38.04 wt% after 30 h, because at pH =7.4, the presence of strong ionic interaction between DOX and nanocomposite hindered the drug release, thanks to the deprotonation of carboxylic acids of poly(SEMA) (negative charge) and protonation of DOX (positive charge). The release rate was accelerated above the LCST (41 − 42 °C) of nanocomposite and at pH =5.4 and 7.4 at T = 43 °C it reached to 64.83 wt% and 49.44 wt% (), because the poly(NIPAM) blocks were collapsed at temperature above the LCST [Citation38]. Based on the cumulative in vitro release studies on DOX@poly(SEMA-b-NIPAM-b-DMAEMA)/Fe3O4 magnetic composite nanospheres, a dual responsiveness was detected which is applicable for tumour target therapy.
In vitro cell cytotoxicity assay
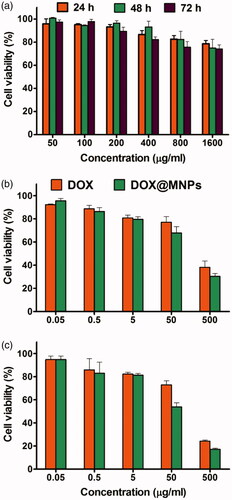
In vitro cellular cytotoxicity of the magnetic nanocomposite and DOX@magnetic nanocomposite to MCF7 cells were investigated by MTT assay. As seen in , the magnetic nanocomposite showed no apparent cytotoxic effect in low concentration after incubation for 48 h. When the concentration of magnetic nanocomposite increased to 1600 µgmL−1, the viability was above 80% after incubation for 48 and 72 h, revealing that the magnetic nanocomposite was biocompatible and suitable for use as a drug nano-carrier. The viability of cell growth of the DOX@magnetic nanocomposite and free DOX at different concentrations were also investigated (). Specifically, the DOX@magnetic nanocomposite exhibited a higher cytotoxic effect in 50 µgmL−1 compared with that of free DOX at the same concentration after incubation for 72 h. This result shows that the DOX@magnetic nanocomposite is suitable for the drug delivery therapeutic effect.
DAPI assay
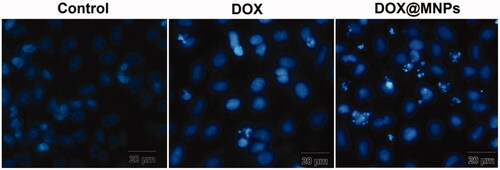
The apoptosis properties of the DOX@magnetic nanocomposite were compared with free DOX against MCF7 cells using the DAPI staining assay, as depicted in . The morphological changes of DOX@magnetic nanocomposite were shown with condensation of chromatin in nuclear and fragmentation of nuclei, i.e., apoptosis. Accordingly, the frequency of chromatin fragmentation in DOX@magnetic nanocomposite-treated cells was more than that of free DOX. In addition, the DOX@magnetic nanocomposite can be used as an excellent candidate for the cancer therapy applications.
Intracellular uptake study
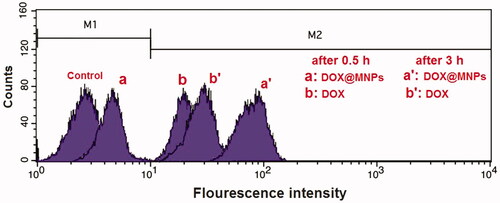
The cellular uptake of DOX@magnetic nanocomposite by MCF7 cell lines was visualized using fluorescence microscopy. The flow cytometry profiles of MCF7 cells incubated for .5 and 3 h with DOX and DOX@magnetic nanocomposite are represented in , and the cells without any treatment were used as the negative control. The magnetic nanocomposite exhibited high fluorescence intensity for 3 h internalized in MCF7 cells.
Conclusions
Stimuli-responsive magnetic iron oxide nanocomposite system was developed for cancer therapy. Poly(HEMA-b-NIPAM-b-DMAEMA) triblock copolymers were prepared by using RAFT technique. The esterification of the hydroxyl groups on poly(HEMA) blocks with excess amount of succinic anhydride resulted in poly(SEMA-b-NIPAM-b-DMAEMA). These copolymers with carboxylic functional groups were then adsorbed onto the surface of Fe3O4 nanoparticles through the interaction with hydroxyl groups on the nanoparticles surface . The well-defined core-shell magnetic nanocomposites ranged in 35 ± 5 nm were synthesized. The VSM analysis depicted the saturation magnetization (δs) of 17 emug−1 for poly(SEMA-b-NIPAM-b-DMAEMA) magnetic nanocomposites. The DLS measurements also confirmed the stimuli-responsiveness of magnetic nanocomposites. The average sizes of poly(SEMA-b-NIPAM-b-DMAEMA) magnetic nanocomposite at pHs of 4.0 and 9.0 were 81.9 and 31.4 nm, respectively. On the other hand, the average diameters of poly(SEMA-b-NIPAM-b-DMAEMA) magnetic nanocomposite at temperatures of 25, 40 and 50 were obtained to be 53, 289 and 137 nm, respectively. The DOX@magnetic nanocomposites demonstrated a high DOX entrapment efficiency. DOX released values after 30 h at pH =7.4 and 5.4 were 53.1 and 38.04%, respectively. The release rate was accelerated above the LCST (41 − 42 °C) of nanocomposite at pH =5.4 and 7.4 and reached 64.83 and 49.44 wt%, respectively. The in vitro cellular cytotoxicity demonstrated that DOX@magnetic nanocomposite was biocompatible and appropriate for use as a drug carrier in cancer therapy. Therefore, DOX@magnetic nanocomposite exhibited a higher cytotoxicity compared with that of free DOX to MCF7 cells. It can be anticipated that DOX@magnetic nanocomposite is an acceptable carrier for cancer therapy.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Espinosa A, Di Corato R, Kolosnjaj-Tabi J, et al. Duality of iron oxide nanoparticles in cancer therapy: amplification of heating efficiency by magnetic hyperthermia and photothermal bimodal treatment. ACS Nano. 2016;10:2436–2446.
- Bayda S, Hadla M, Palazzolo S, et al. Inorganic nanoparticles for cancer therapy: a transition from lab to clinic. Curr Med Chem. 2017.
- Sanchez-Salcedo S, Vallet-Regí M, Shanin SA, et al. Mesoporous core-shell silica nanoparticles with anti-fouling properties for ovarian cancer therapy. Chem Eng J. 2018;340:114–124.
- Scaletti F, Hardie J, Lee YW, et al. Protein delivery into cells using inorganic nanoparticle–protein supramolecular assemblies. Chem Soc Rev. 2018;47(10):3421–3432.
- Abdelaziz HM, Gaber M, Abd-Elwakil MM, et al. Inhalable particulate drug delivery systems for lung cancer therapy: nanoparticles, microparticles, nanocomposites and nanoaggregates. J Controlled Release. 2017;269:374–392.
- Nam J, Won N, Bang J, et al. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv Drug Delivery Rev. 2013;65:622–648.
- Bettini S, Giancane G, Pagano R, et al. A simple approach to synthetize folic acid decorated magnetite @ SiO2 nanostructures for hyperthermia applications. J Mater Chem B. 2017;5:7547–7556.
- Ghorbani M, Hamishehkar H. Redox and pH-responsive gold nanoparticles as a new platform for simultaneous triple anti-cancer drugs targeting. Int J Pharm. 2017;520:126–138.
- Mickoleit F, Borkner CB, Toro-Nahuelpan M, et al. In vivo coating of bacterial magnetic nanoparticles by magnetosome expression of spider silk-inspired peptides. Biomacromolecules 2018;19:962–972.
- Tregubov AA, Sokolov IL, Babenyshev AV, et al. Magnetic hybrid magnetite/metal organic framework nanoparticles: facile preparation, post-synthetic biofunctionalization and tracking in vivo with magnetic methods. J Magn Magn Mater. 2018;449:590–596.
- Vu HC, Dwivedi AD, Le TT, et al. Magnetite graphene oxide encapsulated in alginate beads for enhanced adsorption of Cr (VI) and As (V) from aqueous solutions: role of crosslinking metal cations in pH control. Chem Eng J. 2017;307:220–229.
- Massoumi B, Ghamkhari A, Agbolaghi S. (Dual stimuli-responsive poly (succinyloxyethylmethacrylate-b-N-isopropylacrylamide) block copolymers as nanocarriers and respective application in doxorubicin delivery. Int J Polym Mater Polym Biomater. 2018;67:101–109.
- Zhao R, Li K, Liu R, et al. Reversible 3D self-assembly of graphene oxide and stimuli-responsive polymers for high-performance graphene-based supercapacitors. J Mater Chem A. 2017;5:19098–19106.
- Ghamkhari A, Massoumi B, Agbolaghi S. An in vitro focus on doxorubicin hydrochloride delivery of novel pH‐responsive poly (2‐succinyloxyethylmethacrylate) and poly [(N‐4‐vinylbenzyl), N, N‐diethylamine] diblock copolymers. Polym Int. 2018;67:283–291.
- Berah R, Ghorbani M, Moghadamnia AA. Synthesis of a smart pH-responsive magnetic nanocomposite as high loading carrier of pharmaceutical agents. Int J Biol Macromol. 2017;99:731–738.
- Poorgholy N, Massoumi B, Ghorbani M, et al. Intelligent anticancer drug delivery performances of two poly(N-isopropylacrylamide)-based magnetite nanohydrogels. Drug Dev Ind Pharm. 2018;44(8):1–8.
- Xiang B, Jia X-L, Qi J-L, Yang L-P, et al. Enhancing siRNA-based cancer therapy using a new pH-responsive activatable cell-penetrating peptide-modified liposomal system. Int J Nanomedicine. 2017;12:2385.
- Lin W, Yao N, Qian L, et al. pH-responsive unimolecular micelle-gold nanoparticles-drug nanohybrid system for cancer theranostics. Acta Biomater. 2017;58:455–465.
- Ghorbani M, Hamishehkar H. Decoration of gold nanoparticles with thiolated pH-responsive polymeric (PEG-bp (2-dimethylamio ethyl methacrylate-co-itaconic acid) shell: a novel platform for targeting of anticancer agent. Mater Sci Eng, C. 2017;81:561–570.
- Sun H, Chen X, Han X, et al. Dual thermoresponsive aggregation of schizophrenic PDMAEMA-b-PSBMA copolymer with an unrepeatable pH response and a recycled CO2/N2 response. Langmuir. 2017;33:2646–2654.
- Ghamkhari A, Massoumi B, Salehi R. A new style for synthesis of thermo-responsive Fe3O4/poly (methylmethacrylate-b-N-isopropylacrylamide-b-acrylic acid) magnetic composite nanosphere and theranostic applications. J Biomater Sci Polym Ed. 2017;28:1985–2005.
- de Oliveira TE, Mukherji D, Kremer K, et al. Effects of stereochemistry and copolymerization on the LCST of PNIPAm. J Chem Phys. 2017;146:034904.
- Li Y, Nancy K, Albert G, et al. Supramolecular nanofibrillar thermoreversible hydrogel for growth and release of cancer spheroids. Angew Chem. 2017;129:6179–6183.
- Liu L, Zeng J, Zhao X, et al. Independent temperature and pH dual-responsive PMAA/PNIPAM microgels as drug delivery system: effect of swelling behavior of the core and shell materials in fabrication process. Colloids Surf, A. 2017;526:48–55.
- Yu E, Lo A, Jiang L, et al. Improved controlled release of protein from expanded-pore mesoporous silica nanoparticles modified with co-functionalized poly (n-isopropylacrylamide) and poly (ethylene glycol)(PNIPAM-PEG). Colloids Surf, B. 2017;149:297–300.
- Abbasian M, Mahmoodzadeh F, Salehi R, et al. Chemo-photothermal therapy of cancer cells using gold nanorod-cored stimuli-responsive triblock copolymer. New J Chem. 2017;41:12777–12788.
- Zhang Z, Wang Y, Xu S, et al. Photothermal gold nanocages filled with temperature sensitive tetradecanol and encapsulated with glutathione responsive polycurcumin for controlled DOX delivery to maximize anti-MDR tumor effects. J Mater Chem B. 2017;5:5464–5472.
- Pourbadiei B, Pyadar R, Mansouri F. pH-sensitive nanoscale polymers: highly efficient systems for DOX delivery in cancer treatment. J nanomed Res. 2017;5:1–6.
- Qiao XG, Lambert O, Taveau JC, et al. Nitroxide-mediated polymerization-induced self-assembly of block copolymers at the surface of silica particles: toward new hybrid morphologies. Macromolecules. 2017;50:3796–3806.
- Chmielarz P, Fantin M, Park S, et al. Electrochemically mediated atom transfer radical polymerization (eATRP). Prog Polym Sci. 2017;69:47–78.
- Massoumi B, Mousavi-Hamamlu SV, Ghamkhari A, et al. A novel strategy for synthesis of polystyrene/Fe3O4 nanocomposite: RAFT polymerization, functionalization, and coordination techniques. Polymer-Plastics Technol Eng. 2017;56:873–882.
- Ghamkhari A, Massoumi B, Jaymand M. Novel ‘schizophrenic’ diblock copolymer synthesized via RAFT polymerization: poly (2-succinyloxyethyl methacrylate)-b-poly [(N-4-vinylbenzyl), N, N-diethylamine]. Des Monomers Polym. 2017;20:190–200.
- Wang M, Deng K, Lü W, et al. Rational design of multifunctional Fe@ γ‐Fe2O3@ H‐TiO2 nanocomposites with enhanced magnetic and photoconversion effects for wide applications: from photocatalysis to imaging‐guided photothermal cancer therapy. Adv Mater. 2018;30:1706747.
- Park W, Gordon AC, Cho S, et al. Immunomodulatory magnetic microspheres for augmenting tumor-specific infiltration of natural killer (NK) cells. ACS Appl Mater Interfaces. 2017;9:13819–13824.
- Bories-Azeau X, Armes SP, van den Haak HJ. Facile synthesis of zwitterionic diblock copolymers without protecting group chemistry. Macromolecules 2004;37:2348–2352.
- Magenau AJ, Martinez-Castro N, Savin DA, et al. Polyisobutylene RAFT CTA by a click chemistry site transformation approach: synthesis of poly (isobutylene-b-N-isopropylacrylamide). Macromolecules 2009;42:8044–8051.
- Chomoucka J, Drbohlavova J, Huska D, et al. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62:144–149.
- Hu J, Youssefian S, Obayemi J, et al. Investigation of adhesive interactions in the specific targeting of Triptorelin-conjugated PEG-coated magnetite nanoparticles to breast cancer cells. Acta Biomater. 2018;71:363–378.