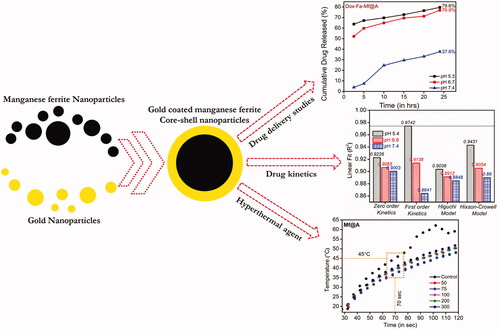
Abstract
Novel materials are explored very often by material scientists to design an efficient drug delivery system to target carcinoma cells. Among various nanosystem, functionalized Iron oxide Nanoparticles (IoNP) were definitely studied especially to target, endocyte and release drug moieties inside the cells. This IoNP platform is usually composed of an inorganic core and a highly biocompatible shell layer in order to perform numerous tasks at the same time, such as drug delivery, multimodal imaging, and instantaneous monitoring, along with collective therapeutic approaches. Hence, in this work, MnFe2O4@Au nanoparticles (Mf@A) are used as a structure for docking anti-cancer drug using a coupling molecule for the precise targeting. The formation of the core–shell structure was corroborated by high-angle annular dark-field scanning transmission electron microscopy and line mapping techniques. Superconducting quantum interference device confirms the fabricated nanostructure is favorably superparamagnetic. The stability of nanoparticles was examined by measuring the zeta-potential measurements. The binding efficiency of the drug onto the Mf@A was found to be >90%. Drug-release was carried out at different pH and found that the release is maximum at lower pH. Finally, at 2.45 GHz we employed as a magneto-hyperthermal agent which produced heat to kill the cancerous cell.
Introduction
In the recent years, nanomedicine research drive to the designing of multifunctional nanoparticles for various biomedical applications in the field of diagnosis, treatment, and therapy [Citation1–6]. In particular, the functionalized magnetic nanoparticles (MNPs) have been widely exploited for several advantages which open up many opportunities in the biomedicine and biotechnology field [Citation7]. It is due to their uncommon chemical and physical features such as magnetic, electronic, and optical properties. And also, MNPs size, shape, surface modification, water dispersity, biocompatibility plays a major role in various applications. Some of the MNPs based applications are magnetic resonance imaging (MRI), hyperthermia, and drug delivery [Citation8–14]. The extensive studies have been carried out using iron oxides, but among ferrites, manganese ferrite (Mf) is prominently used for various biomedical applications which are due to their property such as superparamagnetism, stability, high magnetization, and so on. These properties have attracted the researchers to develop the Mf into a multifunctional nanoparticle by various strategies such as core–shell (CSNPs) formation/bimetallic nanoparticles, surface modifications.
Among the various CSNPs, MNP (core), and GNP (shell) based are always created a niche in the field of biomedicine due to their excellent magnetic and optical properties [Citation15]. The formation of CSNPs is usually carried out using noble metals like gold (Au) or silver. Some of the CSNPs are Fe3O4@Au/Ag and MnFe2O4@Ag [Citation16–19]. Because these coatings offer a very less reactivity, avoiding aggregation of nanoparticles, high stability from the biological environment, less cytotoxic, increasing the chances of surface functionalization with various moieties such as drugs, targeting ligands/markers, and biomolecules (DNA, RNA, and proteins) and finally making the nanoparticles water-dispersible which is one of the important characteristics for biomedical applications [Citation20–22]. These opportunities exposed the advantages of CSNPs and using various techniques, the CSNPs are synthesized by layer-by-layer electrostatic deposition, chemical reduction, and reverse micelle method, and so on [Citation23–25].
CSNPs with various surface modifications act as a multi-tasker for various applications. For example, in case of cancer theranostics, the surface modified CSNPs can be an excellent drug delivery cargo, MRI agent, hyperthermal agent, photothermal agent, and so on [Citation26]. Even though, MNP based CSNPs are widely explored for the past two decades, there are very fewer reports on the Mf based Au CSNPs for biomedical applications [Citation8,Citation26–29].
Based on these advantages, we have designed an Mf coated with GNP along with surface modifications using folic acid (Fa) and doxorubicin (Dox) for the intention to use for biomedical applications especially in the field of cancer. The main purpose of Fa is because it acts as a steering molecule toward the folate receptor expressing cancer cells and enters the cell to deliver the drugs by means of endocytosis [Citation30,Citation31]. And the objective of Dox is acting as an anticancer agent which can be released by CSNPs and affects the cancer cell by two important possible mechanisms either by intercalating with DNA and disrupting topoisomerase-II-mediated DNA repair or by free radical generation leading to collapse of cellular membranes and other biomolecules [Citation32].
Therefore, we present a novel strategy method to synthesize Au-coated MnFe2O4 multifunctional NPs (Mf@A) using the seed-mediated technique to form a CSNPs is demonstrated [Citation33]. We have characterized these CSNPs to analyze the various properties like optical, structural, chemical, magnetic and also the biocompatibility by a combination of ultraviolet (UV)–visible (Vis) spectroscopy, X-ray diffraction, X-ray photoelectron spectroscopy, high resolution transmission electron microscopy (HRTEM) analysis, superconducting quantum interference device (SQUID) measurements. After various confirmations, the NPs are subjected to surface treatment using activated Fa and finally with the Dox moieties. The binding between Fa and Dox was confirmed by using Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analyzer (TGA), and zeta-potential. Later, the toxicity of various complex was tested by MTT assay and also visually analyzed the cellular and nuclear morphologies by confocal microscopy. The above characterizations and testing gave a strong instinct to use it as a nanocargo for drug-delivery study which was carried out at three different pH, and the magnetic property of Mf was taken advantage for the hyperthermal agent under microwave (Mw; ).
Materials and methods
Nanoparticle synthesis
2Mf core nanoparticles
Mf NPs were synthesized using co-precipitation method. The precursors 0.5 M of ferric (III) chloride and 0.25 M of manganese (II) chloride, were taken in the ratio of 1:0.5 which were initially dissolved separately in 10 ml of nitrogen (N2) degassed deionized (DI) water and mixed with 1.5 M solution of 40 ml NaOH. The process was carried out for 1.5 h at 80 °C. The resultant precipitate was separated using a NdFeB strong magnet and washed with DI water. This pure NPs (200 µl) were further dispersed in 1 ml of DI water and 400 µl of dimethyl sulfoxide (DMSO) at 75 °C for 3 h in order to reduce the aggregation and employed for CSNPs formation.
Au nanoseeds
Au coating was carried out using gold seed solution which was prepared freshly by mixing 0.5 ml (1 M) of cetyl trimethylammonium bromide (CTAB), 1 ml (50 mM) of AA and 100 µl (1 M) of the HAuCl4·3H2O solution. This solution complex mixture was sonicated for 30 min.
Mf@A CSNPs
The seed solution of Mf and Au were mixed in the ratio of 1:5 for the synthesis of CSNPs. Initially, the Au seed solution was added dropwise to the Mf core seed solution and stirred for 4 h until the solution turned to purple.
Nanoparticle functionalizations
Fa attachment
The attachment was carried out by mixing 1 ml of activated Fa [Citation34] and 10 ml of CSNPs under N2 atmosphere for 5 h and the stirring is continued for 24 h without N2. After the process, the reaction solution was filtered and dialysed (3000 kDa dialysis membrane) to exclude unreacted Fa using phosphate-buffered saline (PBS) buffer. The pellet was collected and dispersed in DI water after the centrifugation process.
Dox binding
To bind Dox, 5 ml Fa-CSNPs was mixed with 1 ml of triethylamine and 5 ml of DMSO as a solvent; finally, 400 µl of 5 mM Dox solution were added. Then the N2 gas is passed under the condition of continuous stirring at a temperature of 60 °C for 5 h. Finally, the excess of Dox molecules are removed from the solution using dialysis.
Results and discussions of optimization and characterization of CTAB stabilized MnFe2O4@Au CSNPs
Formation mechanism of core/shell nanoparticles
A novel seed-mediated approach for CTAB stabilized Mf@A CSNPs involves gold seed particles, which constitutes a surfactant (CTAB), gold ions and a mild reducing agent AA. Each constituent plays a critical role in determining the surface chemistry of the particles. CTAB is comprised of two head groups: one interacts with the gold anionic surface electrostatically by its quaternary ammonium (cationic) head groups, while the other, which possesses the surfactant head group, interacts with the aqueous solution. There are three distinct interfaces formed that are energetically favorable:
the gold nuclei-CTAB interface,
CTAB bilayer,
outer leaflet of CTAB exposed to the aqueous solution.
The role of CTAB in the formation of CSNPs is as follows. Firstly, it forms a staunch complex with HAuCl4, diminishing its reduction. Secondly, it acts like a protection barrier for gold ions by forming a metallomicellar complex. Thirdly, it adsorbs on the surface of the magnetic core and allows slow reduction of the gold complex to form a shell. When the molar ratio of AA and HAuCl4 exceeds 1.2, the reduction rate is enhanced and a uniform shell layer forms on the surface of the inner Mf core. At room temperature, there was no uniform shell formation on the surface of the core; hence, a mild temperature of 80 °C is very important to form a uniform layer of the outer shell. CSNPs is thermodynamically and potentially very favorable, since the shell metal, [AuBr2], has a higher reduction potential (+0.962 V) as compared to the core metal (−0.447 V). This leads to a reduction-transmetalation reaction, forming a thin gold shell on the surface of the core. Black-coloured Mf NPs became dark-purple in color when the light violet-colored gold seed solution was mixed with the Mf seed solution. Gold seeds supply a large number of small randomly oriented crystalline domains, leading to the seed-mediated growth of the Au shell. The gold seeds initially formed on the core, increase in diameter until a continuous shell is formed on the iron oxide surface, followed by outward growth of the shell. Particle-mediated electron transfer occurs and facilitates the formation of the shell.
Structural and magnetic characterization of Mf@A NPs
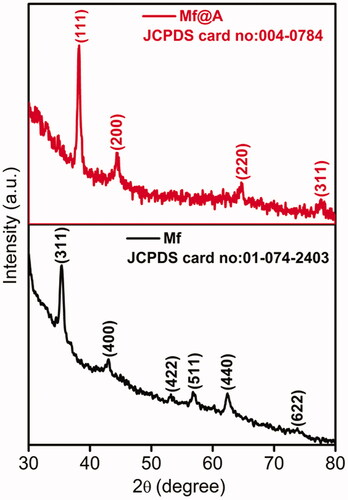
X-ray powder diffraction (XRD) analysis was carried out to detect the purity and phase crystallinity of the synthesized Mf and Mf@A (). The XRD pattern of Mf cubic spinel phase (JCPDS card no. 01-074-2403) exhibiting diffraction peaks with well-defined lattice planes of (311), (400), (422), (511), (440), and (622) respectively [Citation35,Citation36]. The XRD pattern of Mf@A, having diffraction peaks at 2θ 38.15°, 44.32°, 65°, and 77.6° which was indexed to (111), (200), (220), and (311) planes of Au (JCPDS card no. 004-0784) leading to the formation of CSNPs [Citation26]. But the Au peaks are dominant which is due to heavy metal atom effect [Citation37] due to the formation of Mf@A CSNPs. The presence of only one Mf peak denotes that there is a complete layer of Au is formed on the oxide core which can be further proved by our TEM data. Furthermore, the average crystalline size of Mf and Mf@A were calculated by considering full width at half maximum using the Scherrer equation which was found to be about 11.3 and 14.9 nm, respectively, coinciding with those of TEM images.
Figure 2. XRD spectrum representing the formation of Mf and Mf@A. Mf: manganese ferrite; Mf@A: MnFe2O4@Au nanoparticles; XRD: X-ray powder diffraction.
TEM observations of the Mf and Mf@A are shown in and . depicts that the Mf are spherical in shape and the size ranges from 13 to 18 nm with high aggregation due to magnetic nature of core NPs and HRTEM of single Mf nanoparticle. depicts the TEM image of Mf@A. And the Mf@A size is increased ≈1.5-fold from the diameter of the core particle because of the conjugation on nanogold onto the surface of the core NPs leading to the formation of CSNPs. High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) analysis () clearly distinguish the core and the shell. Due to thick Au shell formation continuously onto the surface of core NPs which makes it stable by enhancing steric stabilization and avoiding aggregation of particles.
Figure 3. TEM image of the core (size: 13–18 nm) and HRTEM image of single nanoparticle Mf. HRTEM: high resolution transmission electron microscopy; Mf: manganese ferrite.
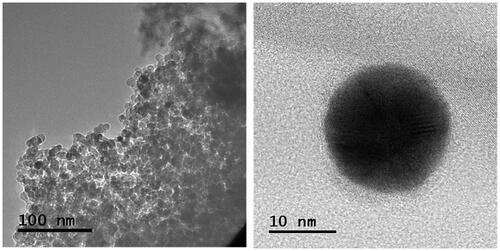
Figure 4. (a) TEM of Mf@A, (b) HRTEM of Mf@A which consists of very thin Au shell (3–4 nm) and a magnetic core, (c) core–shell confirmation by line mapping showing the presence of all elements (inset: scan survey of nanoparticle). HRTEM: high resolution transmission electron microscopy; Mf: manganese ferrite; Mf@A: MnFe2O4@Au nanoparticles.
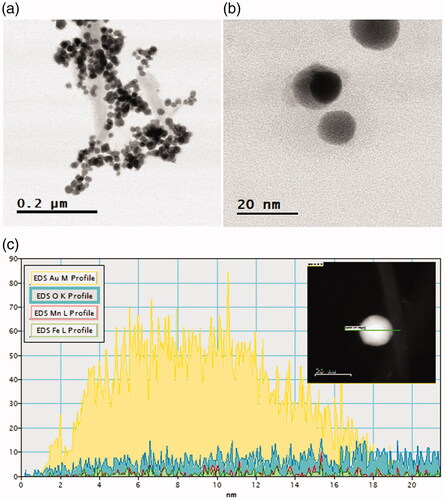
Line scanning for a single CSNPs was collected to determine the composition and distribution of elements. shows the line scan of Mf@A NPs which confirms that Fe and Mn ions are concentrated in the core and Au shell signal is exhibited from the outside of the NPs forming a complex. The inset image represents the scan survey of Mf@A. From the spectra, it interferes that the intensities of the Au were dominated when it is compared with Mf peaks which clearly indicates that the particles are coated with thick layer of Au by correlating with the obtained molar ratios of Au0 to Mf.
Elemental composition of Mf@A NPs
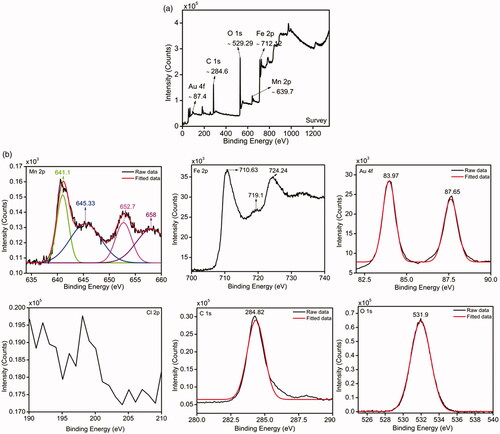
Binding energies and composition of Mf@A nanostructure were detected by using X-ray photoelectron spectroscopy (XPS) measurements. represents the wide-scan of XPS spectrum demonstrating the existence of Mn, Fe, Au, C, and O elements with the range from 0 to ≈1300 eV of binding energies. represents the high-resolution core level spectra of individual elements. The oxidation states of Mn ions are observed which is present in the states of Mn2p3/2 and Mn2p1/2 electrons show binding energies at 641.1 and 652.7 eV [Citation38]. Additionally, there are two more strong peaks observed at 645.33 and 658 eV. These binding energies represent to Mn3+, but due to oxygen, Mn2+ is formed because of oxidation process [Citation39,Citation40]. Fe 2p3/2 and 2p1/2 peaks from Fe3+ ions are situated at around 710.63 and 724.24 eV which are broadened, and a small weak-up were observed at 719.1 eV [Citation41]. The 2p peaks of Mn and Fe are due to the spin-orbit coupling and electrostatic interactions between their core 2p and also because of their unpaired 3d electrons [Citation38,Citation42]. Au binding energies were deconvoluted in two prominent peaks at 83.97 and 87.65 eV which directly denoted the Au state of Au4f7/2 and Au4f5/2 bands respectively with no doublets at 86.8 and 90.5 eV. Interestingly, the spectrum does not show any detectable Cl signal which usually within the bonding energy ranges from 196 to 204 eV. Thus it proves that there is a complete reduction of Au (III) to Au (0) onto Mf which is very well confirmed from TEM analysis [Citation43–46]. The O1s peak (531.9 eV) can be seen which corresponds to the surface component oxygen atoms. In this case of XPS analysis, C1s peak of carbon atom binding energy was taken as a reference which is 284.82 eV. Therefore, from the XPS pattern, it is very well in good agreement with XRD data and TEM results which reveals the formation of Mf@A CSNPs.
Figure 5. (a) Scan survey and (b) elemental analysis of Mf@A by XPS which clearly shows the presence of elements such as Mn, Fe, Au, C, O, and absence of Cl. Mf@A: MnFe2O4@Au nanoparticles; XPS: X-ray photoelectron spectroscopy
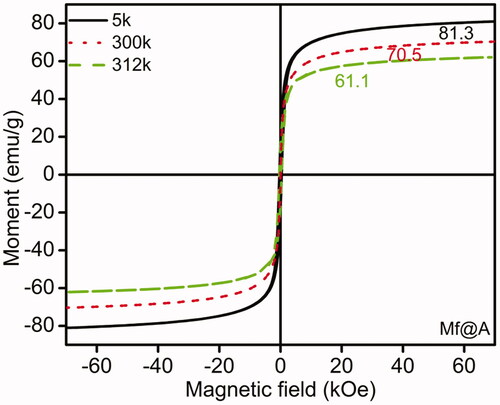
The magnetic properties of Mf@A were evaluated at various temperatures. shows the magnetization field-dependent [M(H)] curves of Mf@A obtained at 5, 300, and 312 K showing the superparamagnetism which is the absence of “magnetic memory”, even at room temperature by retaining the magnetic property of Mf cores. Here it can be noticed a decrease in magnetic saturation (MS), from 81.3 to 61.1 emu/g from 5 to 312 K which is due to Au shell. Even though the MS value is decreased, but it is still high enough to be manipulated under external magnetic field for the applications of MRI and hyperthermia.
Bioconjugation of Fa linker and Dox molecules on Mf@A
Optical analysis
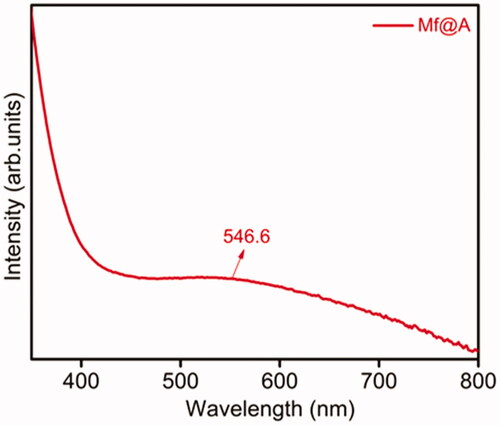
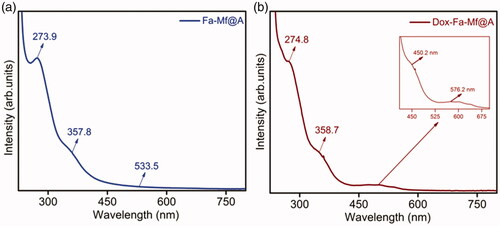
The surface plasmon resonance (SPR) property of CSNPs provides the initial confirmation of formation of Mf@A. and show the UV–vis absorption spectra measured for Mf@A, Fa-functionalized Mf@A and Dox-coated Fa-functionalized Mf@A NPs. The synthesized Mf@A shows prominent optical plasmon absorption band at λmax = 546.6 nm () which was red-shifted from GNPs values because of the Au nanoshell formation onto the Mf cores. Mf@A CSNPs are functionalized with Fa onto the surface to enhance the target specificity of NPs to the laryngeal carcinoma cells which express high folate receptors [Citation47]. Fa functionalization on Mf@A NPs () was confirmed from the optical analysis which shows a peak at 273.9 and 357.8 nm which is Fa signature marker absorption wavelength [Citation48] with minor blue-shift due to Au shell. And the distinct intensity of Mf@A NPs was seen at 533.5 nm which proves the Fa-Mf@A complex formation. Finally, Dox attachment was confirmed from the peak (inset) at 450.2 nm and Mf@A peak at 576.2 nm and Fa attachment from the two clear peaks at 274.8 nm a slight blue-shift and 358.7 nm which ensure the formation of Dox-Fa-Mf@A NPs complex ().
Spectroscopy analysis
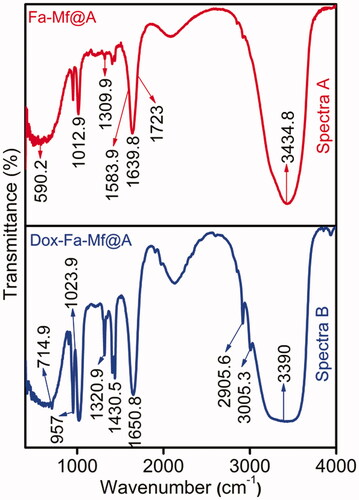
FTIR spectra have been carried out for the samples to confirm the chemical design and attachment of organic molecules onto the NPs surface at frequency 400–4000 cm−1. After the removal of excess CTAB from the Mf@A CSNPs surface, it is bioconjugated with Fa and encapsulated with Dox (). Spectra A depicts the peaks of activated Fa showing bands at 1639.8 and 1723 cm−1 of –CH and –NH stretch respectively. Fa conjugation to Mf@A NPs is by asymmetric stretching of primary amines –NH and bending vibrations of –CO confirming the formation of an amide linkage between Fa and Mf@A NPs at 1583.9 cm−1. Spectra B represents the immobilization of Dox moieties to Fa-Mf@A NPs. The interaction between these moieties are purely based on –NH amide group and –COOH carboxylic groups of Fa and Dox respectively. Bands representing the attachments are 1430.5 cm−1 anhydride and 1650.8 cm−1 amide stretching of =CO. The peaks at 2905.6 cm−1 are of secondary and 3005.3 cm−1 of secondary and primary –NH2 bending respectively [Citation34].
Zeta potential, cytotoxicity studies of nanoparticle complexes
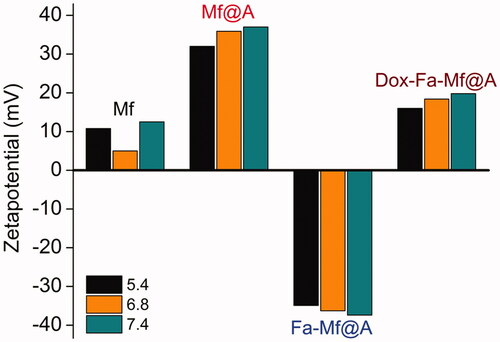
depicts zeta (ζ) potential measurements as a function of pH for bare Mf, Mf@A, Fa-functionalized Mf@A, and Dox-coated Fa-functionalized Mf@A NPs. All these NPs pHs are adjusted to 5.4, 6.8, and 7.4 as the drug delivery study is mainly carried out in these ranges and the measurements are recorded after 24 h. In case of bare Mf NPs, it is inferred that in all three different pH the ζ potential value lies in the range of positive, due to heavy aggregation because of superparamagnetic behavior which is already confirmed by means of SQUID analysis (+10.8 to +12.5 mV). But, interestingly after the Au coating, the ζ value is increased to the positive range by stabilizing the NPs ranging from +32 to +37 mV which is due to the presence of CTA+ ions on the surface of CSNPs. The functionalization of Fa increases the ζ value in the negative mV depicting that the protein moieties attachment changes the surface charge which confirms the stabilization of Fa-Mf@A complex. Finally, Dox-Fa-Mf@A complex also shows the ζ value to be in the positive range of +16 to +19.8 mV because of Dox attachment which is inherent with a positive charge which provides the perfect stability and avoiding aggregation of NPs complex by electrostatic repulsion [Citation34]. So, this favors the use of this complex as a drug delivery vehicle.
Figure 10. Zeta potential of Mf, Mf@A, Fa-functionalized Mf@A and Dox immobilized Fa-Mf@A. Dox: doxorubicin; Mf: manganese ferrite; Mf@A: MnFe2O4@Au nanoparticles.
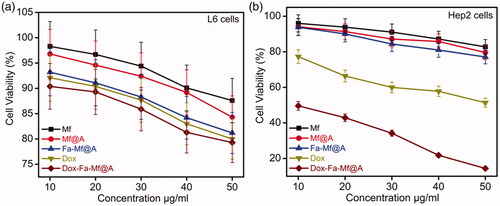
MTT assay was carried out to assess the cell viability by incubating L6 and Hep2 cells with bare Mf and Mf@A NPs for a period of 24 h in the concentration range of 10–50 μg/ml. shows the viability of cells which is more than 85% even at a relatively higher dose of NPs. Therefore, it is well proven that these NPs are highly biocompatible for biomedical applications. But as expected there is negligible cell death in case of Fa-functionalized Mf@A nanocluster. The cell viability was evaluated by inoculating free Dox and Dox-coated Fa-functionalized Mf@A in both L6 and Hep2 cells. depicts that free Dox shows minor cell death which confirms the side-effect of Dox to normal cells and negligible cell death with Dox-Fa-Mf@A complex. But clearly proves the purpose of Dox in the Hep2 cancer cell line by killing them and the activity of Dox is increased in case of Dox-Fa-Mf@A complex by showing apoptosis of 12%. This proves that the entry of Dox inside the nucleus is higher which induces DNA damage causing cell death by efficient Fa-functionalized Mf@A nanocargos.
Figure 11. MTT assay of (a) L6 cells with Mf, Mf@A, Fa-Mf@A, Dox, and Dox-Fa-Mf@A showing no apoptosis at even at higher concentrations, (b) Hep2 cells with only Dox and Dox-Fa-Mf@A showing around 88% cell death at a high concentration. Dox: doxorubicin; Mf: manganese ferrite; Mf@A: MnFe2O4@Au nanoparticles.
Nanoparticles internalization studies
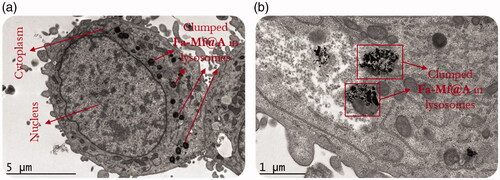
Further cryoEM analysis has been carried out to confirm NPs internalization inside the cell instead of just interaction with the cytoplasm is shown in . This entry of NPs is based on a mechanism as follows [Citation49]:
Figure 12. (a) CryoEM image showing the Fa-Mf@A clusters, (b) High-resolution CryoEM image showing the engulfed cluster of Fa-Mf@A inside the lysosome by the process of endocytosis. Mf@A: MnFe2O4@Au nanoparticles.
Initially the NPs attach onto the surface of the cell which causes intussusception of the plasma membrane,
Then forming endocytic cavities,
Finally internalized into the cell.
shows the high-magnification image of NPs clumping inside the lysosomes. This is because in the tumor region the pH is acidic (pH 4–6) causing aggregation of NPs. This proves that Fa-Mf@A NPs passed through the cellular membrane by process of an endocytic mechanism [Citation50,Citation51].
Dox intracellular distribution
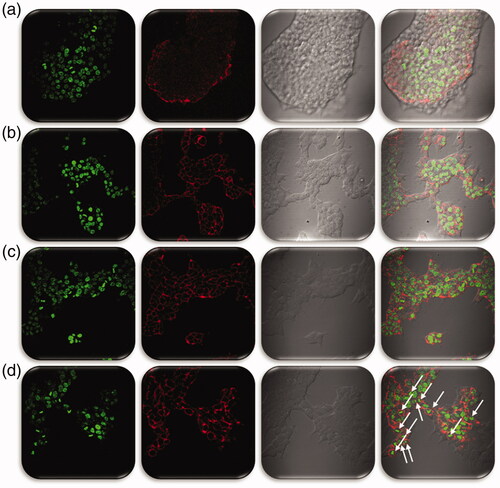
From the cryoEM studies, we confirmed the entry of Fa attached NPs. So, furthermore to evaluate the process of Dox internalization inside the Hep2 cells were studied by incubating Dox-Fa-Mf@A NPs and exploiting the fluorescent character of Dox moieties at various time intervals by confocal microscopy which is shown in .
Figure 13. Confocal microscopy images of Hep2 cells incubated with Dox-Fa-Mf@A NPs for 0, 6, 12, and 24 h (BF: bright field, scale bar: 25 µm). Dox: doxorubicin; Mf@A: MnFe2O4@Au nanoparticles.
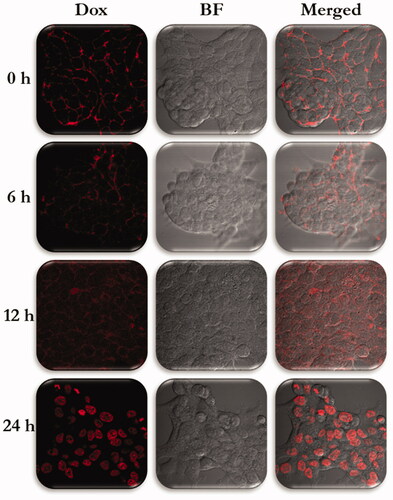
0 h: Initially, after incubation of NPs, a lot of fluorescent spots were observed around the cellular membrane with no florescence seen in the nucleus.
6 h: After 6 h, the weak signal of Dox is seen from the cytoplasm which confirms the endocytosis pathway of NPs internalization.
12 h: The fluorescent intensity of Dox is increased all over the cell and the intensity difference is seen very clearly from the 0 and 12 h.
24 h: After prolonged incubation of 24 h, Dox florescence in nuclei became very strong, with no signal from the cytoplasm. This is due to change in pH which cleaves the bond between the Fa and Dox moieties.
These results indicated that Dox attached NPs complex showed Mf@A acts a potential nanocargo and specifically targets the Hep2 cells.
Cellular and nuclear morphology studies
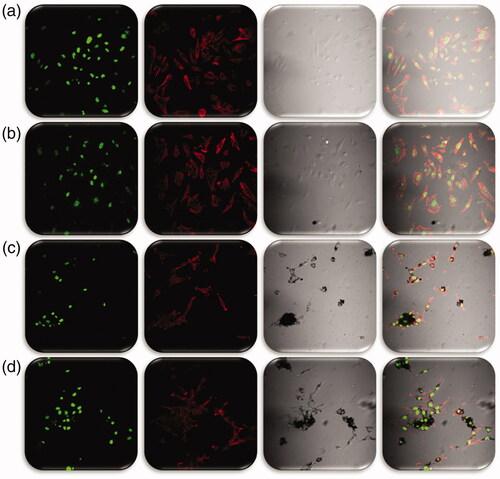
To evaluate the cellular and nuclear morphology of L6 and Hep2 cells incubated with Mf, Mf@A, Fa-Mf@A and also Dox-Fa-Mf@A potential application it was visualized under confocal microscopy. shows the morphologies treated with Mf, Mf@A, and Fa-Mf@A which does not show any noticeable changes in both the type of cell lines. But the activity of Dox-Fa-Mf@A was shown in and , in which L6 cells are not been affected much but the Hep2 cells show major apoptosis by disruption of nuclear membrane and the whole cell damage (). This proves that the Dox-Fa-Mf@A acts as an efficient nanocargo in delivering the Dox which causes cell death.
Applications of Mf@A
In vitro Dox release
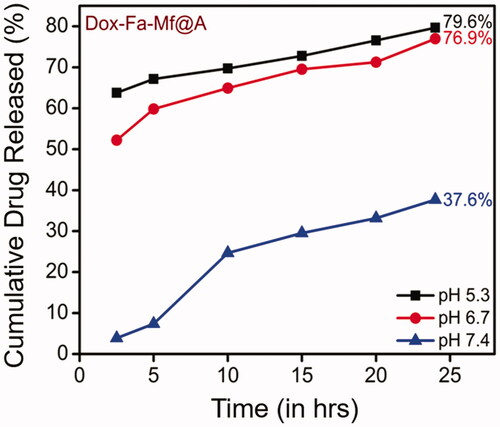
The drug loading efficiency (%) was calculated before initiating the drug release studies which was found to be 97.5% (calculation is shown in ESI). Then, the in vitro drug release performance of Dox-Fa-Mf@A NPs was investigated by employing at three different pH of PBS buffers (5.4, 6.8, and 7.4) at room temperature for 24 h. The pH based studies were evaluated because cancer cells show lower pH profile when compared to normal cells due to the formation of lactic acid because of a lesser amount of blood flow which decreases both the oxygen and nutrients content which is known as Warburg effect [Citation52]. We determined that our NPs system is pH sensitive which makes it a distinctive drug delivery system. Therefore, as the pH decreases in the cellular organelles thereby increasing the release of drugs which is shown in . The major benefits of pH depend drug delivery system is that the early release of drug moieties inside the cell can be avoided, increased drug loading and release rate and finally dosage [Citation53,Citation54]. The Dox cumulative release at three different pH after 24 h was found to be 79.6% at pH 5.4, 76.9% at pH 6.8 and 37.6% at pH 7.4. At pH 5.4 shows the maximum amount of drug release when compared to pH 6.8 and 7.4. This difference is due to pH decrement inside the cell organelle (acidic pH) which lead to dissociation of the drug from the complex by the breaking the amide bond between Fa and Dox molecules [Citation55].
Drug kinetic models
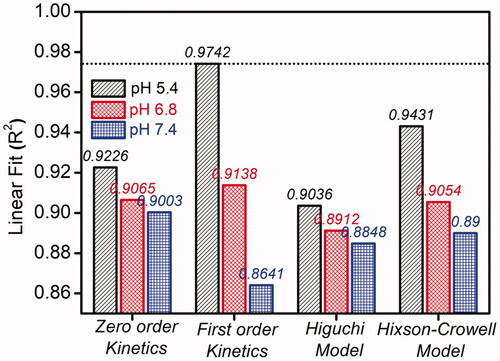
By obtaining the above in vitro release elucidation, these results were analyzed with different mathematical drug kinetics models such as zero order, first order, Higuchi and Hixon-Crowell model to predict the drug release rates and diffusion characteristics () by calculating the R2 (coefficient of determination) values for each model employing the formula [Citation56] which is shown in ESI.
Usually, the various kinetic models denote the amount of dissolved drug corresponding to the function of time [Citation57]. From the calculations, it is found that the first order rate kinetics expressed very high regression coefficient value of R2 = 0.9742. Thus, this model is considered to be the best model for this NPs based drug release of Dox which clearly signifies that the drug release rate depends on its concentration. So, this proves Dox-Fa-Mf@A NPs system is more efficient as a nanocargo for drug delivery.
Microwave ablation therapy
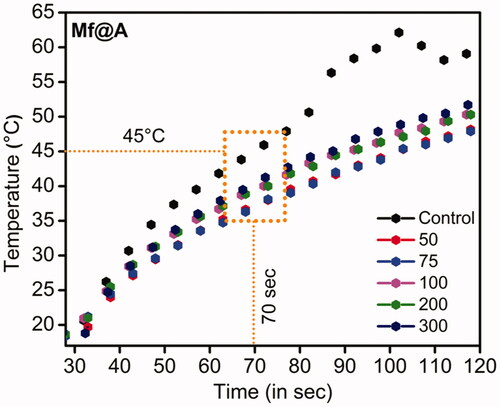
Mf@A particles were tested as a hyperthermal agent under Mw irradiation. The choice of Mw was because the higher temperature produced by electromagnetic field leads to complete annihilation of tissues by causing irretrievable cell injury by the mechanism of apoptosis and necrosis. The increment of temperature is produced by the concept of rotating dipoles or dielectric hysteresis [Citation58]. So, the increment of temperature as a function of time has been carried out at a frequency of 2.45 GHz to induce localized hyperthermia by employing an interstitial antenna which acts as a source from the generator power to the NPs sample. The Mf@A were dispersed in PBS, the applicator was inserted with the temperature probe, and Mw was irradiated using home-made setup for 120 s at 6 W. It is observed that the temperature increment was very rapid and reached 45 ± 1 °C around 70 s and temperature raised up to 51 ± 1.5 °C within 120 s for Mf@A which is shown in . Here the water was used as a control which shows the maximum absorption of Mw [Citation59] and the temperature increment was maximum. This temperature completely depends on the Au iteration on Mf particles. The Au coating on the core surface retains the superparamagnetic behavior of Mf NPs which leads to increment of energy by magnetic anisotropy within the Au nanoshell [Citation60]. The obtained result is compared with gold-coated maghemite CSNPs where the temperature increment was 38 °C (10 min, 120 W) at 2.45 GHz in water [Citation61].
Conclusion
In the present work, a biomedical platform of Mf@A comprised of an inorganic core NPs and a biocompatible surface coating was engineering which can provide enough stabilization under physiological conditions to perform multiple functions simultaneously, such as in multimodal imaging, drug delivery, and real-time monitoring, as well as combined therapeutic approaches.
Therefore, Mf@A CSNPs were used as able paraphernalia for the docking of an anti-cancer drug such as Dox using Fa as a linker for the attachment. The attachment could be monitored using UV–vis spectroscopy. HAADF-STEM and line mapping confirm the formation of CSNPs structure. SQUID confirms the CSNPs are highly superparamagnetic. The stability of Mf@A NPs was scrutinized by measuring the zeta potential measurements. The amalgamation of the drug along with activated Fa as a navigational molecule is the critical phase for targeted drug delivery. Attachments were verified using FTIR which confirmed the formation of non-covalent interactions. Mf@A-Fa-Dox complex was found to be non-toxic for normal cells and considerably toxic for Hep2 cells detected by confocal microscopy and MTT assay. Then the complex was employed for drug delivery and hyperthermia studies. The drug loading capacity was found to be more than 90%. Drug-release was carried out at three different pH like 5.4, 6.8, and 7.4 and found that at pH 5.4 and 6.8 the release is maximum which is favorable for cancer cell treatment. The mathematical modeling studies show it follows the first order rate kinetics with high R2 values. Finally, under the Mw, the CSNPs produced enough heat to cause both apoptosis and necrosis in a shot span of time. This proves the designed CSNPs can be a potential candidate for various biological applications especially in the field of cancer theranostics.
Acknowledgements
M. Ravichandran is thankful and also acknowledge his posdoctoral fellowship from Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México (UNAM). The authors would like to thank Carlos Vazquez Calzada for performing confocal microscopy, Norma Barragan Andrade for carrying out cellular experiments, Dr. Francisco Garcia-Sierra for interpreting cellular experiments studies, and Dr. Marco A. Garza-Navarro for SQUID analysis. We greatly thank Dr. Sergio Armando Tomas for XPS, Jaime E Lara & J. H. Zepeda Peralta for hyperthermia technique, Marcela Guerrero for XRD analysis & FTIR spectroscopy measurements, Alvaro Angeles Pascual from LANE, for HRTEM and related techniques.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aftab S, Shah A, Nadhman A, et al. Nanomedicine: an effective tool in cancer therapy. Int J Pharm. 2018;540:132–149.
- Chen G, Wang Y, Xie R, et al. A review on core–shell structured unimolecular nanoparticles for biomedical applications. Adv Drug Deliv Rev. 2018;130:58–72.
- Sau S, Alsaab HO, Bhise K, et al. Multifunctional nanoparticles for cancer immunotherapy: a groundbreaking approach for reprogramming malfunctioned tumor environment. J Control Release. 2018;274:24–34.
- Zhou Q, Zhang L, Wu H. Nanomaterials for cancer therapies. Nanotechnol Rev. 2017;6:473–496.
- Ataee-Esfahani H, Wang L, Nemoto Y, et al. Synthesis of bimetallic Au@Pt nanoparticles with Au core and nanostructured Pt shell toward highly active electrocatalysts. Chem Mater. 2010;22:6310–6318.
- Wang L, Yamauchi Y. Strategic synthesis of trimetallic Au@Pd@Pt core–shell nanoparticles from poly(vinylpyrrolidone)-based aqueous solution toward highly active electrocatalysts. Chem Mater. 2011;23:2457–2465.
- Reddy LH, Arias JL, Nicolas J, et al. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112:5818–5878.
- Oh Y, Lee N, Kang HW, et al. In vitro study on apoptotic cell death by effective magnetic hyperthermia with chitosan-coated MnFe2O4. Nanotechnology. 2016;27:115101.
- Wang G, Chen G, Wei Z, et al. Multifunctional Fe3O4/graphene oxide nanocomposites for magnetic resonance imaging and drug delivery. Mater Chem Phys. 2013;141:997–1004.
- Wang G, Chang Y, Wang L, et al. Preparation and characterization of PVPI-coated Fe3O4nanoparticles as an MRI contrast agent. J Magn Magn Mater. 2013;340:57–60.
- Chalkidou A, Simeonidis K, Angelakeris M, et al. In vitro application of Fe/MgO nanoparticles as magnetically mediated hyperthermia agents for cancer treatment. J Magn Magn Mater. 2011;323:775–780.
- Laurent S, Dutz S, Häfeli UO, et al. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23.
- Majeed MI, Lu Q, Yan W, et al. Highly water-soluble magnetic iron oxide (Fe3O4) nanoparticles for drug delivery: enhanced in vitro therapeutic efficacy of doxorubicin and MION conjugates. J Mater Chem B. 2013;1:2874–2884.
- Chomoucka J, Drbohlavova J, Huska D, et al. Magnetic nanoparticles and targeted drug delivering. Pharmacol Res. 2010;62:144–149.
- Goza M, Ravichandran P, Jagadale G, et al., Chapter 14 – inorganic nanoflotillas as engineered particles for drug and gene delivery A2. In: Grumezescu AM, editor. Engineering of nanobiomaterials. Amsterdam, Netherlands: Elsevier Inc.; 2016. p. 429–483.
- Ye M, Wei Z, Hu F, et al. Fast assembling microarrays of superparamagnetic Fe3O4@Au nanoparticle clusters as reproducible substrates for surface-enhanced Raman scattering. Nanoscale. 2015;7:13427–13437.
- Wang C, Xu J, Wang J, et al. Polyethylenimine-interlayered silver-shell magnetic-core microspheres as multifunctional SERS substrates. J Mater Chem C. 2015;3:8684–8693.
- Kouassi GK, Irudayaraj J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal Chem. 2006;78:3234–3241.
- Wang J, Wu X, Wang C, et al. Magnetically assisted surface-enhanced Raman spectroscopy for the detection of Staphylococcus aureus based on aptamer recognition. ACS Appl Mater Interfaces. 2015;7:20919–20929.
- Sun Z, Du J, Yan L, et al. Multifunctional Fe3O4@SiO2-Au satellite structured SERS probe for charge selective detection of food dyes. ACS Appl Mater Interfaces. 2016;8:3056–3062.
- Malynych S, Luzinov I, Chumanov G. Poly(vinyl pyridine) as a universal surface modifier for immobilization of nanoparticles. J Phys Chem B. 2002;106:1280–1285.
- Arvizo RR, Bhattacharyya S, Kudgus RA, et al. Intrinsic therapeutic applications of noble metal nanoparticles: past, present and future. Chem Soc Rev. 2012;43:2970.
- Spasova M, Salgueiriño-Maceira V, Schlachter A, et al. Magnetic and optical tunable microspheres with a magnetite/gold nanoparticle shell. J Mater Chem. 2005;15:2095–2098.
- Cho SJ, Shahin AM, Long GJ, et al. Magnetic and mossbauer spectral study of core/shell structured Fe/Au nanoparticles. Chem Mater. 2006;18:960–967.
- Wang L, Luo J, Fan Q, et al. Monodispersed core–shell Fe3O4@Au nanoparticles. J Phys Chem B. 2005;109:21593–21601.
- Wang J, Wu X, Wang C, et al. Facile synthesis of Au-coated magnetic nanoparticles and their application in bacteria detection via a SERS method. ACS Appl Mater Interfaces. 2016;8:19958–19967.
- Pal M, Rakshit R, Mandal K. Surface modification of MnFe2O4nanoparticles to impart intrinsic multiple fluorescence and novel photocatalytic properties. ACS Appl Mater Interfaces. 2014;6:4903–4910.
- Ahmad T, Iqbal Y, Bae H, et al. Relaxivities of hydrogen protons in aqueous solutions of gold-coated manganese ferrite nanoparticles. J Korean Phys Soc. 2013;62:1696–1701.
- Gallo J, García I, Padro D, et al. Water-soluble magnetic glyconanoparticles based on metal-doped ferrites coated with gold: synthesis and characterization. J Mater Chem. 2010;20:10010–10020.
- Pandey S, Oza G, Mewada A, et al. Folic acid mediated synaphic delivery of doxorubicin using biogenic gold nanoparticles anchored to biological linkers. J Mater Chem B. 2013;1:1361.
- Kamen BA, Capdevila A. Receptor-mediated folate accumulation is regulated by the cellular folate content. Proc Natl Acad Sci USA 1986;83:5983–5987.
- Kong G, Anyarambhatla G, Petros WP, et al. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: importance of triggered drug release. Cancer Res 2000;60:6950–6957.
- R. Manisekaran Nano-flotillas MnFe2O4@Au core–shell nanoparticles: an efficient MRI contrast agent, magneto-hyperthermal and drug-delivery armada for cancer. Cham: Springer; 2018, pp. 139–159.
- Ravichandran M, Oza G, Velumani S, et al. Plasmonic/magnetic multifunctional nanoplatform for cancer theranostics. Sci Rep. 2016;6:1–15.
- Stolyarchuk IL, Dolgikh LY, Vasilenko IV, et al. Catalysis of steam reforming of ethanol by nanosized manganese ferrite for hydrogen production. Theor Exp Chem. 2012;48:129–134.
- Kardar ZS, Beyki MH, Shemirani F. Takovite-aluminosilicate@MnFe2O4 nanocomposite, a novel magnetic adsorbent for efficient preconcentration of lead ions in food samples. Food Chem. 2016;209:241–247.
- Teng X, Black D, Watkins NJ, et al. Platinum-maghemite core–shell nanoparticles using a sequential synthesis. Nano Lett. 2003;3:261–264.
- Nesbitt HW, Banerjee D. Interpretation of XPS Mn(2p) spectra of Mn oxyhydroxides and constraints on the mechanism of MnO2precipitation. Am Mineral. 1998;83:305–315.
- Huang CC, Khu NH, Yeh CS. The characteristics of sub 10 nm manganese oxide T1 contrast agents of different nanostructured morphologies. Biomaterials 2010;31:4073–4078.
- Kim T, Cho E-J, Chae Y, et al. Urchin-shaped manganese oxide nanoparticles as pH-responsive activatable T1 contrast agents for magnetic resonance imaging. Angew Chem Int Ed. 2011;50:10589–10593.
- Li Z, Wang SX, Sun Q, et al. Ultrasmall manganese ferrite nanoparticles as positive contrast agent for magnetic resonance imaging. Adv Healthc Mater. 2013;2:958–964.
- Grosvenor AP, Kobe BA, Biesinger MC, et al. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf Interface Anal. 2004;36:1564–1574.
- Li X, Zhu X-H, Fang Y, et al. Programmed synthesis of magnetic mesoporous silica nanotubes with tiny Au nanoparticles: a highly novel catalyst system. J Mater Chem A. 2014;2:10485.
- Zhu Y, Shen J, Zhou K, et al. Multifunctional magnetic composite microspheres with in situ growth Au nanoparticles: a highly efficient catalyst system. J Phys Chem C. 2011;115:1614–1619.
- Xiong R, Wang Y, Zhang X, et al. In situ growth of gold nanoparticles on magnetic γ-Fe2O3@cellulose nanocomposites: a highly active and recyclable catalyst for reduction of 4-nitrophenol. RSC Adv. 2014;4:6454–6462.
- Fernandes C, Pereira C, Guedes A, et al. Gold nanoparticles decorated on bingel-thiol functionalized Multiwall carbon nanotubes as an efficient and robust catalyst. Appl Catal A Gen. 2014;486:150–158.
- Xie M, Zhang H, Xu Y, et al. Expression of folate receptors in nasopharyngeal and laryngeal carcinoma and folate receptor-mediated endocytosis by molecular targeted nanomedicine. Int J Nanomed. 2013;8:2443–2451.
- Dántola ML, Denofrio MP, Zurbano B, et al. Mechanism of photooxidation of folic acid sensitized by unconjugated pterins. Photochem Photobiol Sci. 2010;9:1604–1612.
- Zhang C, Wängler B, Morgenstern B, et al. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir. 2007;23:1427–1434.
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625.
- Dausend J, Musyanovych A, Dass M, et al. Uptake mechanism of oppositely charged fluorescent nanoparticles in Hela cells. Macromol Biosci. 2008;8:1135–1143.
- Karimi M, Ghasemi A, Sahandi Zangabad P, et al. Smart micro/nanoparticles in stimulus-responsive drug/gene delivery systems. Chem Soc Rev. 2016;45:1457.
- Rasouli S, Davaran S, Rasouli F, et al. Synthesis, characterization and pH-controllable methotrexate release from biocompatible polymer/silica nanocomposite for anticancer drug delivery. Drug Deliv. 2014;21:155–163.
- Wang Y, Chen L, Tan L, et al. PEG-PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials 2014;35:6972–6985.
- H.D.G and VRWA, Yong S. Cho Encyclopedia of nanoscience and nanotechnology, Vol.1 California, USA: American Scientific Publishers; 2004.
- Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–133.
- Shaikh HK, Kshirsagar RV, Patil SG. Mathematical models for drug release characterization: a review. World J Pharm Pharm Sci. 2015;4:324–338.
- Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208.
- Kim DK, Amin MS, Elborai S, et al. Energy absorption of superparamagnetic iron oxide nanoparticles by microwave irradiation. J Appl Phys. 2005;97:10J510–10J513.
- Mohammad F, Balaji G, Weber A, et al. Influence of gold nanoshell on hyperthermia of super paramagnetic iron oxide nanoparticles (SPIONs). J Phys Chem C Nanomater Interfaces. 2010;114:19194–19201.
- Pearce JA, Cook JR, Emelianov SY. Ferrimagnetic nanoparticles enhance microwave heating for tumor hyperthermia therapy. 2010 Annu Int Conf IEEE Eng Med Biol Soc EMBC. 2010;10:2751–2754.