Abstract
Background
Achieved Silica Nanoparticles (SiNPs) to encapsulate the photosensitizer [Protoporphyrin IX (PpIX)] in photodynamic therapy (PDT) application was reported in this research.
Materials and Methods
Cytotoxicity for five different concentrations of encapsulated and naked PpIX was measured. Optimum concentration and optimum exposure time of encapsulated and naked PpIX that needed to destroy the cells (Osteosarcoma cells) was measured.
Results
The results showed that the encapsulated PpIX has more efficacy compared to the naked PpIX and the applicability of the encapsulated PpIX-SiNPs was proved on osteosarcoma cells.
Conclusion
The results established the important in-vitro photodynamic effectiveness of PpIX-SiNP, which may open a new application for PpIX in its clinical and in-vitro studies.
Introduction
Photodynamic therapy (PDT) is a new cancer treatment that depends on photosensitizer (PS) that absorbs the energy of the used light. The energy is transferred to produce an excited singlet state (1O2) after radiations interact with the PS. The singlet oxygen is reactive with the DNA of the cells and cause cell death [Citation1]. The main problem of using PDT is that the PS will be cleared rabidly by the reticulo-endothelial system in the biological system. To resolve this problem, nanoparticles were used to encapsulate the PS as a drug delivery system. The suitable design of the nanoparticle makes it has highly efficacious to encapsulate the used PS. PSs will be concentrated inside the nanoparticles. The PS will be kept longer in the biological system at needed concentrations [Citation2].
The main focus in choosing the suitable nanoparticles was on its high biocompatibility and its low toxicity. Silica nanoparticles (SiNPs) have suitable characteristics to be a delivery system and it can be easily synthesis at low temperatures [Citation3]. The outer surface of SiNPs can be contacted with biological compounds and the inner surface can be encapsulate the used PS [Citation4–6].
The aims of our work were to check the efficacy of the encapsulation on the PS compared to the naked PS and to measure the optimal exposure time of measured optimum concentration for the used PS. Protoporphyrin IX (PpIX) is one of the most important porphyrin derivatives to be PS in our research [Citation7–9]. PpIX begins its effect by absorbing the energy of the light; series of chemical reactions will produce the singlet oxygen. It induces cell destruction leading to destroy cancer cells [Citation10–12]. PpIX encapsulated by SiNPs was used in vitro to test its effect on osteosarcoma cells. Optimum concentration, optimal exposure and cytotoxicity of PpIX-SiNPs were measured. The efficacy of encapsulated PpIX was compared with naked PpIX after studying which was the most effective for different concentrations of PpIX and for different exposure times.
Materials and methods
Reverse-micellar method was used to achieve the encapsulate PpIX by SiNPs [Citation13,Citation14]. Different concentrations of PpIX (0.974, 0.478, 0.244, 0.122 and 0.061 µM as final concentrations) were encapsulated by SiNPs.
Osteosarcoma cell line was used in all of our experiments. Osteosarcoma cells were cultured in McCoy’s Medium which contains 10% fetal bovine serum (FBS) and 1% antibiotic (Penicillin), and under appropriate conditions (37 °C in a 5% CO2 environment). The cells were grown overnight; the cells were washed twice by using Phosphate buffered saline (PBS) and then were incubated in 5 ml fresh McCoy’s Medium contains PpIX-SiNPs for 6 h. All samples were exposed to the light source (Intensity ∼0.89 mW/cm2 arc lamp, 40 cm separating the sample and the light source) for 60 min. The medium was removed and the cells were washed twice with PBS. The flask was placed back in the incubator overnight after adding 3 ml of fresh McCoy’s Medium and then were counted. The cytotoxicity of the PpIX-SiNPs was measured by using haemocytometer.
After measuring the optimum concentration, optimum exposure time was measured by exposing it to the light source for various times (0, 15, 30, 45, 60, 75, 90, 105 and 120 min). Optimal concentration and optimal exposure time were measured for naked PpIX and all PpIX-SiNPs results were compared to results of naked PpIX.
Results
Cytotoxicity of encapsulated and naked PpIX on osteosarcoma cells
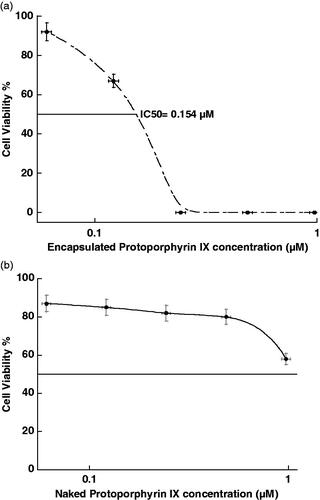
Cytotoxicity of the PpIX-SiNPs and naked PpIX for different concentrations was first tested on osteosarcoma cells to study the in vitro PDT effect of the encapsulated and naked PpIX. The cytotoxicity of PpIX-SiNPs and naked PpIX and half maximal inhibitory (IC50) concentration are shown in .
The optimum concentration of encapsulated and naked PpIX
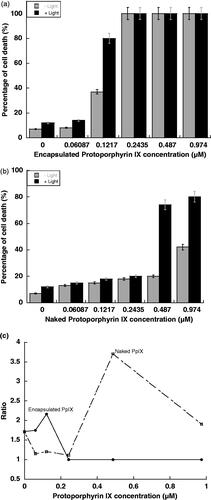
Haemocytometer was used to measure the percentage of cell death. Analysis of the cells treated by PpIX-SiNP at the different concentrations was implemented. With and without exposure to light for 120 min, the percentage of cell’s death was measured at different concentrations of PpIX-SiNPs and naked PpIX as shown in . The ratio of dead cell percentage between with and without light irradiation was measured and shown in .
The optimum exposure time for naked and encapsulated PpIX
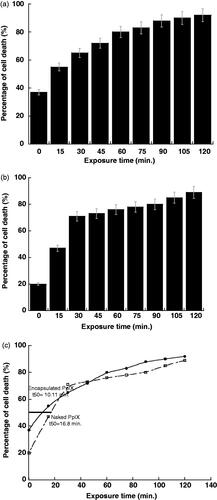
A series of experiments on ostersarcoma cells were done with treatment by 0.122 µM and 0.478 µM of PpIX-SiNP and naked PpIX, respectively to measure the optimal exposure time. The optimal concentration of encapsulated PpIX and naked PpIX were exposed to the light source for different exposure times and the changes in cell death are shown in . The optimum exposure time to destroy 50% of our cells was at 10.11 min for the encapsulated PpIX as shown in . But for naked PpIX, the optimum exposure time was 16.8 min.
Discussion
Encapsulated PpIX has more cytotoxicity than naked PpIX as shown in , the reason for that is the cytotoxicity of naked SiNPs in addition to the used chemicals (such as 1-Biotanol and Tween 80) that still contact with the outer surface of SiNPs after synthesis. Then we can use any of our concentrations of naked PpIX but for encapsulated PpIX we can use only below 0.154 µM concentration. However, the encapsulated PSs have high optimal concentration and exposure time toxicity efficacy compared to naked PSs to produce similar toxicity efficacy.
The ratio of dead cell percentage between with and without light irradiation in proved that the optimal concentration of PpIX-SiNP was 0.122 µM (optimal concentration at maximum ratio) and for naked PpIX was at 0.478 µM. That means the osteosarcoma need to be treated at least four times the concentration of naked PpIX to achieve approximately the same efficacy of PpIX-SiNPs.
shows that the PpIX encapsulated SiNPs destroyed the cells faster than naked PpIX where naked PpIX need more exposure time comparing to the encapsulated PpIX to destroy the 50% of the cells. That means PpIX-SiNPs has higher efficacy in destroying the cells compared to the naked PpIX. The reason for that is the PpIX molecules were collected together by the encapsulation which causes more absorbance of energy light, also the outer surface of SiNPs was applicable with used cells. Comparing to the naked PpIX, the molecules of naked PpIX were spread in the solution and its interaction with light was dispersed.
We can conclude that the encapsulation by SiNPs should be greatly increasing the ability and the efficiency of PpIX as a PS to use in PDT. As explained above, encapsulated PpIX not only 1O2 generator, but also it has destroyed the target cells. And we can conclude that encapsulated PpIX-SiNPs have more efficacy than naked PpIX because the osteosarcoma needs to be treated at less concentration and less exposure time by using encapsulated PpIX-SiNPs to achieve same efficacy and effect compared to the naked PpIX.
The physical effects of encapsulated of the PS by SiNPs to be one of the main photodynamic applications were discussed. The encapsulated PSs have more efficacies compared to the naked PSs and the applicability of the encapsulated PSs was proved on osteosarcoma cells [Citation15]. The results showed the importance of in vitro photodynamic effectiveness for encapsulated PSs. Encapsulated PS has cytotoxicity on the osteosarcoma cells higher than naked PSs, which can be used to conclude that the encapsulation reduced the PS toxicity but the chemicals, which used for SiNPs synthesis, increased the cytotoxicity [Citation14].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Al-Akhras MAH, Aljarrah K, Makhadmeh GN, et al. Introducing the effect of Chinese Chlorella as a photosensitizing drug at different temperatures. J Mol Pharm Org Process Res. 2013;1(3):e109 doi:10.4172/2329-9053.1000e109
- Vargas A, Pegaz B, Debefve E, et al. Improved photodynamic activity of porphyrin loaded into nanoparticles: an in vivo evaluation using chick embryos. Int J Pharm. 2004;286:131–145.
- Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–11544.
- Kneuer C, Sameti M, Haltner EG, et al. Silica nanoparticles modified with aminosilanes as carriers for plasmid DNA. Int J Pharm. 2000;196:257–261.
- Zhao Y, Sadtler B, Lin M, et al. Nanoprobe implantation into mammalian cells by cationic transfection. Chem Commun. 2004;784–785 doi:10.1039/b317061f
- Santra S, Yang H, Dutta D, et al. TAT conjugated, FITC doped silica nanoparticles for bioimaging applications. Chem Commun. 2004;2810–2811.
- Lee J, Choi J, Chun J, et al. Relationship of protoporphyrin IX synthesis to photodynamic effects by 5‐aminolaevulinic acid and its esters on various cell lines derived from the skin. Br J Dermatol. 2008;159:61–67.
- Berlin N, Neuberger A, Scott J. The metabolism of δ-aminolaevulic acid. 1. Normal pathways, studied with the aid of 15N. Biochem J. 1956;64:80.
- Kloek J, Akkermans W, Henegouwen GM. Derivatives of 5‐aminolevulinic acid for photodynamic therapy: enzymatic conversion into protoporphyrin. Photchem Photbio. 1998;67:150–154.
- Georgakoudi I, Foster TH. Singlet oxygen‐versus nonsinglet oxygen‐mediated mechanisms of sensitizer photobleaching and their effects on photodynamic dosimetry. Photchem Photbio. 1998;67:612–625.
- Gross G. Therapy of human papillomavirus infection and associated epithelial tumors. Intervirology. 1997;40:368–377.
- Beutner M, Karl R, Ferenczy M. Therapeutic approaches to genital warts. Am J Med. 1997;102:28–37.
- Chatterjee DK, Fong LS, Zhang Y. Nanoparticles in photodynamic therapy: an emerging paradigm. Adv Drug Deliv Rev. 2008;60:1627–1637.
- Makhadmeh GN, Abdul Aziz A, Abdul Razak K. The efficacy of methylene blue encapsulated in silica nanoparticles compared to naked methylene blue for photodynamic applications. Artif Cells Nanomed Biotechnol. 2016;44:1–1022.
- Makhadmeh GN, Aziz AA, Razak KA, et al. Encapsulation efficacy of natural and synthetic photosensitizers by silica nanoparticles for photodynamic applications. IET Nanobiotechnol. 2015;9:381–385.