Abstract
Ephrin type-A receptor 2 (EphA2) is a transmembrane receptor which is upregulated in injured lungs, including those treated with bleomycin. YSA peptide (YSAYPDSVPMMS), a mimic of ephrin ligands, binds to EphA2 receptors on cell surface with high affinity. In this study, we assessed the ability of YSA-functionalized and non-functionalized poly (dl-lactide-co-glycolide) (PLGA) nanoparticles to enhance delivery to bleomycin treated cultured vascular endothelial cells and, in a bleomycin induced lung injury mouse model. Nanoparticles were loaded with a lipophilic fluorescent dye. Human umbilical vein endothelial cells (HUVEC) with or without 2-day bleomycin pretreatment (25 µg/ml) and adult mice with or without intratracheal instillation of bleomycin (0.1 U) were dosed with nanoparticles. Mice received nanoparticles via tail vein injection 4 days after bleomycin treatment. Three days after nanoparticle injection, tissues (lung, heart, kidney, spleen, liver, brain, eyes and whole blood) were harvested and quantified for fluorescence using IVIS imaging. Mean particle uptake increased with time and concentration for both types of particles in HUVEC, with the uptake being higher for YSA-functionalized nanoparticles. Bleomycin treatment increased the 3-h uptake of both types of nanoparticles in HUVEC by about two-fold, with the YSA-functionalized nanoparticle uptake being 1.66-fold compared to non-functionalized nanoparticles (p < .05). In mice, bleomycin injury resulted in 2.3- and 4.7-fold increase in the lung levels of non-functionalized and YSA-functionalized nanoparticles (p < .05), respectively, although the differences between the two particle types were not significant. In conclusion, PLGA nanoparticle delivery to cultured vascular endothelial cells and mouse lungs in vivo is higher following bleomycin treatment, with the delivery tending to be higher for YSA functionalized nanoparticles.
Introduction
Acute respiratory distress syndrome (ARDS) is a life threatening disease defined by non-cardiogenic pulmonary edema and hypoxemia [Citation1]. During ARDS the alveolar-capillary barrier breaks down leading to increased vascular permeability and severe tissue inflammation [Citation2]. Despite improvements in supportive care for patients with ARDS, mortality remains close to 40%, with no disease-modifying treatments currently available [Citation1,Citation3]. Computed tomography scanning demonstrated that the lung injury seen in ARDS is often heterogeneous, with some lung regions severely damaged and others seemingly spared [Citation4]. Thus, therapeutic delivery methods that target the injured lung may be more effective than non-targeted treatment approaches [Citation5].
Ephrin (Eph) receptors, members of receptor protein tyrosine kinase family, are responsible for the maintenance of the cell-cell interactions [Citation6]. Eph receptors of type A2 (EphA2) are present on lung endothelial cells, and upon activation by the ligand Eph, alter tight junction proteins and increase vascular permeability () [Citation7,Citation8]. Our group and others have previously shown that EphA2 is upregulated in lung tissue of bleomycin lung injury mouse model [Citation9] as well as in other models [Citation10,Citation11] of lung injury, leading to increased vascular permeability and inflammation. In vivo studies demonstrated that systemically administered ephrin-A1 suppresses EphA2, thereby decreasing tumorigenicity and invasiveness of carcinoma xenografts [Citation12]. Upregulation of a receptor such as EphA2 in a diseased tissue makes the receptor a suitable target to localize targeted nanoparticle delivery systems to the receptor [Citation13]. Indeed, in prostate cancer cells, targeting EphA2 receptors to deliver therapeutic molecules has shown promising results [Citation14]. Thus, lung tissue expressing increased EphA2 after injury can potentially be targeted by ligands capable of recognizing the EphA2 receptor.
Figure 1. Schematic representation of ephrin type-A receptor 2 (EphA2) expression dependent uptake of YSA functionalized nanoparticles. Bleomycin increases the expression of EphA2 surface receptors, which may increase the localization and/or uptake of YSA functionalized nanoparticles. Increased vascular permeability and increased epithelial cell expression of EphA2 in bleomycin injured lungs was previously reported by Carpenter et al. [Citation9].
![Figure 1. Schematic representation of ephrin type-A receptor 2 (EphA2) expression dependent uptake of YSA functionalized nanoparticles. Bleomycin increases the expression of EphA2 surface receptors, which may increase the localization and/or uptake of YSA functionalized nanoparticles. Increased vascular permeability and increased epithelial cell expression of EphA2 in bleomycin injured lungs was previously reported by Carpenter et al. [Citation9].](/cms/asset/fe3d8be4-5cf1-4112-b5e5-bc6d9908ad69/ianb_a_1528984_f0001_c.jpg)
YSA peptide (sequence – YSAYPDSVPMMS) is a mimic of Eph ligands that specifically bind to EphA2 receptors and increases receptor internalization [Citation15–17]. Ligand binding leads to phosphorylation of EphA2, followed by dephosphorylation of FAK (focal adhesion kinase), leading to dissociation of FAK-EphA2 complex and subsequent suppression of tumor growth [Citation18]. YSA peptide has high specificity and binding affinity to EphA2 receptors with a Kd = 187 ± 7 nM [Citation15], and it can easily be linked to the nanoparticle surface to achieve targeted drug delivery. Prostate cancer (PC3) cells showed 60%–470% increased uptake of YSA functionalized gold nanorods compared to reversed YSA peptide [Citation19]. Additionally, MDA-MB-231 breast cancer xenograft tumor mouse model showed sustained accumulation of YSA peptide modified liposomes in tumor tissue [Citation20].
Poly (dl-lactide-co-glycolide) (PLGA) is a biodegradable copolymer approved by US Food and Drug Administration (FDA) for the use in various drug products. PLGA nanoparticles can be used to achieve prolonged and sustained delivery of therapeutic agents including DNA, RNA, protein, peptide and small molecules to their respective target sites [Citation21–23]. These nanoparticles can also be surface modified using specific ligands and cross-linking chemistry for targeted drug delivery [Citation24,Citation25].
Based on the above background, we prepared PLGA nanoparticles functionalized with YSA peptide as an intravenously targeted nanoparticle for EphA2 receptors in injured lung. We hypothesized that PLGA nanoparticles surface functionalized with YSA peptide would show higher uptake in injured endothelial cells in vitro as well as in vivo models. We used human umbilical vein endothelial cells (HUVEC) for in vitro experiments and a bleomycin injured lung in mouse caused by intratracheal instillation of bleomycin for in vivo studies.
Materials and methods
Materials
RESOMER RG 503 H poly (d,l-lactide-co-glycolide) acid terminated with 50:50 monomer ratio with a molecular weight of 24,000–38,000 Da (Cat. No. 719870), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC; Cat. No. E7750), 2-(N-morpholino)ethanesulfonic acid (MES; Cat No. M3671), N-hydroxysuccinimide (NHS; Cat. No. 130672) and polyvinyl alcohol (PVA; molecular weight 31,000–50,000 Da; 87%–89% hydrolyzed; Cat. No. 363073) were obtained from Sigma-Aldrich (St. Louis, MO). Dichloromethane (DCM) and Nile red (99%, ACROS Organics) were purchased from Fisher Scientific (Waltham, MA). DiR dye (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindotricarbocyanine iodide; Cat. No.60017) was purchased from Biotium (Fremont, CA). HUVEC (Cat. No. C2517A Lot No. 0000439577), endothelial growth medium (EGM-2; Cat. No.CC-3162) and heparin were purchased from Lonza (Basel, Switzerland). Hoechst stain (Hoechst 33342) and CellMask deep red stain (Cat. No. C10046) were procured from Life Technologies (now part of Thermo Fisher Scientific, Waltham, MA). YSA (sequence-YSAYPDSVPMMS; purity >98% theoretical pI/Mw: 3.80/1347.52) was purchased from GenScript (Piscataway, NJ). Bleomycin was purchased from Hospira, Inc. (Lake Forest, IL).
Preparation of Nile red and DiR loaded PLGA nanoparticles
A typical batch process for nanoparticles is described below. PLGA polymer (120 mg) was dissolved in 3.6 ml of DCM in glass vials and mixed with 0.4 ml of a 3 mg/ml Nile red solution in DCM at 4 °C. The polymer and Nile red solution were sonicated for 2 min at 40% amplitude in an ice bath with the addition of 0.8 ml of water to generate a primary emulsion. This primary emulsion was then transferred to 20 ml of 2% PVA solution in water and sonicated for 4 min at 80% amplitude on ice bath (with the pulse cycling on and off every 30 s) using a probe sonicator (Q700, QSonica, Newtown, CT). The emulsion was magnetically stirred for 6 h at room temperature to evaporate DCM and reduce its residual content in the product [Citation26,Citation27]. Formed nanoparticles (NP) were centrifuged at 32,000 g for 30 min and then washed twice with 5 ml double distilled water at 4 °C to remove any excess PVA. After washes, nanoparticles (PLGA-NR-NP) were resuspended in 5 ml water and lyophilized using a FreeZone 2.5 plus (Labconco Corporation, Kansas City, MO). For the preparation of DiR loaded nanoparticles (PLGA-DiR-NP), a 0.4 ml of a 3 mg/ml Xenolite DiR dye solution in DCM at 4 °C was used instead of Nile red solution.
Peptide functionalization of PLGA nanoparticles
In a typical batch, NHS (3 mg) and EDC (8 mg) were added to 8 mg of PLGA-NR-NP in 1.5 ml of phosphate buffered saline (PBS; pH 6.5). The preparation was stirred at room temperature (23 °C) for 15 min and washed thrice with double distilled water (10 ml) at 32,000g for 30 min at 4 °C to isolate nanoparticles with the YSA peptide (PLGA-NR-YSA-NP) and the pellet was suspended in 0.5 ml double distilled water. The formed nanoparticles were frozen, lyophilized, and stored at −20 °C for future use. This procedure was also used to functionalize PLGA-DiR-NP to generate PLGA-DiR-YSA-NP.
Characterization of nanoparticles
Nile red or DiR dye loading in the nanoparticles was determined by spectroscopy method. A calibration plot was generated by dissolving Nile red dye in DCM in the concentration range of 0.625–20 µg/ml. The calibration curve for DiR dye was prepared in DCM in the range of 0.001–0.15 µg/ml. Polymer particles loaded with dye were dissolved in DCM for analysis. The samples were quantified using a Molecular Devices SpectraMax M5 instrument (Sunnyvale, CA).
Nanoparticles suspensions (1 mg/ml) were prepared in double distilled water for size and zeta potential measurements using Malvern Zetasizer Nano-ZS instrument (Westborough, MA), which employs dynamic light scattering approach with the v7.12 software.
In vitro uptake of nanoparticles
Around 5000 HUVEC cells per well were seeded in a 96-well plate in EGM-2 medium and allowed to grow at 37 °C under 5% CO2/95% air in a humidified atmosphere for 24-h till the cells reached ∼70% confluency. PLGA-NR-NP or PLGA-NR-YSA-NP (200 µl of 0.1 or 0.5 mg/ml) suspensions were prepared separately in serum-free medium and added to wells. After 1, 3 and 6 h of incubation, the cells were washed thrice with ice-cold PBS at pH 6.5. Cells were stained with 100 µl of Nuclear Stain in culture medium (1 µg/ml) for 15 min at 37 °C followed by cell incubation with 100 µl CellMask red membrane stain (1 µg/ml medium) for 5 min at 37 °C. Cells were fixed with 3.7% paraformaldehyde in PBS (100 µl/well) for 20 min at room temperature after washing with ice-cold PBS. Following washing, cell images and total Nile red fluorescence intensity for each well were obtained using Operetta High-Content Imaging System (PerkinElmer, Inc., Waltham, MA). Cells were then lysed using 3% sodium dodecyl sulfate and total cell protein content was measured using a micro BCA protein assay kit (ThermoFisher Scientific, Waltham, MA).
To evaluate the effect of bleomycin injury on the uptake of nanoparticles, ∼70% confluent HUVEC cells were pre-treated with bleomycin (25 µg/ml) for 48 h and then incubated further for 3 h with 0.5 mg/ml of nanoparticles in serum-free medium at 37 °C. The uptake of nanoparticles was measured as Nile red intensity in DCM extract of whole cell lysate using fluorescence spectroscopy and normalized to protein concentration.
In vivo distribution of nanoparticles
Animal handling and all the procedures were conducted as per the approval and guidelines of Institutional Animal Care and Use Committee (IACUC), University of Colorado Denver. Wild type adult mice (20–30 g) originally obtained from Jackson Laboratories (Bar Harbor, ME) and inbred at University of Colorado were used for in vivo studies. All animals had ad libitum access to standard animal chow and water. Animals were housed under controlled condition at 22 ± 1 °C with a relative humidity of 30%–35% and dark/light cycling every 12 h. To induce lung injury, one group of animals were intra-tracheally instilled with bleomycin at a dose of 0.1 U in 100 µl PBS at pH 7.4. The control group animals received only 100 µl PBS. Four days after bleomycin administration, a 100 µl suspension of 2 mg nanoparticles (DiR-loaded, with or without YSA-functionalization) dispersed in PBS was injected via the tail vein. DiR as opposed to Nile red was used for tissue imaging after in vivo dosing, due to greater sensitivity of the former dye for tissue imaging. Three days after the injection of nanoparticles, animals were anesthetized with inhaled isoflurane (1.5%–4%) and sacrificed by exsanguination through right ventricle aspiration followed by cervical dislocation. The chest was opened, and lungs were flushed with 5–10 ml of cold PBS (pH 7.4) via the right ventricle to remove blood. The lung, spleen, liver, kidney, brain, heart, eyes and whole blood were harvested and immediately used for imaging.
Fluorescence imaging of the organs was performed immediately after isolation using a Xenogen IVIS 2000 imaging system with the Living Image Software (Xenogen Corp., Alameda, CA). Other instrument parameters used for image acquisition were as follows; indocyanine green (ICG) excitation and emission filters, 0.5 s exposure time, medium binning, f-stop (2) and field of view C. The region of interest was manually drawn around the tissue and radiant efficiency was obtained for all the tissues studied. While several studies report radiant efficiency as a measure of tissue concentrations, to determine whether this measure changes by tissue weight even when the area of measurement is controlled for, measures were made with and without weight normalization in a pilot study with liver tissue. Representative control liver tissue from a PLGA-DiR-NP injected animal was used for this purpose. Weights of whole control liver tissue as well as three uneven dissected pieces of same tissue, were recorded and imaged using IVIS. Total counts, total and average radiant efficiency, were obtained from the whole tissue as well as pieces. The total and average radiant efficiency with or without weight normalization for the three tissue pieces was compared to the whole tissue to determine whether tissue weight normalization is required for average radiant efficiency.
Statistical analysis
The experimental results were presented as a mean ± SD. Statistical analysis was conducted using One-way ANOVA with Bonferroni post-test analysis using GraphPad Prism software (v 4.5) to determine the significance for in vitro experiments. Unpaired t test was used for in vivo experiment. Data with p < .05 was considered significant.
Results
Characterization of nanoparticles
The Nile red and DiR dye loaded PLGA-NP with or without YSA functionalization were characterized for dye loading, size and zeta potential. The dye loading was 1.04% ± 0.05% in Nile red particles and 0.86% ± 0.02% for DiR dye particles. The size and zeta-potential of PLGA-NR-NP, PLGA-NR-YSA-NP, PLGA-DiR-NP and PLGA-DiR-YSA-NP are shown in . The average particle sizes for these nanoparticles ranged from 219 to 279 nm. Zeta-potential indicated that all particles are negatively charged.
Table 1. Size and zeta-potential of nanoparticles.
In vitro cell uptake study
Nanoparticle uptake in HUVEC was increased with time and dose for both YSA-functionalized and non-functionalized nanoparticles, based on Nile red intensity per mg protein (). The cell uptake was generally higher for YSA-functionalized particles relative to non-functionalized nanoparticles, with the differences being statistically significant for the 0.5 mg/ml treatment at 3 h (p < .001). No statistical difference between the twonanoparticles was observed at 1, 3 and 6 h for 0.1 mg/ml and 1 and 6 h for 0.5 mg/ml treatments. The nanoparticles were localized throughout the cell without being concentrated in any particular cellular region, as evident from the Operetta High content cell images ().
Figure 2. Dose- and time-dependent uptake of nanoparticles in human umbilical vein endothelial cells (HUVEC) cells. Cell imaging based quantification of Nile red dye in HUVEC cells at 1, 3 and 6 h following exposure of 0.1 or 0.5 mg/ml nanoparticles with and without YSA-functionalization. Fluorescence intensity is normalized to protein concentration. Data represents mean ± SD for n = 3. *p < .001 compared to the uptake of 0.5 mg/ml PLGA-NR-NP at 3 h. PLGA: poly (dl-lactide-co-glycolide).
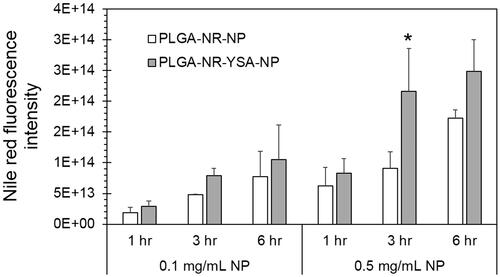
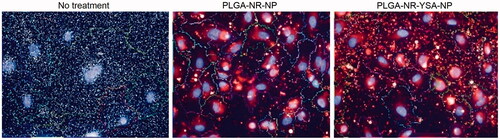
Figure 3. Fluorescence images showing 6-h uptake of nanoparticles by human umbilical vein endothelial cells (HUVEC) cells. High magnification images of HUVEC cells obtained using Operetta high-content imaging system (PerkinElmer, Waltham, MA) at 6 h after treatment with 0.5 mg/ml of nanoparticles with or without YSA-functionalization. Blue stain indicates the cell nucleus, red indicates the Nile red dye, and multicolored lines indicate the cell boundaries.
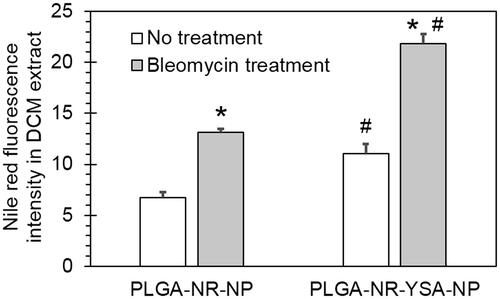
Bleomycin (25 µg/ml) pre-treatment for 48-h increased 3-h HUVEC uptake of non-functionalized nanoparticles to 1.95-fold (p < .0001) compared to untreated group (). Also, the HUVEC uptake of YSA-functionalized nanoparticles was 1.98-fold (p < .0001) higher than non-functionalized nanoparticles in bleomycin group.
Figure 4. Effect of bleomycin pre-treatment on nanoparticle uptake by human umbilical vein endothelial cells (HUVEC) cells. Spectrofluorometric quantification of 3-h uptake of 0.5 mg/ml PLGA- nanoparticles with and without functionalization by YSA peptide. Data shows Nile red fluorescence in DCM extract of HUVEC cell lysate normalized to protein concentration with or without a 48-h pretreatment with 25 µg/ml bleomycin. Data represents mean ± SD for n = 3. *p < .0001 compared to the corresponding no treatment group. #p < .001 compared to the corresponding PLGA-NR-NP group. DCM: dichloromethane; PLGA: poly (dl-lactide-co-glycolide).
In vivo distribution of nanoparticles
The average of average radiant efficiency of individual liver pieces is similar to that of whole tissue ( and ), confirming that it is a concentration measure. Hence, we have used the average radiant efficiency from experimental tissue as a measure for the concentration of nanoparticles. IVIS imaging from PLGA-DiR-NP injected animals showed detectable fluorescence signal in all isolated tissues except eyes at 3 days post-administration (). There was no difference in the average radiance efficiency levels between all the tissues of control as well as injured animals except lung (). In the lungs of bleomycin-injured animals, there was 1.3-fold increase in the average radiance efficiency levels of YSA-functionalized nanoparticles relative to non-functionalized nanoparticles. Also, lung of bleomycin injured animals showed increased levels of average radiance efficiency for non-functionalized (2.3-fold, p < .05) and YSA-functionalized (4.7-fold, p < .01) nanoparticles compared to normal. In general, the average radiance efficiency levels (relative concentration) of both nanoparticles followed the trend of liver>spleen>>>lung∼kidney∼brain>heart∼whole blood>>eye in control animals and liver>spleen>lung>>kidney∼brain>heart∼whole blood>eye in bleomycin injured animals. brain > heart ∼ whole blood > eye in bleomycin injured animals.
Figure 5. Radiant efficiency, a measure of tissue distribution of nanoparticles, in whole liver and dissected pieces. Whole liver tissue from control animal intravenously injected with 2 mg of non-functionalized DiR dye loaded nanoparticles and three uneven pieces of the same tissue were imaged in the fluorescence mode using Xenogen IVIS 2000 (PerkinElmer). Same area was imaged and counted for each tissue. ICG excitation and emission filters were set to a 0.5 s exposure time. Tissue areas were marked by the contour selection method and the total counts and radiant efficiency were measured. ICG: indocyanine green.
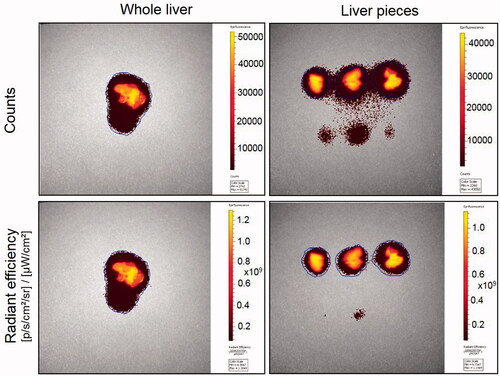
Figure 6. Tissue distribution of functionalized and non-functionalized nanoparticles visualized using IVIS imaging. Representative IVIS images of isolated tissues from mice, 3 days after intravenous injection of nanoparticles with and without YSA-functionalization. Images were obtained in the fluorescence mode using the Xenogen IVIS 2000 (PerkinElmer). The ICG excitation and emission filters were set to a 0.5 s exposure time. Tissue areas were marked by the contour selection method and the radiant efficiency was measured. ICG: indocyanine green.
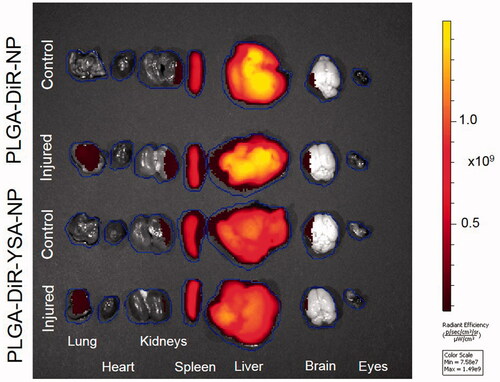
Figure 7. Relative tissue distribution of functionalized and non-functionalized nanoparticles. Mice were dosed intravenously with nanoparticles and sacrificed 3 days after dosing. Tissues were isolated and imaged using Xenogen IVIS 2000 (PerkinElmer). The ICG excitation and emission filters were set to a 0.5 s exposure time. Tissue areas were marked by the contour selection method and the average radiant efficiency was obtained after the subtraction of signal from blank tissues. Data represents mean ± SD for n = 5 or 6. *p < .05 compared to the corresponding control group for the same tissue. ICG: indocyanine green.
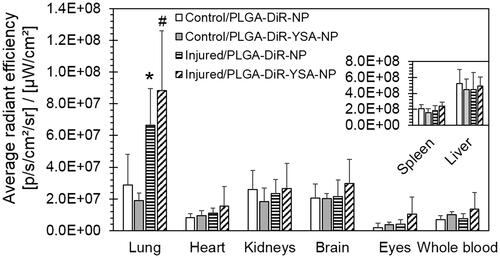
Table 2. Counts, total radiant efficiency and average radiant efficiency of nanoparticle distribution in whole liver versus pieces of the same liver.
Discussion
Polymeric nanoparticles intended for the treatment of various diseases are currently under investigation and several of them are capable of sustained drug delivery [Citation28]. These nanoparticles can be functionalized to target specific tissues, to increase the delivery and efficacy, while minimizing side effects of therapeutic molecules [Citation29,Citation30]. In the current study, we tested nanoparticles functionalized with YSA peptide to achieve targeted delivery to injured lung tissue by utilizing the natural overexpression of EphA2 receptors in lung after bleomycin injury. Specifically, PLGA nanoparticles were surface coated with YSA peptide and studied for their uptake relative to control nanoparticles in HUVEC cells as well as in a mouse model of bleomycin-induced lung injury.
PLGA is a commonly used biodegradable polymer, mainly because its hydrolysis products (lactic acid and glycolic acid) can easily be metabolized by the body. US FDA and the European Medicine Agency (EMA) already approved various forms of PLGA in drug delivery systems [Citation31,Citation32]. Hence, we selected this polymer as a model carrier material for nanoparticles. We used negatively charged nanoparticles in this study because of their relatively higher accumulation and safety in lung [Citation33] as compared to positively charged nanoparticles, based on some studies. Functionalization of nanoparticles with YSA peptide did not dramatically alter particle size, which is consistent with previously reported studies [Citation34]. There was approximately 4.3-fold increase in negative zeta potential observed in DiR dye loaded nanoparticles relative to Nile red dye loaded nanoparticles. Peptide functionalization increased the electronegativity of Nile red loaded nanoparticles by 1.8-fold and DiR dye loaded nanoparticles by 1.2-fold, may be because of the contribution of negatively charged peptide on the surface (). YSA peptide displays negative charge at neutral pH, and as the charge measurements were conducted in distilled water, the peptide might have contributed to the increased negative charge of nanoparticles. Previous studies also showed slight elevation of negative charge of liposomes after YSA functionalization [Citation20,Citation35].
Nanoparticles functionalized with targeting ligands can improve drug delivery to target cells. There are many ligands that can be attached to nanoparticle surface for targeted drug delivery [Citation36]. Here we used the YSA peptide as a targeting ligand because of its ability to enter cells via EphA2 receptors [Citation20,Citation37], which allows targeted intracellular delivery of the associated nanoparticles and their payload. It is very important for the nanoparticles to achieve cellular uptake to show desired efficacy of an intracellularly acting drug that is otherwise poorly permeable. To determine intracellular delivery, we evaluated nanoparticle uptake in HUVEC cells. It has been previously demonstrated that HUVEC cells express EphA2 receptors even in normal culture conditions [Citation38]. This makes HUVEC cells a suitable candidate to study in vitro uptake of YSA functionalized particles. Entrapped Nile red dye in the nanoparticles allowed us to visualize and quantify nanoparticle delivery to HUVEC cells. As evident in fluorescence images of HUVEC cells, the nanoparticles were present in clusters of varying sizes spread throughout the cell mass (). Also, some diffuse dye intensity was evident within the cells. Transmission electron microscope studies on polymeric nanoparticles elsewhere have demonstrated that nanoparticles accumulate in cytoplasm through endocytosis and phagocytosis [Citation39]. Our finding of increased uptake of YSA functionalized nanoparticles by HUVEC cells is consistent with previous studies wherein HUVEC cells demonstrated increased selectivity for YSA peptide functionalized liposomes via an EphA2 mediated mechanism [Citation20]. We saw dose- and time-dependent increase in the uptake of nanoparticles, indicating that the cell surface receptors are not yet saturated and there may be scope for dose escalation to achieve further increase in nanoparticle uptake (). In addition, we found even greater uptake of the YSA functionalized nanoparticles in bleomycin-injured HUVEC cells (), consistent with elevated expression of EphA2. Significant increase of EphA2 receptor expression upon bleomycin injury was observed previously [Citation9]. This led us to test the uptake of YSA functionalized nanoparticles in EphA2 overexpressing injured lung tissue in a mouse model.
Bleomycin-induced lung injury mouse model is widely used to study lung fibrosis. In this model, there is an initial development of pulmonary edema followed by fibrosis and this resembles many characteristics of human acute lung injury [Citation40–42]. Bleomycin induces acute alveolitis within 2–4 days of intratracheal instillation in rodents, which subsequently progresses to interstitial inflammation and fibrosis by 14 days [Citation43,Citation44]. During bleomycin induced lung injury, molecular oxygen gets reduced to superoxide and hydroxyl radicals, causing single or double strand DNA breakdown, blocking regeneration of epithelial cells, and initiating inflammation and lung fibrosis [Citation45]. Significantly increased expression of EphA2 receptors was seen after 4 days of intratracheal bleomycin administration in mice [Citation9]. Hence, we utilized this model and chose the 4-day time point after bleomycin administration to inject the nanoparticles and determine nanoparticle distribution to lung tissue during the early inflammatory phase [Citation40,Citation41].
IVIS instrument was used in this study to quantify fluorescence of nanoparticles in tissues. Radiant efficiency is a commonly used parameter to report tissue fluorescence using this instrument. Following injection of DiR loaded nanoparticles in a mouse in pilot studies, comparison of average radiant efficiency in whole liver tissue versus pieces of the same liver indicated that average radiant efficiency without the normalization to tissue weight might be a good indicator of tissue concentration of nanoparticles, relative to total radiant efficiency or counts alone ( and ).
The liver and spleen being highly metabolic organs responsible for clearance, showed the highest distribution of nanoparticles but exhibited no difference in the uptake after intra-tracheal bleomycin administration relative to untreated animals, as minimal or no effect of bleomycin is anticipated in these organs (). Following systemic dosing of nanoparticles, high levels of nanoparticles were observed in liver and spleen tissues, where they undergo clearance through the mono-nuclear phagocytic system [Citation46]. Although the nanoparticles were administrated intravenously, the blood levels of these nanoparticles were low as compared to liver and spleen. This indicates that majority of nanoparticles were cleared from blood circulation within 3 days administration. Moreover, kidney and blood levels of the two types of nanoparticles in animals with or without injury were relatively similar, indicating that YSA functionalization did not likely affect the blood circulation time or clearance of these nanoparticles through kidney. There was higher uptake of nanoparticles in bleomycin-injured lung, irrespective of YSA functionalization. Selectively, only lung tissue out of all the tissues collected, showed increased levels of YSA functionalized nanoparticles after bleomycin injury, possibly due to enhanced vascular permeability and/or selective binding of these particles to pulmonary vascular endothelium in these animals. Nanoparticles are expected to enter lung microcapillaries, escape the vasculature, and penetrate the cell membrane of lung epithelial cells. Blake and Staub [Citation47] reported three types of pores in the lung vascular endothelium of sheep, with the pore sizes being 3.4, 12.5 and 100 nm. Thus, particles as large as 100 nm may escape normal lung vasculature. In our studies, with bleomycin injury, there is a possibility that the effective pore sizes may be larger. The particles we used had a mean size of 219–279 nm, with a distribution that spans sizes smaller and higher than this mean size. Thus, the lower size particles in the mix are expected to cross the lung vasculature better than the larger particles in the mixture, with the overall permeability being greater in the injured lung. Besides pore transport, endocytosis and transcytosis may allow uptake and transport of large particles across cell membranes. One likely site of interaction of YSA nanoparticles is EphA2-overexpressing [Citation9] lung cells, resulting in improved internalization (). Lung EphA2 is also elevated in other animal models of lung injury including viral infection, hypoxia and lipopolysaccharide models [Citation10,Citation11]. However, lack of significant differences in vivo () and in some in vitro groups () between YSA functionalized and non-functionalized nanoparticles suggests that there is room for further improving the ligand used in targeting EphA2 receptors. Alternatively, the differences in distribution of particles may have dissipated by day 3, the time of our measurement in vivo.
In summary, intravenously administered polymeric nanoparticle delivery to lung tissue is enhanced in bleomycin-induced lung injury animals. Thus, nanoparticles might improve the benefit-risk ratio of any associated therapeutic agents.
Acknowledgements
The authors are thankful to Dr. Ruchit Trivedi for preliminary studies related to this work. The authors are also thankful to Rachel Hartman for assistance with data analysis for this manuscript.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Pierrakos C, Karanikolas M, Scolletta S, et al. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res 2012; 4:7–16.
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349.
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788–800.
- Gattinoni L, Caironi P, Pelosi P, et al. What has computed tomography taught us about the acute respiratory distress syndrome? Am J Respir Crit Care Med. 2001;164:1701–1711.
- Millar FR, Summers C, Griffiths MJ, et al. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax 2016;71:462–473.
- Coulthard MG, Morgan M, Woodruff TM, et al. Eph/Ephrin signaling in injury and inflammation. Am J Pathol. 2012;181:1493–1503.
- Cheng N, Brantley DM, Liu H, et al. Blockade of EphA receptor tyrosine kinase activation inhibits vascular endothelial cell growth factor-induced angiogenesis. Mol Cancer Res 2002;1:2–11.
- Fang BW, Ireton RC, Zhuang GL, et al. Overexpression of EPHA2 receptor destabilizes adherens junctions via a RhoA-dependent mechanism. J Cell Sci. 2008;121:358–368.
- Carpenter TC, Schroeder W, Stenmark KR, et al. Eph-A2 promotes permeability and inflammatory responses to bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2012;46:40–47.
- Cercone MA, Schroeder W, Schomberg S, et al. EphA2 receptor mediates increased vascular permeability in lung injury due to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L856–L863.
- Hong JY, Shin MH, Chung KS, et al. EphA2 receptor signaling mediates inflammatory responses in lipopolysaccharide-induced lung injury. Tuberc Respir Dis. 2015;78:218–226.
- Duxbury MS, Ito H, Zinner MJ, et al. Ligation of EphA2 by Ephrin A1-Fc inhibits pancreatic adenocarcinoma cellular invasiveness. Biochem Biophys Res Commun. 2004;320:1096–1102.
- Singh SR, Grossniklaus HE, Kang SJ, et al. Intravenous transferrin, RGD peptide and dual-targeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659.
- Gobin AM, Moon JJ, West JL. EphrinA I-targeted nanoshells for photothermal ablation of prostate cancer cells. Int J Nanomedicine. 2008;3:351–358.
- Koolpe M, Dail M, Pasquale EB. An ephrin mimetic peptide that selectively targets the EphA2 receptor. J Biol Chem. 2002;277:46974–46979.
- Scarberry KE, Dickerson EB, McDonald JF, et al. Magnetic nanoparticle-peptide conjugates for in vitro and in vivo targeting and extraction of cancer cells. J Am Chem Soc. 2008;130:10258–10262.
- Mitra S, Duggineni S, Koolpe M, et al. Structure-activity relationship analysis of peptides targeting the EphA2 receptor. Biochemistry. 2010;49:6687–6695.
- Lee HY, Mohammed KA, Kaye F, et al. EphA2 targeted intratumoral therapy for non-small cell lung cancer using albumin mesospheres. Am J Transl Res. 2017;9:3293–3303.
- Alkilany AM, Boulos SP, Lohse SE, et al. Homing peptide-conjugated gold nanorods: the effect of amino acid sequence display on nanorod uptake and cellular proliferation. Bioconjugate Chem. 2014;25:1162–1171.
- Guo ZM, He B, Yuan L, et al. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int J Pharm. 2015;493:380–389.
- Li J, Zhang C, Fan L, et al. Brain delivery of NAP with PEG-PLGA nanoparticles modified with phage display peptides. Pharm Res. 2013;30:1813–1823.
- Kompella UB. Nanotechnology and drug delivery. J Ocul Pharmacol Ther. 2013;29:89.
- Kompella UB, Lee VH. Delivery systems for penetration enhancement of peptide and protein drugs: design considerations. Adv Drug Deliv Rev. 2001;46:211–245.
- Sundaram S, Trivedi R, Durairaj C, et al. Targeted drug and gene delivery systems for lung cancer therapy. Clin Cancer Res. 2009;15:7299–7308.
- Scheinman RI, Trivedi R, Vermillion S, et al. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine (Lond). 2011;6:1669–1682.
- Shelke NB, Kadam R, Tyagi P, et al. Intravitreal poly(L-lactide) microparticles sustain retinal and choroidal delivery of TG-0054, a hydrophilic drug intended for neovascular diseases. Drug Deliv Transl Res. 2011;1:76–90.
- Koushik K, Kompella UB. Preparation of large porous deslorelin-PLGA microparticles with reduced residual solvent and cellular uptake using a supercritical carbon dioxide process. Pharm Res. 2004;21:524–535.
- Li J, Wang Y, Zhu Y, et al. Recent advances in delivery of drug-nucleic acid combinations for cancer treatment. J Control Release. 2013;172:589–600.
- Zhang Q, Neoh KG, Xu LQ, et al. Functionalized mesoporous silica nanoparticles with mucoadhesive and sustained drug release properties for potential bladder cancer therapy. Langmuir. 2014;30:6151–6161.
- Almeida PV, Shahbazi MA, Makila E, et al. Amine-modified hyaluronic acid-functionalized porous silicon nanoparticles for targeting breast cancer tumors. Nanoscale. 2014;6:10377–10387.
- Danhier F, Ansorena E, Silva JM, et al. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–522.
- Semete B, Booysen L, Lemmer Y, et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed Nanotechnol Biol Med. 2010;6:662–671.
- Schleh C, Semmler-Behnke M, Lipka J, et al. Size and surface charge of gold nanoparticles determine absorption across intestinal barriers and accumulation in secondary target organs after oral administration. Nanotoxicology. 2012;6:36–46.
- Thasneem YM, Sajeesh S, Sharma CP. Effect of thiol functionalization on the hemo-compatibility of PLGA nanoparticles. J Biomed Mater Res. 2011;99A:607–617.
- Wang JL, Liu YL, Li Y, et al. EphA2 targeted doxorubicin stealth liposomes as a therapy system for choroidal neovascularization in rats. Invest Ophthalmol Vis Sci. 2012;53:7348–7357.
- Friedman AD, Claypool SE, Liu R. The smart targeting of nanoparticles. Curr Pharm Des. 2013;19:6315–6329.
- Wang S, Noberini R, Stebbins JL, et al. Targeted delivery of paclitaxel to EphA2-expressing cancer cells. Clin Cancer Res. 2013;19:128–137.
- Chan B, Sukhatme VP. Receptor tyrosine kinase EphA2 mediates thrombin-induced upregulation of ICAM-1 in endothelial cells in vitro. Thromb Res. 2009;123:745–752.
- Costanzo M, Carton F, Marengo A, et al. Fluorescence and electron microscopy to visualize the intracellular fate of nanoparticles for drug delivery. Eur J Histochem. 2016;60:2640.
- Kim JW, Rhee CK, Kim TJ, et al. Effect of pravastatin on bleomycin-induced acute lung injury and pulmonary fibrosis. Clin Exp Pharmacol Physiol. 2010;37:1055–1063.
- Aono Y, Nishioka Y, Inayama M, et al. Imatinib as a novel antifibrotic agent in bleomycin-induced pulmonary fibrosis in mice. Am J Respir Critical Care Med 2005;171:1279–1285.
- Szapiel SV, Elson NA, Fulmer JD, et al. Bleomycin-induced interstitial pulmonary-disease in the nude, athymic mouse. Am Rev Respir Dis 1979;120:893–899.
- Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol 1983;112:170–177.
- Smith RE, Strieter RM, Zhang K, et al. A role for C-C chemokines in fibrotic lung disease. J Leukoc Biol. 1995;57:782–787.
- El-Khouly D, El-Bakly WM, Awad AS, et al. Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor Kappa-B in rats. Toxicology 2012;302:106–113.
- Anselmo AC, Gupta V, Zern BJ, et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7:11129–11137.
- Blake LH, Staub NC. Pulmonary vascular transport in sheep. A mathematical model. Microvasc Res. 1976;12:197–220.
