 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In recent years, natural and synthetic polymers have attracted much attention due to their great potentials in medical science. In the present study, we have investigated the effect of chitosan-bulk (Ch-bulk), chitosan nanoparticles (ChNP), chitosan nanoparticles conjugated with glutaraldehyde (ChNP-GA) with an average size of 300–400 nm on human colorectal carcinoma cells (HCT-116) to examine their cytotoxic and anti-cancer abilities. We have evaluated the effects of Ch-bulk, ChNP, ChNP-GA on cancer cells by morphometric and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays respectively. Our results revealed that the Ch-bulk, ChNP, ChNP-GA decreased cell viability, cell proliferation and caused cell death in a concentration-dependent manner. Both morphometric and quantitative analyses confirmed that (Ch-bulk) and Chitosan nanoparticles (ChNP and ChNP-GA) induced concentration-dependent effects on the cancer cells. Among these three, ChNP-GA produced a more profound effect on the survivability with compared to each-bulk and Ch-NP treated groups. A dose of 2 mg/mL did not produced much effect on the cancer cell death, however, a dose of 4 mg/mL–6 mg/mL produced significant morphological changes like nuclear condensation and augmentation. Interestingly, a dose of 8 mg/mL produced significant cell death 48 hours post-treatment. In addition, during our morphometric analysis, we found that (Ch-bulk) and Chitosan nanoparticles (ChNP and ChNP-GA) treated cells underwent nuclear disintegration and fragmentation which lead to programmed cell death. Our studies demonstrate that the Ch-bulk, ChNP and ChNP-GA holds a great potential in the treatment of colon cancer.
Background
In recent years, natural and synthetic polymers have attracted much attention due to their great potentials in overcoming the physiological barriers and to protect the active molecules such as oligonucleotides, proteins or peptides from degradation, while working as delivery systems for delivering the bioactive molecules, protecting and targeting the loaded substances to specific sites [Citation1,Citation2]. Owing to their advantages such as the availability of natural resources, stability and ease of surface modification, biodegradability, biocompatibility and nontoxicity, natural polymeric materials are studied for controlled release of active molecules and also for stabilization of biological molecules [Citation3,Citation4]. Among the various kinds of polymers, chitin (C8H13O5N) is considered one of the most abundant polymers in nature. It is one of the interesting polymers which have been widely applied in the medical field. Complexes and gels can be formed by interacting chitosan with polyanions and manufactured commercially on a huge scale by alkaline N-deacetylation of chitin [Citation5].
Many parameters like moisture, heavy metals content and crystallinity, play an essential part in the application of chitosan. Other major parameters like molecular weight and viscosity improvement in aqueous solution also employed in the biochemical and pharmacological applications [Citation6,Citation7]. Two of the most important subjects to obtain distinctive physical-mechanical properties are molecular weight and degree of deacetylation. Moreover, low molecular weight chitosan nanoparticles are used to deliver the non-viral vector gene, because they are biocompatibility, have more solubility and show high activity in biological systems [Citation8].
Chitosan is used in drug delivery by forming beads (1–2 mm) via contact a gel with anions. Due to the large size of these beads, its application is still limited [Citation6,Citation9]. The first description of chitosan nanoparticles (ChNP) was by Ohya and co-workers in 1994. They applied ChNP synthesized by emulsifying and crosslinking for intravenous delivery of anticancer drug 5-fluorouracil [Citation6,Citation10]. Since then, many approaches have been applied for preparation ChNP. Currently, five techniques are available, including microemulsion, polyelectrolyte complex, ionotropic gelation, reverse micellar and emulsification solvent method [Citation11]. Furthermore, ChNP are natural materials with unique biological, physicochemical and antimicrobial properties, which make them a distinctive environmentally friendly material and safe bioactivities on the humans. The distinctive features of ChNP encouraged for the improvement of therapeutic drug delivery systems which have been extensively employed in the sustained drug-containing nanocarriers [Citation12,Citation13]. Consequently, they are used in biomedical areas, including tissue engineering, cancer diagnosis, antioxidant activity, drug delivery, encapsulation of biologically active compounds, enzyme immobilization support and antimicrobial agent [Citation14]. Over the past few years, several studies have been conducted to study the effect of various nanoparticles on cancer treatments. Interestingly, it has been shown that most of the nanoparticles possess and exhibit anti-cancer properties [Citation15–26]. The purpose of this study to examine the effect of Ch-bulk, ChNP and ChNP-GA on HCT-116 cancer cells by using different concentrations and evaluate their cytotoxic effects after 6 h, 24 h and 48 h post-treatments.
Methods
Synthesis and characterization of ChNP and ChNP-GA
Materials: Chitosan with 75–85% degree of deacetylation with low molecular weight (Sigma-Aldrich, Taufkirchen, Germany), glutaraldehyde - C5H8O2 (Sigma-Aldrich, Taufkirchen, Germany), sodium hydroxide - NaOH (Sigma-Aldrich, Taufkirchen, Germany), and hydrochloric acid - HCl (Sigma-Aldrich, Taufkirchen, Germany) were used as precursors without further purification.
Preparation: The chitosan powder was first dissolved in 1 Molar of HCl and the solution was then stirred for more than 24 h until a pure viscous solution of chitosan was gained. Chitosan stock solution was prepared by dissolving chitosan in 1 Molar of HCl. ChNP was prepared by adjusting the pH of a chitosan stock solution to 8.0 by adding NaOH and thereafter sample was washed and dried at 50 °C for 24 h. ChN-GA was prepared by adjusting the pH of a chitosan stock solution to 8.0 and then GA was added to the solution (6 μl GA/5 ml Ch). The crosslinking formation between amine groups of chitosan polymer and glutaraldehyde were occurred to form stable amine bonds. ().
Figure 1. Schematic representation of glutaraldehyde cross-linked with chitosan copolymer: The crosslinking formation between amine groups of chitosan polymer and glutaraldehyde were occurred to form stable amine bonds.
Characterization: The crystalline structure of Ch-bulk, ChNP and ChNP-GA was confirmed by X-Ray Diffraction (XRD) (Shimadzu XRD-7000, Kyoto, Japan). The chitosan particles were scanned with a beam of monochromatic CuKα X-radiation of wavelength (λ = 1.5406 Ǻ), and the glancing angles X-ray diffraction was in the range of 5° ≤ 2θ ≤ 80° with a step size of 0.1°. We have used Fourier Transform Infrared Spectroscopy (FT-IR) (PerkinElmer, Ohio, USA) to study the functional groups present in the Ch-bulk, ChNP and ChNP-GA. The differences in spectral peaks of the Ch-bulk, ChNP and ChNP-GA were then evaluated. The morphology, distribution and shape of the samples were obtained directly by Transmission Electron Microscopy (TEM) (FEI, MORGAGNE.68, Check Republic). The chitosan nanoparticles were applied onto a copper grid and air dried for 3 min at room temperature. The nanoparticles loaded grid was then negatively stained using 1% uranyl acetate for 90 s and air dried at room temperature and processed for TEM analysis. Thermogravimetric analysis (TGA) was done to investigate the thermal behaviour of the chitosan particles. TGA was performed using the Simultaneous Thermal Analyzer (STA6000, PerkinElmer, Ohio, USA). The samples were treated with increasing temperature from 25 °C to 700 °C at a heating rate of 10 °C/min under flowing nitrogen atmosphere.
Treatment of ChNP and ChNP-GA on cancer cells
Human colorectal carcinoma (HCT-116) cells were cultured under controlled conditions and we have used passage number 44 in the study. In brief, HCT-116 cells were first cultured in the media containing DMEM, L-glutamine, 10% fetal bovine serum, selenium chloride, 120 U/mL penicillin, and 120 μg/mL streptomycin and were grown in the CO2 incubator with 37º C and 5% CO2 condition (Heracell 150i, Thermo-scientific, Waltham, MA, USA). Once the cells reached 80% confluency, the cells were then grown into 96-well cell plates. Before treating Ch-bulk, ChNP and ChNP-GA to the cancer cells, they were first autoclaved to remove the contaminations. We have treated the cancer cells with different concentrations (2.0 mg/mL, 4.0 mg/mL, 6 mg/mL and 8 mg/mL) of Ch-bulk, ChNP and ChNP-GA. The treated cancer cells were microscopically observed after 6 h, 24 h and 48 h intervals. We have taken triplicate samples for each group to obtain statistical calculations.
Cell morphology
After the treatments of Ch-bulk, ChNP and ChNP-GA, cancer cells from different concentrations were removed from the CO2 incubator and were observed under an inverted microscope (TS100F Eclipse, Nikon, Tokyo, Japan) to evaluate the morphological changes and each sample was observed under 200 and 400 magnifications respectively.
MTT assay
The cancer cells were seeded with 6 × 104 cells/mL concentration in 96-well cell culture plates and incubated in CO2 incubator. The cells were cultured in the media containing DMEM, 10% Fetal bovine serum, 1% penicillin (100 IU/mL) and streptomycin (100 μg/mL) and kept in the CO2 incubator with 37° C and 5% CO2 condition. Once cells reached to 80% confluency, they were treated with Ch-bulk, ChNP and ChNP-GA with these concentrations 2.0 mg/mL, 4.0 mg/mL, 6 mg/mL and 8 mg/mL respectively. No Ch-bulk, ChNP and ChNP-GA were not added to the control groups. Thereafter 20 μL of MTT (5 mg/mL) solution was added to each well and were further incubated for 4 h, then the media was changed with the addition of DMSO. The samples were then measured using an ELISA plate reader (Synergy NEO2, Biotek Instruments, Winooski, VT, USA) at 570 nm wavelength.
Percentage (%) of cell viability was calculated as per below-given formula:
Statistical analysis
The mean ± standard deviation (SD) from control and Ch-bulk, ChNP and ChNP-GA group was calculated. All statistical analyses were completed with GraphPad Prism 6 (GraphPad Software). The difference between control and Ch-bulk, ChNP and ChNP-GA group by a one-way analysis of variance (ANOVA) test (p < .05). p < .05 was considered statistically significant.
Results
Characterizations of Ch-bulk, ChNP and ChNP-GA
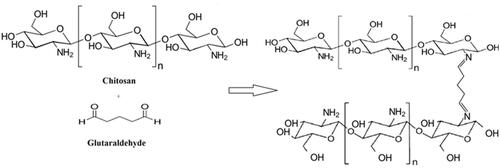
XRD Measurement: The XRD pattern of pure Ch-bulk and ChNP showed the characteristic amorphous structure of chitosan (). The first crystalline peaks of Ch-bulk and ChNP were found to be around 2θ of 10.33° and 9.94°, respectively. The main peak for Ch-bulk was at 20.53° with d = 4.32 Å, while for ChNP it was 20.02° and d = 4.43 Å. On the other hand, crosslinked chitosan glutaraldehyde (ChNP-GA) showed the peak of chitosan that around 10° completely disappeared ().
Figure 2. The X-ray diffraction patterns of (a) Ch-bulk, (b) ChNP and (c) ChNP-GA: First crystalline peaks of Ch-bulk and ChNP was found to be around 2θ of 10.33° and 9.94°, respectively. The main peak for Ch-bulk was at 20.53° with d = 4.32 Å, while for ChNP it was 20.02° and d = 4.43 Å. ChNP-GA showed the peak of chitosan that around 10° completely disappeared.
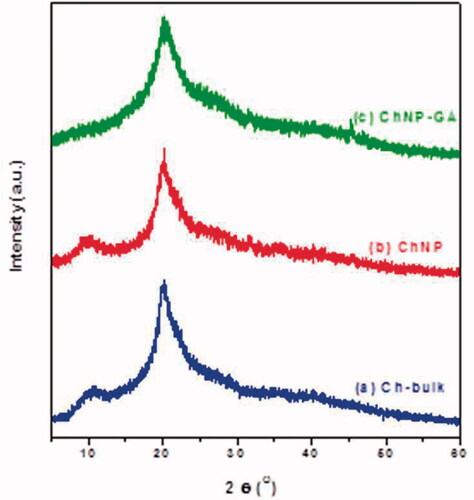
FT-IR analysis: FT-IR spectrum of Ch-bulk, ChNP and ChNP-GA were shown in . We have observed that Ch-bulk and ChNP showed broad absorption peaks at 3292 cm−1 and 3315 cm−1 respectively. Interestingly, ChNP-GA exhibited a significantly lower absorbance than Ch-bulk and ChNP. In addition, the broader and stronger peak shifted significantly to higher wave number at 3341 cm−1, while the peak at 1561 cm−1 refers to the amide II band. Other characteristic peaks of CH3 and CH2 symmetric stretch of chitosan polymer were present 2860 cm−1, 2861 cm−1 and 2874 cm−1 for Ch-bulk, ChNP and ChNP-GA, respectively. A FTIR spectrum depicts markedly shift in various peaks of ChNP-GA.
Figure 3. FTIR Spectra of (a) Ch-bulk, (b) ChNP and (c) ChNP-GA: Ch-bulk and ChNP showed broad absorption peaks at 3292 cm−1 and 3315 cm−1 respectively. ChNP-GA exhibited a significantly lower absorbance than Ch-bulk and ChNP. Other characteristic peaks of CH3 and CH2 symmetric stretch of chitosan polymer were present 2860 cm−1, 2861 cm−1 and 2874 cm−1 for Ch-bulk, ChNP and ChNP-GA, respectively.
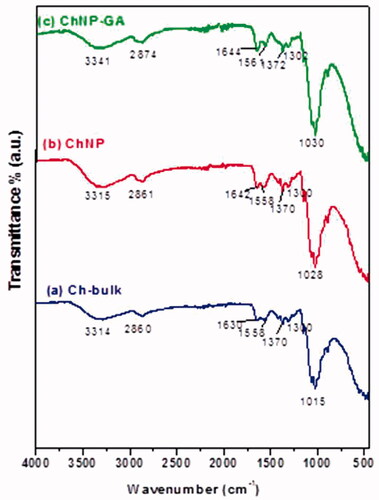
TGA analysis: TGA analysis showed a simple and smooth diagram of the thermal degradation of ChNP and ChNP-GA (). In the case of ChNP, a degradation temperature was found to be directly related to overall weight loss. The first weight loss was in between 100 °C and 240 °C in case of ChNP, whereas the major degradation was observed in the range 240–400 °C. The last range (temperature higher than 450 °C) was attributed to the thermal decomposition of pyranose ring and the destruction of the remaining carbon with weight loss. In case of ChNP-GA, there was an additional weight loss observed at after 400 °C, which referred to thermal degradation of glutaraldehyde. Before 200 °C, the weight loss of water molecules of ChNP-GA (9 wt.%) was higher than ChNP, referring that the sample prefers to keep the more amount of water molecules and removes it more easily. A dramatic decomposition process started in the second stage, and crosslinked glutaraldehyde sample showed a higher temperature (up to 390 °C) with a weight loss of 24%. Finally, a long curve of ChNP-GA exhibited and reached 700 °C (18 wt.%) with different evident features.
Figure 4. TGA curves of (a) ChNP and (b) ChNP-GA: In case of ChNP, a degradation temperature was found to be directly related to overall weight loss. The first weight loss was in between 100 °C and 240 °C in case of ChNP, whereas the major degradation was observed in the range 240–400 °C. The last range (temperature higher than 450 °C) was attributed to the thermal decomposition of pyranose ring and the destruction of the remaining carbon with weight loss. In case of ChNP-GA, there was an additional weight loss observed at after 400 °C, which referred to thermal degradation of glutaraldehyde.
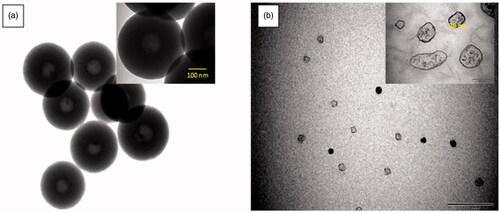
TEM analysis: TEM analysis showed that ChNP and ChNP-GA were spherical in shapes (). The particle size and morphology of chitosan nanoparticles has markedly changed without (a) and with (b) glutaraldehyde. TEM images of pure chitosan nanoparticles ChNP () showed a uniform size distribution of a spherical hollow shape with an average diameter 350 nm, However, revealed characterization of ChNP-GA, the particles sizes were lower than ChNP and the diameter of spherical nanoparticles was to be decreased significantly to around 30 nm. ChNP-GA showed to have fewer regular surfaces and edges.
Figure 5. TEM images of (a) ChNP and (b) ChNP-GA: (a) showed a uniform size distribution of a spherical hollow shape with an average diameter 350 nm, however, (b) revealed characterization of ChNP-GA, the particles sizes were lower than ChNP and the diameter of spherical nanoparticles was to be decreased significantly to around 30 nm. ChNP-GA showed to have fewer regular surfaces and edges.
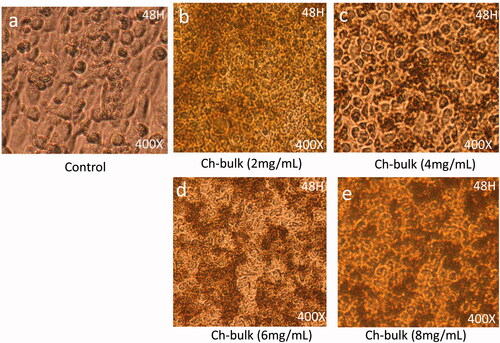
Effect of chitosan-bulk (Ch-bulk) on cancer cell morphology
Post 48-h treatment of each-bulk with dose 2 mg/mL showed significant levels of nucleus condensation and nuclear augmentation of the HCT-116 cells () with compared to control cells (). The dose of 4 mg/mL showed strong nuclear condensation and augmentation and showed the beginning of cell membrane disruption (). The dosages of 6 mg/ML and 8 mg/mL respectively, showed a significant loss of cell population as many cancer cells were found dead ().
Figure 6. Cancer Cell morphology after treatment of Ch-bulk: The HCT-116 cells analyzed 48 h post-treatments (a) is control; (b) is treated with dose 2 mg/mL, (c) is treated with 4 mg/mL, whereas (d) and (e) are treated with 6 mg/mL and 8 mg/mL. The dose of 4 mg/mL showed strong nuclear condensation and augmentation and showed beginning of cell membrane disruption. 400× magnifications.
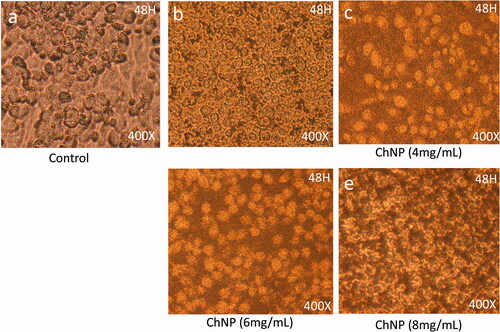
Effect of chitosan nanoparticles (ChNP) on cancer cell morphology
Post 48-h treatment of ChNP-GA with dose 2 mg/mL showed significant levels of nucleus condensation and nuclear augmentation of the HCT-116 cells () with compared to control cells (). The dose of 4 mg/mL showed strong nuclear condensation and augmentation and showed strong cell membrane disruption (). The dosages of 6 mg/ML and 8 mg/mL respectively, showed a significant loss of cell population as large number of cancer cells were found dead ().
Figure 7. Cancer Cell morphology after treatment of ChNP: The HCT-116 cells analyzed 48 h post-treatments (a) is control; (b) is treated with dose 2 mg/mL, (c) is treated with 4 mg/mL, whereas (d) and (e) are treated with 6 mg/mL and 8 mg/mL. The dose of 4 mg/mL showed strong nuclear condensation and augmentation and showed strong cell membrane disruption. 400× magnifications.
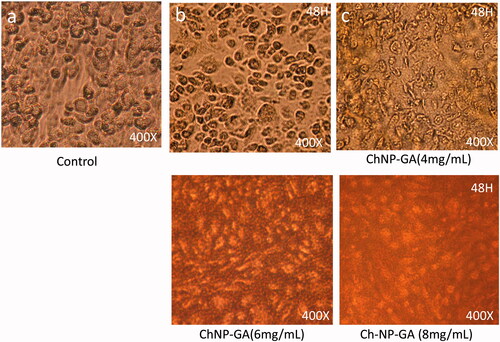
Effect of chitosan nanoparticles (ChNP) conjugated with glutaraldehyde (GA) on cancer cell morphology
Post 48-h treatment of ChNP with dose 2 mg/mL showed significant levels of nucleus condensation and nuclear augmentation of the HCT-116 cells () with compared to control cells (). The dose of 4 mg/mL showed complete loss of cell internal organelles and complete disappearance of the nucleus (). The dosages of 6 mg/ML and 8 mg/mL respectively, showed a complete loss of cell population as almost all the cancer cells were found dead ().
Figure 8. Cancer Cell morphology after treatment of ChNP-GA: The HCT-116 cells analyzed 48 h post-treatments (a) is control; (b) is treated with dose 2 mg/mL, (c) is treated with 4 mg/mL, whereas (d) and (e) are treated with 6 mg/mL and 8 mg/mL. The dose of 4 mg/mL showed complete loss of cell internal organelles and complete disappearance of nucleus. 400× magnifications.
Effect of Ch-bulk, ChNP, and ChNP-GA on cancer cell survivability:
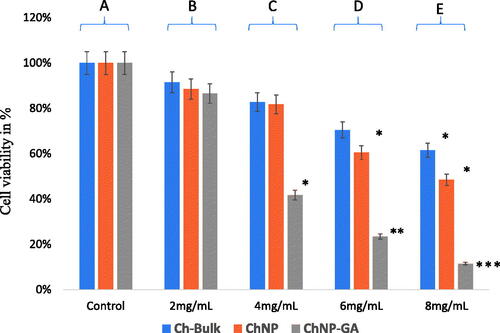
Ch-bulk, ChNP, and ChNP-GA decrease cell viability of HCT-116 cells by dose-dependent manner. MTT assays were carried out to examine cell viability and the inhibition rate of HCT-116 cell line. MTT assay was carried with different concentrations of Ch-bulk, ChNP-GA and ChNP, and for 48 h and treatment with 2 mg/mL dose showed 91.50%, 88.50% and 86.50% cell viability, whereas treatment with 4 mg/mL dose showed 82.75%, 81.75% and 41.75% cell viability. When cancer cells were treated with 6 mg/mL, cell viability decreased by 70.50%, 60.50% and 23.50%, respectively. The most profound effect was observed with 8 mg/mL, where cancer cells survivability was decreased by 61.50%, 48.50% and 11.50%, respectively. ().
Figure 9. Cancer cell viability by MTT Assay. The HCT-116 cells were treated with Ch-bulk, ChNP, and ChNP-GA. Ch-bulk ChNP, and ChNP-GA treated with concentrations (2 mg/mL, 4 mg/mL, 6 mg/mL, 8 mg/mL for 48 h. Data are the means ± SD of three different experiments. Difference between two treatment groups was analyzed by Student’s t-test where (*p < .05, **p < .01; ***p < .001), p values were calculated by Student’s t-test. No changes were observed in control group (A); 2 mg/mL treatments did not show any significant decrease in cell survivability (B); 4 mg/ml treatments (ChNP-GA) showed a significant decrease in cell survivability, whereas Ch-bulk and ChNP did show significant impact (C); 6 mg/mL4mg/ml treatments (ChNP-GA and ChNP) showed a significant decrease in cell survivability, whereas Ch-bulk did show significant impact (D); and 8 mg/ml treatments (Ch-bulk) showed a significant decrease in cell survivability, whereas Ch-bulk and ChNP highly significant impact on cell survivability (E).
Discussion
In the present study, we have synthesized three different chitosan-based particles (Ch-bulk, ChNP, ChNP-GA) with different size and shapes. The XRD analysis showed that main peak for Ch-bulk was at 20.53° with d = 4.32 Å, while for ChNP it was 20.02° and d = 4.43 Å. ChNP-GA showed the peak of chitosan that around 10° completely disappeared. The disappearance of peak is due to the reaction between chitosan and glutaraldehyde by cross-linking mechanism. It has been reported that the deformation of hydrogen bonds of hydroxyl and amino groups leads to strongly change in the arrangements of original chitosan chain [Citation27]. Consequently, the main peak of chitosan shifted to 20.24° and became more amorphous and board due to reduction in the crystallinity after cross-linking with GA. This agrees with the results reported by Oyrton et al. [Citation28]. Furthermore, an irregular chitosan molecular structure as well leads to decreased d-spacing to 4.38 Å.
FT-IR analysis demonstrates that many functional groups of absorption bands have similar frequency. The spectral bands for chitosan and the copolymers produce due to several bands from multiple functional groups. As a result, a detailed characterization of the particles by FTIR analysis is complicated [Citation29]. It has been reported that pure chitosan (Ch-bulk and ChNP), broad absorption peaks at 3292 cm−1 and 3315 cm−1 attributed to O–H stretching mode [Citation30]. Moreover, the increased numbers of hydrogen bonds were formed between chitosan chins leads to the narrow peak of OH band [Citation31]. The absorption peaks 1630 cm−1, 1558 cm−1, 1370 cm−1, and peaks 1642 cm−1, 1558 cm−1, and 1370 cm−1 were assigned to the C = O stretching vibration of amide I, bending vibrations of the N–H (N-acetylated residues, amide II band), and OH bending, respectively [Citation32,Citation33]. Further, the peaks were observed at 1015 cm−1 and 1028 cm−1 corresponding to C–O–C bending vibration and C–OH stretching vibration [Citation34]. For ChNP-GA sample, interestingly, the sample exhibited a significantly lower absorbance than pure chitosan. In addition, the broader and stronger peak shifted significantly to higher wavenumber at 3341 cm−1, while the peak at 1561 cm−1 refers to amide II band. This confirms cross-linking of chitosan with the glutaraldehyde [Citation29]. These peaks that due to the presence of major amine groups reduced noticeably in ChNP-GA is indicating that the glutaraldehyde molecules were bound to these groups [Citation35]. These results agree with XRD results. Other characteristic peaks of CH3 and CH2 symmetric stretch of chitosan polymer were present 2860 cm−1, 2861 cm−1 and 2874 cm−1 for Ch-bulk, ChNP and ChNP-GA, respectively. An FTIR spectrum depicts markedly shift in various peaks of ChNP-GA.
TGA analysis showed a smooth thermal degradation of pure and glutaraldehyde cross-linked to chitosan nanoparticles. The decrease in the thermal degradation is attributed to loss of particles weight. For example, it has been reported that weight loss between 100 °C and 240 °C indicated to remove water molecules with equivalent 4% of the total weight [Citation36]. Furthermore, the major degradation in the range 240–400 °C can be ascribed to the thermal and oxidative decomposition of chitosan, specifically the decomposition of amine groups and polymer chain degradation through the split of glycosidic linkages and caused a weight loss of 15 wt.% [Citation37]. The last range (temperature higher than 450 °C) was attributed to the thermal decomposition of pyranose ring and the destruction of the remaining carbon with weight loss around 6% [Citation38]. However, for ChNP-GA, there was an additional weight loss observed at after 400 °C, which referred to thermal degradation of glutaraldehyde. Before 200 °C, the weight loss of water molecules of ChNP-GA (9 wt.%) was higher than ChNP, referring that the sample prefers to keep more the amount of water molecules and removes it more easily. This result indicates that the amounts of amino groups are very few at glutaraldehyde-crosslinked chitosan [Citation35]. A dramatic decomposition process started in the second stage, and crosslinked glutaraldehyde sample shows higher temperature (up to 390 °C) with a weight loss of 24%. Finally, a long curve of ChNP-GA exhibited and reached 700 °C (18 wt.%) with different evident features. TGA analysis allowed to observe the effect of glutaraldehyde on the thermal stability, that the total weight loss corresponds to 51% and thereby confirms enhanced thermal stability of the ChNP-GA in comparison with the pure chitosan (ChNP).
TEM analysis of pure ChNP showed a uniform size distribution of a spherical hollow shape with an average diameter 350 nm. The formation of hollow structure could be due to method of fitting and self-assembly process might be another reason, which usually produces hollow nanosphere [Citation37]. ChNP-GA showed fewer regular surfaces and edges. Furthermore, the advantage of the nanoparticles with smaller sizes that they have small volumes to larger surface area. Thus, may have a high capacity of drug-loading and a slow rate of drug-diffusion. Benefits of applying small-size particles as a carrier system involve the high cellular uptake, able to penetrate arterial walls and good suspensibility [Citation39].
Once we have confirmed physical and structural properties of Ch-bulk, ChNO and ChNP-GA, we wanted to study whether chitosan nanoparticles have any biological activities. In the study, we have shown for the first time that Chitosan nanoparticles significantly decreased the cancer cell survivability with dose-dependent manner. The morphological and microscopic data showed that Ch-bulk, ChNP, and ChNP-GA not only affected the cancer cell membrane but also induced nuclear condensation, augmentation and disintegration with dose-dependent manner. There are a couple of studies which have demonstrated that chitosan nanoparticles have cytotoxic effects on the cancer cells [Citation40,Citation41]. In the present study, we have used human colorectal carcinoma cells (HCT-116) to evaluate anti-cancer properties of Ch-bulk, ChNP and ChNP-GA, respectively. Moreover, HCT-116 cells have been widely for testing anti-cancer drugs and molecules [Citation42–46].
In the present study, we have examined the effects of three types of chitosan such as Ch-bulk, ChNP, and ChNP-GA on cancer cells. Among these, ChNP-GA produced more profound effect on cancer cell morphology than Ch-bulk and ChNP respectively, while we do not reason for a profound impact of ChNP-GA on cancer cells. The role of glutaraldehyde conjugated with ChNP in inducing the cell death cannot be ruled out. The major morphological changes observed after 48 h of ChNP-GA treatments were nuclear condensation, nuclear augmentation and chromatin condensation. Strikingly, the dose with 6 mg/mL and 8 mg/mL showed significant decrease in the cancer cell population. Similar nature of effects was observed when cancer cells were treated with FMSP-nanoparticles [Citation25] and other nanoparticles [Citation47].
To examine the impact of Ch-bulk, ChNP and ChNP-GA on cancer cell survivability, we have compared the dead cells with live cells by staining with MTT. We have found that all three-chitosan showed dose-dependent effects on cancer cell survivability. The dose 2 mg/mL did not affect cell survivability significantly, whereas the cell survivability decreases with dose-dependent manner with 4 mg/mL, 6 mg/mL and 8 mg/mL dosages. These results confirm that chitosan bulk (Ch-bulk) and chitosan nanoparticles (ChNP and ChNP-GA) have dose-dependent effects on the cancer cells. It has been previously observed that FMSP-nanoparticles showed dose-dependent response on cancer cells [Citation25,Citation16].
Conclusions
In the present study, we have shown that chitosan nanoparticles (Ch-bulk, ChNP and ChNP-GA) decreased cancer cell proliferation in a dose-dependent manner. Lower dosage (2 mg/mL) produced no significant impact on the cancer cell survival, whereas higher dosages 4 mg/mL, 6 mg/mL 8 mg/mL caused a highly significant cell death during the same period. In addition, we have shown Ch-bulk, ChNP and ChNP-GA treated cancer cells undergo nuclear disintegration and fragmentation. These results suggest that Ch-bulk, ChNP and ChNP-GA are potential biomaterials could be used in the treatment of colon cancer.
| Abbreviations | ||
| CO2 | = | Carbon dioxide |
| DMSO | = | Dimethyl sulfoxide |
| DMEM | = | Dulbecco's Modified Eagle Medium |
| FBS | = | Fetal bovine serum |
| IU | = | International Unit |
| FMSP-nanoparticles | = | Fluorescent magnetic submicronic polymer nanoparticles |
| MTT | = | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| nm | = | Nanometer |
| NPs | = | Nanoparticles |
| OD | = | Optical density |
| TEM | = | Transmission electron microscopy |
| µg | = | Microgram |
| µl | = | Microliter |
| mg | = | Milligram |
| mL | = | Millilitre |
Acknowledgements
Authors are thankful to the entire management of the Institute for Research & Medical Consultations (IMRC), Imam Abdulrahman Bin Faisal University, Dammam, Kingdom of Saudi Arabia for their support and encouragement. We are thankful to Mr Dionecio Jr. Bagon Dela Roca for assisting the cell culture and bioassay related work. We are also thankful to Dr Khaldoon M. Alsamman, Clinical Laboratory Science, College of Applied Medical Science, Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia for providing HCT-116 cell line.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Zia KM, Tabasum S, Khan MF, et al. Recent trends on gellan gum blends with natural and syntheticpolymers. Int J Biol Macromol. 2018;109:1068–1087.
- James RS, Lillard W. Jr, Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223.
- Ghormade V, Deshpande MV, Paknikar KM. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol Adv. 2011;29:792–803.
- Zeng M, Zhu L, Li L, et al. miR-378 suppresses the proliferation, migration and invasion of colon cancer cells by inhibiting SDAD1. Cell Mol Biol Lett. 2017;22:12.
- Zhao L-M, Shi L-E, Zhang Z-L, et al. Preparation and application of chitosan nanoparticles and nanofibers. Braz J Chem Eng. 2011;28:353–362.
- Divya K, Jisha MS. Chitosan nanoparticles preparation and applications. Environ Chem Lett. 2018;16:101–112.
- Rinaudo M. Chitin and chitosan: properties and applications. Prog Poly Sci. 2006;31:603–632.
- Csaba N, Köping-Höggård M, Alonso MJ. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int J Pharm. 2009;382:205–214.
- Shiraishi S, Imai T, Otagiri M. Controlled release of indomethacin by chitosan polyelectrolyte complex: optimization and in vivo/in vitro evaluation. J Control Rel. 1993;25:217–225.
- Grenha A. Chitosan nanoparticles: a survey of preparation methods. J Drug Target. 2012;20:291–300.
- Kamat V, Marathe I, Ghormade V, et al. Synthesis of monodisperse chitosan nanoparticles and in situ drug loading using active microreactor. ACS Appl Mater Interfaces. 2015;7:22839–22847.
- Al-Remawi MMA. properties of chitosan nanoparticles formed using sulfate anions as crosslinking bridges. Am J Appl Sci. 2012;9:1091–1100.
- Larbi F, García A, del Valle LJ, et al. Comparison of nanocrystals and nanofibers produced from shrimp shell α-chitin: from energy production to material cytotoxicity and pickering emulsion properties. Carbohydr Poly. 2018;196:385–397.
- Malmiri HJ, Jahanian MAG, Aydin B. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am J Biochem Biotechnol. 2012;8:203–219.
- Gottlieb E, Armour SM, Harris MH, et al. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717.
- Khan FA, Akhtar S, Almohazey D, et al. Fluorescent magnetic submicronic polymer (FMSP) nanoparticles induce cell death in human colorectal carcinoma cells. Artif Cells Nanomed Biotechnol. 2018;25:1–7.
- Unfried K, Albrecht C, Klotz LO, et al. Cellular responses to nanoparticles: target structures and mechanisms. Nanotoxicology. 2007;1:52–71.
- Hanley C, Layne J, Punnoose A. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19:295103.
- Turcotte S, Chan DA, Sutphin PD, et al. A molecule targeting VHL-deficient renal cell carcinoma that induces autophagy. Cancer Cell. 2008;14:90–102.
- Wang H, Wick RL, Xing B. Toxicity of nanoparticulate and bulk ZnO, Al2O3 and TiO2 to the nematode Caenorhabditis elegans. Environ Pollut. 2009;157:1171–1177.
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Del. 2010;7:1063–1077.
- Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011;21:113–119.
- Wang X, Wang W, Li L, et al. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2014;1842:1240–1247.
- Yaffee P, Osipov A, Tan C, et al. Review of systemic therapies for locally advanced and metastatic rectal cancer. J Gastrointest Oncol. 2015;6:185–200.
- Khan FA, Akhtar S, Almofty SA, et al. FMSP-nanoparticles induced cell death on human breast adenocarcinoma cell line (MCF-7 Cells): morphometric analysis. Biomolecules. 2018;8:32.
- Smalley KS, Herlyn M. Towards the targeted therapy of melanoma. Mini Rev Med Chem. 2006;6:387–393.
- Nagireddi S, Katiyar V, Uppaluri R. Pd(II) adsorption characteristics of glutaraldehyde cross-linked chitosan copolymer resin. Int J Biol Macromol. 2017;94:72–84.
- Monteiro OAC, Jr, Airoldi C. Some studies of crosslinking chitosan–glutaraldehyde interaction in a homogeneous system. Int J Biol Macromol. 1999;26:119–128.
- Poon L, Wilson LD, Headley JV. Chitosan-glutaraldehyde copolymers and their sorption properties. Carbohydr Poly. 2014;109:92–101.
- Skwarczynska AL, Kuberski S, Maniukiewicz W, et al. Thermosensitive chitosan gels containing calcium glycerophosphate. Spectrochim Acta A Mol Biomol Spectrosc. 2018;201:24–33.
- Pratt DY, Wilson LD, Kozinski JA. Preparation and sorption studies of glutaraldehyde cross-linked chitosan copolymers. J Colloid Interface Sci. 2013;395:205–211.
- Mauricio-Sánchez RA, Salazar R, Luna-Bárcenas JG, et al. FTIR spectroscopy studies on the spontaneous neutralization of chitosan acetate films by moisture conditioning. Vibrational Spectrosc. 2018;94:1–6.
- Yasmeen S, Kabiraz M, Saha B, et al. Chromium (VI) ions removal from tannery effluent using chitosan-microcrystalline cellulose composite as adsorbent. Irjpac. 2016;10:1–14.
- Branca C, Khouzami K, Wanderlingh U, et al. Effect of intercalated chitosan/clay nanostructures on concentrated pluronic F127 solution: a FTIR-ATR, DSC and rheological study. J Colloid Interface Sci. 2018;517:221–229.
- Baroni P, Vieira RS, Meneghetti E, et al. Evaluation of batch adsorption of chromium ions on natural and crosslinked chitosan membranes. J Hazardous Mater. 2008;152:1155–1163.
- Habiba U, Siddique TA, Lee JJL, et al. Adsorption study of methyl orange by chitosan/polyvinyl alcohol/zeolite electrospun composite nanofibrous membrane. Carbohydr Poly. 2018;191:79–85.
- El-Sawy NM, Abd El-Rehim HA, Elbarbary AM, et al. Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr Poly. 2010;79:555–562.
- Moussout H, Ahlafi H, Aazza M, et al. Kinetics and mechanism of the thermal degradation of biopolymers chitin and chitosan using thermogravimetric analysis. Poly Degrad Stab. 2016;130:1–9.
- Bhattara N, Hassna RR, Shinn-Huey C, et al. Chitosan and lactic acid-grafted chitosan nanoparticles as carriers for prolonged drug delivery. Int J Nanomed. 2006;1:181–187.
- Sadreddini S, Safaralizadeh R, Baradaran B, et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol Lett. 2017;181:79–86.
- Siahmansouri H, Somi MH, Babaloo Z, et al. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT-29 colorectal cancer cell line. J Pharm Pharmacol. 2016;68:1119–1130.
- Sudeep HV, Gouthamchandra K, Venkatesh BJ, et al. Viwithan, a Standardized Withania somnifera Root extract induces apoptosis in murine melanoma cells. Pharmacogn Mag. 2018;13:S801–S806.
- Mazewski C, Liang K, Gonzalez de Mejia E. Comparison of the effect of chemical composition of anthocyanin-rich plant extracts on colon cancer cell proliferation and their potential mechanism of action using in vitro, in silico, and biochemical assays. Food Chem. 2018;242:378–388.
- Asif M, Shafaei A, Abdul Majid AS, et al. Mesua ferrea stem bark extract induces apoptosis and inhibits metastasis in human colorectal carcinoma HCT 116 cells, through modulation of multiple cell signalling pathways. Chin J Nat Med. 2017;15:505–514.
- Zheng Z, Jiang J, Guo J, et al. Bio-inspired coplanar-gate-coupled ITO-free oxide-based transistors employing natural nontoxic bio-polymer electrolyte. Org Electron. 2016;37:474–478.
- Sinha A, Banerjee K, Banerjee A, et al. Induction of apoptosis in human colorectal cancer cell line, HCT-116 by a vanadium- Schiff base complex. Biomed Pharmacother. 2017;92:509–518.
- Zielinska E, Zauszkiewicz-Pawlak A, Wojcik M, et al. Silver nanoparticles of different sizes induce a mixed type of programmed cell death in human pancreatic ductal adenocarcinoma. Oncotarget. 2018;9:4675–4697.
