?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Supercritical fluid (SCF) technology offers a potential green alternative to organic solvent-based methods for drug formulation. Albendazole (ABZ) has promising anticancer activity when formulated to increase its cellular uptake. Herein, a static volume method was used to determine the solubility of ABZ in supercritical carbon dioxide (scCO2) for the future development of such ABZ formulations. The solubility of ABZ in scCO2 (250 bar, 37 °C) was approximately 12 mg/100 mL. The extent of dissolution was measured at various time points to determine when saturation solubility occurred, which was demonstrated from 9 h. In order to determine if scCO2 processing induced ABZ polymorphism, DSC/TGA, FTIR and XRD were used, which demonstrated no change in its solid state. Following this, ABZ loaded liposomes were manufactured using SCF technology. The liposomes diameter was 167.2 ± 5.3 nm as determined by Zetasizer, and confirmed by cryo-transmission electron microscopy. In conclusion, scCO2 was used successfully to solubilize ABZ, and to manufacture liposomes of nano-sized range. This study provides insight into use of green technology for future ABZ liposomal formulation without the need for organic solvents.
Graphical Abstract

Introduction
The literature is silent on the solubility data of albendazole (ABZ) in supercritical carbon dioxide (scCO2). In order to develop an ABZ dosage form, such as liposomes (in current study) prepared by a supercritical fluid (SCF) method, the solubility of ABZ in SCF (scCO2 in the current study) must be determined. This would help to set experimental parameters such as the amount of ABZ to be added in relation to the SCF as solvent and equally important the required SCF conditions (i.e. temperature and pressure). Moreover, SCF processing can drive polymorphic changes in organic compounds, but no data is available regarding the effects of scCO2 on the solid-state properties of ABZ [Citation1]. Such questions need to be investigated as part of the pre-formulation studies to provide basic but important information before formulating ABZ loaded liposomes.
ABZ is a colorless, non-ionizable, lipophilic (Log P 2.7), low molecular weight (265.33 g/mol) crystalline compound, which is insoluble in water (<1.3 µg/mL) and displays polymorphism in its solid state [Citation2–5]. ABZ is registered as an anthelmintic agent and was patented in 1975 [Citation6]. It is used for the treatment of various infections caused by parasitic worms [Citation7–9]. Recently, it has been found that ABZ may also have anticancer properties, when prepared in formulations that enhance its cellular uptake [Citation10]. The different types of conventional methods (e.g. thin film method for production of liposomes) currently utilize a substantial amount of organic solvent/s, which are highly undesirable and unsustainable, and very likely to have an increased focus from a regulatory standpoint. The need for alternative preparation methods that do not require organic solvents has become important as their impact on the environment becomes increasingly problematic. With that in mind, the interest in alternative solvents expected to grow, along with determining the solubility of therapeutic compounds in such solvents. SCF-based methods have the potential to provide a low energy alternative process that is environmentally friendly and sustainable. SCFs are compounds, which form a single phase with properties of both a liquid and gas when subjected to conditions above their critical point: critical temperature (Tc) and critical pressure (Pc) [Citation11,Citation12]. They have diffusivity higher than a liquid and viscosity higher than a gas, creating high solvent power for many hydrocarbons that can be manipulated by changing temperature and pressure. This solvent tuneability makes SCF superior as a solvent compared to conventional solvents. The suitability of the SCF as a solvent, must be determined as early as possible for a given therapeutic in order to proceed and develop a formulation prepared by SCF processing. A simple gravimetric analysis, which has previously been validated for determining the solubility of naphthalene and phenanthrene in scCO2, was adapted in this study to determine the solubility of ABZ in scCO2 [Citation13]. Following this, the SCF technology was used to manufacture ABZ loaded liposomes.
The crystallization process of a given solute can result in the formation of different polymorphic forms and this process is driven by conditions such as temperature, solvent properties, and stirring rate. Given scCO2 is a unique solvent, and a process controlled by temperature, a range of solid state characterization methods were used to assess polymorphic changes using Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) analysis, diffraction scanning calorimetry (DSC) and thermogravimetric analysis (TGA) on the commercial and scCO2 processed ABZ. The result of the solid-state characterization provided direction for the manufacturing of liposomes; hence, SCF technology was further employed to manufacture liposomes. The manufactured liposomes were analysed for particle sizing and surface charge of liposomes. Cryo transmission electron microscopy (Cryo-TEM) was used to confirm morphology and particle size. In order to quantify the drug loading capacity, a method was developed using high performance liquid chromatography (HPLC). The time dependent drug release of the liposomes was performed at 37° C and compared with the control. This study provides original data on the solubility of ABZ in scCO2, changes in solid state due to scCO2 processing, production of liposomes using scCO2, drug loading capacity, particle size and morphology, and drug release using dialysis membranes, which is essential pre-formulation information and will be useful for subsequent research into ABZ loaded delivery system using SCF-assisted methods.
Materials and methods
Materials
ABZ (analytical standard; ≥98%) was purchased from Sigma-Aldrich NSW, Australia (CAS number: 54965–21-8), liquid CO2 bottle (having liquid draw tube inside) from BOC gas Australia, Ethanol was purchased from Merck Millipore (Kilsynth, VIC, Australia). Orthophosphoric acid aqueous solution (85% w/w) and dipotassium hydrogen ortho-phosphate from Chem-supply (Gillman, SA, Austrailia), dialysis bags (Cut-off M.W 10 K Daltons) from ThermoFischer SCIENTIFIC, Phosphate buffered saline from Sigma Aldrich, Australia, DSPC (1,2-distearoyl-sn-glycero-3-phosphocholine), DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine), and DSPE-PEG-2000 (1,2-distearoyl-sn-glycero-3-phospho- ethanolamine-N-[methoxy(polyethylene glycol)-2000] (ammonium salt)) were purchased from Avanti Polar Lipids (Alabama, USA). All solvents used in the study were of analytical and high-pressure liquid chromatography (HPLC) grade.
Determination of ABZ solubility in scCO2
The solubility of ABZ in scCO2 was determined using the already established method by Sherman, et al. [Citation13]. ABZ (50 mg) was weighed in a stainless steel (SS) sample holder (internal diameter of 19 mm), covered with SS mesh (21 µm pore size) and clamp. The sample was then placed into a 60 mL SS vessel (Nottingham, UK). Liquid CO2 was then pumped into the closed SS vessel using a syringe pump (Teledyne Isco Series 260 D with controller, Lincoln, USA) and compressed to make scCO2 at predetermined pressure of 250 bar. The temperature was controlled using a Watlow (St Louis, Missouri, USA) heating band (600 W) to 37 °C. An overhead stirring motor and paddle was used to stir at 200 RPM. A series of scCO2 processing experiments were performed on separate samples (with a mesh cover) for up to 18 h. After processing, the heating unit was switched off and CO2 (as gas) was vented. A 12-h experiment was also performed separately without mesh to assess if the presence of the mesh hindered the dissolution of ABZ in scCO2.
Once the system was completely depressurized, the SS vessel was opened and the sample holder was removed and weighed. The solubility of the ABZ was determined by calculating the difference in the weight of the sample holder containing ABZ before and after processing in scCO2.
DSC and TGA
DSC and TGA analyses were carried out with Mettler DSC equipment (Mettler Toledo TGA/DSC, 2 STAR System) using ranges based on previously reported data [Citation14,Citation15]. Samples of ABZ (commercial grade and scCO2 processed) of approximately 2–3 mg were transferred into aluminum crucibles. Heating runs were carried out under compressed air at a 10 °C min−1 rate over 30–225 °C and 30–400 °C for DSC and TGA, respectively.
FTIR
FTIR spectroscopy of ABZ, (pure commercial and scCO2 processed) was conducted to examine the spectrum for any change in the structure. The IR spectra of samples were recorded on a Bruker Tensor (Bruker Optik GmbH, Germany) with a triple bounce diamond. Scanning was performed between the 600 and 4000 cm−1 region with 20 scans/sample and a resolution of 4 cm−1. Data was analyzed using OPUS software (version 4) and graphed as the reciprocal of wavelength (i.e. wavenumbers or cm−1).
X-Ray diffraction
XRD patterns of the commercial and scCO2 processed ABZ were recorded using an XG X-Ray Diffractometer (Rigaku Smart Lab) at 9 kW. The source was Cu Kα radiation (λ = 1.5406 Å) and scanning angle 2θ ranged from 5° to 35° with a speed of 0.058°min−1 [Citation6]. The current and voltage used during operation were 200 mA and 45 kV, respectively. The results were analyzed using Diffrac (plus) EVA (Bruker AXS Inc., USA) software.
Production of ABZ liposomes using SCF technology
To manufacture ABZ loaded liposomes, DSPC and DSPE-PEG-2000 (94:6 molar ratio with 5 mM concentration) and 3 mg of ABZ (active drug) were added in the high-pressure SS vessel. The experiments were performed at 37 °C, 250 bar, 200 RPM, in a 60-mL-high-pressure SS vessel for 1 h. The apparatus used to manufacture liposomes is similar, as used and explained in above sections to determine saturation solubility of ABZ. The system was depressurised slowly at the end of experiment. The sudden drop of pressure led to the formation of CO2 gas from scCO2 and released back into the air at the end of experiments. The thin layer formed on the walls of vessels hydrated using 5 mL of phosphate buffer saline (PBS) (pH 7.4, 10 mM) by mixing and shaking at 65 °C for 30 min. Liposomes were later bath sonicated (Grant XUB18 Ultrasonic Water Baths) for 15 min above the transition temperature to determine the effect on particle size before and after sonication.
Free drug separation and determination of loading capacity
Free ABZ was separated from DSPC-PEG-ABZ liposomes by centrifugation (1,000 g for 30 min at 4 °C) in a refrigerated centrifuge (Eppendorf Centrifuge 5804 R). The loading capacity from the supernatant having ABZ loaded DSPC-PEGylated liposomes was calculated by lysis of the manufactured liposomes with absolute methanol and sonicated for 10 min to dissolve completely [Citation14]. The concentration of ABZ was calculated by HPLC (method described in the following sections). The clear solution of liposomes and methanol containing ABZ was used for analysis by HPLC after centrifuging the sample at very high speed (14,000 g for 2 min). This method was developed at the assessed conditions to pellet free ABZ and supernatant was quantified. The following formula was used to calculate the drug loading.
Release profile
The in-vitro drug release of ABZ from liposomes was studied by dialysis (10,000 MW cut off; Sigma-Aldrich) method [Citation16]. Two mL of the DSPC-PEG-ABZ and standard ABZ formulation in PBS (control) were added into two separate dialysis bags and immersed in 2 × 50 mL of PBS (pH 7.4). The beakers containing 50 mL of PBS and dialysis bags were incubated on stirrer (200 RPM, 37 °C). One hundred microliters of PBS was taken at the different time intervals (0.5, 1, 1.5, 2, 3, 4, 8, 16, 24, 32, and 48 h) and replaced by fresh PBS. The method used was as previously published with some modifications [Citation16]. The samples were analysed by HPLC to quantify the drug using the standard calibration curve.
Quantification of ABZ by HPLC
Fifteen mM of dipotassium hydrogen orthophosphate buffer was prepared and the pH was adjusted by orthophosphoric acid to 3.8. Kinetex® C18 column was used (LC 250 × 4.6 mm, 5 μm (Phenomenex, USA) for quantification analysis. HPLC was run under isocratic conditions, eluting with 50% (v/v) acetonitrile, buffered with 15 mM dipotassium hydrogen orthophosphate buffer pH 3.8 in water. The elution was performed at 1 mL/min and 25 °C. All analyses were done at 292 nm in triplicate [Citation17]. The concentration of ABZ in liposomes was calculated using a standard calibration curve.
Characterization of liposomes
Determination of mean particle size and distribution by dynamic light scattering
The average diameter, polydispersity index (PDI) and zeta potential were measured by dynamic light scattering (DLS) with Zeta-Sizer Nano ZS (Malvern, UK). Disposable cuvettes were used to determine the Zeta-average particle size and PDI, and disposable folded zeta cells (DTS1070 cells from Malvern) were used to determine the zeta potential of the liposomes. All runs were performed in triplicate at 25 °C.
Morphological analysis by cryo-TEM
The liposome samples were imaged by Cryo-TEM. The samples were transferred grids with 300 mesh EM carbon coated film (EMS, Hatfield PA,USA) using a FEI Vitrobot Mark IV plunge freezer set (100% humidity) at 22 °C. The samples were vitrified using thin ice and plunged by liquid ethane at −180 °C. Frozen grids were cryo-transferred into a FEI Tecnai F30 TEM (FEI, Einhoven, Netherlands) using a Gatan cryo holder. The microscope operated at 300 kV and the samples were imaged at −179 °C under low dose conditions using a Gatan K2 summit camera (Gatan, Pleasonton CA, USA). This was operated in counting mode at a dose rate of 9 e/px/s, a total exposure 10 s and a dose fractionation of 0.2 s. For the acquisition of the image, the Serial EM was used. The 50 frame motion was corrected using the ‘Align’ function of Serial EM [Citation18–20].
Results
Determination of ABZ solubility in scCO2
Solubility data of ABZ in scCO2 is shown in . The mass of solute (mg) dissolved in scCO2 is plotted against time (h). A thin solid layer of ABZ attached on the wall of high-pressure SS vessel showed that ABZ was solubilized during processing and re-crystallized on the wall during decompression. The solubility of ABZ in scCO2 under the assessed experimental conditions (250 bar and 37 °C) was approximately 12 mg/100 mL after 9 h, which is where the solubility curve started to become linear. The solubility of ABZ after 12, 15, and 18 h was similar (97.7%) to that obtained after 9 h. As a precaution, one experiment without the SS mesh cover (21 µm pore size) was performed and the results were similar to those with the SS mesh cover, indicating that the mesh cover did not provide any hindrance for the mass transfer of ABZ into solution.
Figure 1. Equilibrium curve for ABZ in scCO2 at 37 °C and 250 bar. (●) with mesh and (▲) without mesh using 60 mL high-pressure vessel. Image demonstrates that the thickness of the thin layer of recrystallized ABZ in the apparatus increases over time up until (a) 9 h (b) 12 h and (c) 18 h. The grey dotted curve is the best fit line (y = 1.3392ln(x) + 3.7545) and dashed line is saturation solubility. Each data point is obtained from 11 separate experiments.
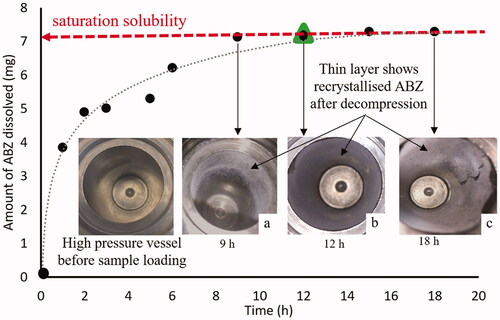
The data points were obtained from 11 separate experiments to determine saturation solubility of ABZ. The last 5 data points from 9 h, including the run without a mesh on the sample holder, showed a flattening of the dissolution curve, meaning that saturation solubility of ABZ in scCO2 was achieved.
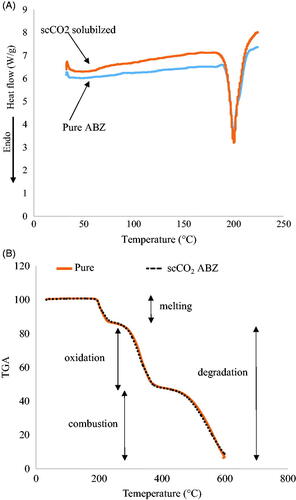
Solid state characterization by thermoanalysis
Thermograms of stock (commercial ABZ) and scCO2-processed ABZ are shown in . The melting point (MP) peaks overlapped with each other and showed no change, indicating that no polymorphism was observed in commercially available form of ABZ. In addition to DSC (), the TGA thermograms () also confirmed no change in the polymorphic state of commercially available form of ABZ after processing with scCO2. shows that the MP of ABZ (approx. 200 °C) did not change after processing with scCO2.
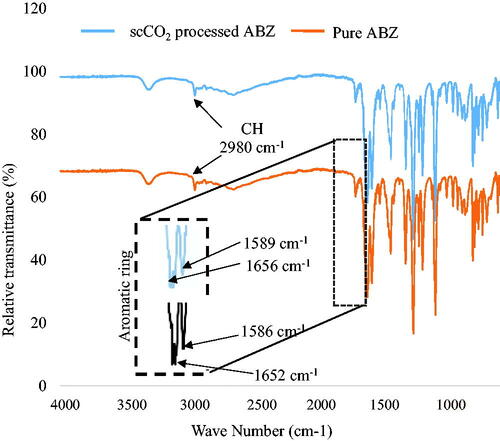
FTIR
The FTIR spectra of the commercial and scCO2 processed ABZ showed characteristic bands at similar positions (). The major functional groups of ABZ appeared at frequency of 1435 and 1464 cm−1 (indicating C-H stretching from the benzene ring), at 2980 cm−1 (C-H stretching of the alkane), at 1656 cm−1 (C = C stretching in the aromatic ring), at 1589 cm−1 (COOH), at 3304 cm−1 (N-H stretching of amine), and at 1177 cm−1 (C-N vibrations) ().
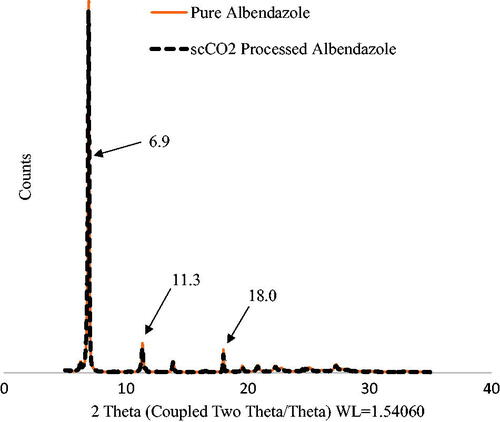
X-Ray diffraction
The XRD patterns obtained from commercial and re-crystallized, the scCO2 treated ABZ are shown in . It shows both samples having peak intensities at characteristic 2θ angles; 6.9°, 11.3°, and 18° were superimposable.
Loading capacity
The absorbance of different concentrations of ABZ was determined at 292 nm to draw a standard curve and followed by determination of loading capacity of ABZ entrapped by liposomes. The loading capacity of liposomes using SCF technology was 4.4 ± 0.25% w/w (n = 3) ().
Table 1. Loading capacity and particle size of ABZ loaded PEGylated liposomes.
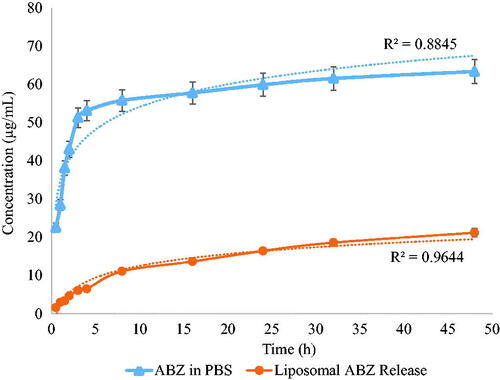
Release profile
The results obtained from in-vitro ABZ release experiments at 37 °C from liposomal ABZ and the unformulated ABZ control group at the different time points is reported in . It is clear that the release rate of ABZ from standard formulation in PBS was higher and faster (control group than that of ABZ loaded liposomes.
Characterization of liposomes
Liposomes prepared using SCF technology were characterized in terms of particle size and zeta potential. The particle size of DSPC-PEG-ABZ liposomes was reduced from 675.2 ± 15.3 nm to 167.2 ± 5.3 nm and PDI from 0.41 ± 0.03 to 0.26 ± 0.02 after 15-min sonication. The morphology and particle size of liposomes were confirmed using cryo-TEM ().
Discussion
The solubility of ABZ in scCO2 was approximately 12 mg/100 mL which is “very slightly soluble” and comparatively better than the solubility of ABZ in water (2.2 mg/100 mL) which is “practically insoluble” [Citation5]. The advantage of the higher ABZ solubility in scCO2 could play an important role in developing ABZ formulations using SCF technology. The low dielectric constant of CO2 along with its zero dipole moment makes it nonpolar. As a rule of thumb, solvation occurs between two compounds when “like dissolves like”. The observed solubility can be attributed to the quadrupole moment of the CO2 molecule (i.e. non-polar nature) and the partly non-polar molecular structure of ABZ, suggesting a hydrophobic interaction [Citation13,Citation21].
As published previously, ABZ recrystallized from methanol and DMF, provided the two polymorphic forms: Form I and II. Form I had an endothermic peak at 220 °C, whereas Form II yielded a peak at 160 °C [Citation6]. In the current study, the MPs were observed as 200 °C for both samples, (commercial and scCO2 processed ABZ) which could be due to co-mixture of both crystal forms. Given that the MP was closer to that of Form I, it could be that the mixture was predominately of the Form I. The higher MP of Form I is an indication that it is the more stable of the two forms. The DSC result showed that polymorphic state of ABZ did not change ().
The TGA thermograms after melting was followed by degradation, including oxidation and reduction, and showed the same pattern for both the commercial and scCO2 processed ABZ samples [Citation22]. The TGA result supports the DSC data (). For further confirmation and clarification of any chemical change in crystal habit, FTIR and XRD methods were used. FTIR helps to determine the functional group changes of a molecule by using the light absorbance at different frequencies, and XRD is a quick analytical method providing information about the unit cell dimensions and crystal form.
The results of FTIR further confirmed no change before and after processing with scCO2. showed that the double bonds of the aromatic rings for commercial ABZ and scCO2 treated ABZ at 1589 and 1656 cm−1, and 1586 and 1652 cm−1, respectively, did not show any change. The band differences were less than 5 wavenumbers, suggesting no likely change of crystal structure [Citation10,Citation20]. The absence of any new bands indicated that scCO2 processed ABZ did not show any change in the polymorphic state. Moreover, the lack of any shifts in the frequency of the scCO2 processed ABZ () compared to pure ABZ indicated absence of any significant interaction between the pure and scCO2 ABZ. Furthermore, change in the peak intensities or emergence of any new peak because of XRD was not observed in the scCO2-processed ABZ ().
The loading capacity of ABZ using SCF liposome formulation was calculated as 4.4% ± 0.25 (n = 3). It was previously reported that the entrapment of hydrophilic molecules into liposomes was higher due to larger area of aqueous core of liposomes and strong bonding between hydrophilic chains bonds, compared to hydrophobic molecules. While gaps between lipid bilayer (where a hydrophobic drug is loaded) are very small [Citation23]. The concentration of ABZ loaded into the liposomes might have promising anticancer effects, as shown by recently published studies [Citation3,Citation23]. The liposomal formulation in current study had 70.8 µg/mL ±0.17 (n = 3) (i.e. 4.4% ± 0.25) of ABZ loaded into liposomes and might be of interest with potential anticancer properties and sustained release effect [Citation3].
The PEGylated DSPC liposomes used for the manufacturing of liposomes might have effects on delayed release, when compared to control ABZ, as reported previously [Citation24]. The delayed and sustained effect of ABZ from SCF manufactured liposomes is shown in . The hydrophobic alkyl chains of the polymer create a barrier against the flow of drug, which might remain entrapped for a long period and provide a possible reason for the observed slow release. In addition, it was reported that phosphorylated PEG polymer in DSPEPEG-2000 provided stealth effect, and also reduced the particle size with improved shelf stability [Citation25,Citation26]. Herein, the developed SCF technology demonstrated to be an inexpensive and green alternative method and provides unilamellar and spherical liposomes with better PDI compared to the conventionally used Bangham method (or thin film method). The zeta potential of liposomes (−4.23 mv ±0.09 mV) obtained in the current study showed that liposomes were negatively charged, which may improves suspension stability with less flocculation of liposomes, as similar charges repel each other. The composition of stealth liposomes in the current project, such as DSPC and DSPE-PEG-2000 (94:6 molar ratio), is similar to the market formulation (Doxil®) [Citation27,Citation28]. Thus, using a similar molar ratio of DSPC and DSPE-PEG-2000 (94:6) in the current study, the liposomes would be expected to have a stealth effect with better shelf stability.
The particle size of liposomes determined using DLS was further supported and confirmed by morphology and particle size data obtained by Cryo-TEM (). Electron microscopy results showed that liposomes were unilamellar and spherical in shape with particle size in the range of 140–150 nm, and DLS results showed 167.2 nm ±5.346 nm. The difference in the particle size provided by DLS and Cryo-TEM could be due to hydration shells surrounding liposomes and could explain the larger particle size than that measured using the DLS zetasizer. The effect on size from hydration shells has been explained elsewhere in the literature [Citation29–31]. This has been explained elsewhere in the literature. In addition, the particle size of liposomes <400 nm has been reported for extravasation into tumors [Citation32,Citation33]. If the enhanced permeability effect is based on a size exclusion phenomenon around 400 nm or less (depending on the cancerous tissue), then the liposomes prepared in this study would be expected to exploit this phenomenon.
Conclusions
A static gravimetric method was used to determine the saturation solubility of ABZ in scCO2 and found to be ‘very slightly soluble’ (∼12 mg/100 mL). Solid-state characterization of untreated and scCO2-processed ABZ showed the absence of polymorphism, as proved by using DSC/TGA, FTIR, and XRD. The ABZ loaded liposomes in nanoparticle size range were manufactured and provided a controlled release effect on ABZ release profile. The data from this study provides original information about ABZ solubility and manufacturing of DSPC-PEG-ABZ liposomes using scCO2 and offers a good starting point for future ABZ and dosage form research that wishes to use scCO2 as a solvent.
Acknowledgements
Faheem Maqbool is a recipient of Australian Government Research Training Program Scholarship from The University of Queensland, Brisbane, Australia, for which we are very grateful. The authors also thank Professor Andrew K. Whittaker of the Australian Institute for Bioengineering and Nanotechnology (AIBN), The University of Queensland, Brisbane, QLD 4072 Australia, for his support and access to specialized equipment, including the SCF unit used in this research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Yeo S-D, Kiran E. Formation of polymer particles with supercritical fluids: a review. J Supercrit Fluid. 2005;34:287–308.
- Brusau E, Camí G, Narda G, et al. Synthesis and characterization of a new mebendazole salt: mebendazole hydrochloride. J Pharm Sci. 2008;97:542–552.
- Noorani L, Stenzel M, Liang R, et al. Albumin nanoparticles increase the anticancer efficacy of albendazole in ovarian cancer xenograft model. J Nanobiotechnol. 2015;13:1–2.
- Schamp K, Schreder S-A, Dressman J. Development of an in vitro/in vivo correlation for lipid formulations of EMD 50733, a poorly soluble, lipophilic drug substance. Eur J Pharm Biopharm. 2006;62:227–234.
- Vogt M, Kunath K, Dressman JB. Dissolution improvement of four poorly water soluble drugs by cogrinding with commonly used excipients. Eur J Pharm Biopharm. 2008;68:330–337.
- Pranzo MB, Cruickshank D, Coruzzi M, et al. Enantiotropically related albendazole polymorphs. J Pharm Sci. 2010;99:3731–3742.
- Cook G. Use of benzimidazole chemotherapy in human helminthiases: indications and efficacy. Parasitology Today. 1990;6:133–136.
- Tamarozzi F, Nicoletti GJ, Neumayr A, et al. Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: a systematic review. Curr Opin Infect Dis. 2014;27:425–431.
- Torrado S, Torrado S, Torrado JJ, et al. Preparation, dissolution and characterization of albendazole solid dispersions. Int J Pharm. 1996;140:247–250.
- Horton J. Albendazole: a review of anthelmintic efficacy and safety in humans. Parasitol. 2000;121:S113–S132.
- Kim M-S, Jin S-J, Kim J-S, et al. Preparation, characterization and in vivo evaluation of amorphous atorvastatin calcium nanoparticles using supercritical antisolvent (SAS) process. Eur J Pharm Biopharm. 2008;69:454–465.
- Otake K, Imura T, Sakai H, et al. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir. 2001;17:3898–3901.
- Sherman G, Shenoy S, Weiss R, et al. A static method coupled with gravimetric analysis for the determination of solubilities of solids in supercritical carbon dioxide. Ind Eng Chem Res. 2000;39:846–848.
- Moriwaki C, Costa G, Ferracini C, et al. Enhancement of solubility of albendazole by complexation with β-cyclodextrin. Braz J Chem Eng. 2008;25:255–267.
- Moyano J, Liro J, Perez J, et al. Thermal analysis of albendazole investigated by HSM, DSC and FTIR. Resonance (NMR). 2014;4:1043–1050.
- Alavi SE, Esfahani MKM, Ghassemi S, et al. In vitro evaluation of the efficacy of liposomal and pegylated liposomal hydroxyurea. Ind J Clin Biochem. 2014;29:84–88.
- Marciocha D, Kalka J, Turek-Szytow J, et al. A pretreatment method for analysing albendazole by HPLC in plant material. Water Air Soil Pollut. 2013;224:1646.
- Hyatt JA. Liquid and supercritical carbon dioxide as organic solvents. J Org Chem. 1984;49:5097–5101.
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36–51.
- Vuitton DA. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus?. Exp Rev anti-Infect Ther. 2009;7:145–149.
- Ingold K, Bigler P, Thormann W, et al. Efficacies of albendazole sulfoxide and albendazole sulfone against in vitro-cultivated Echinococcus multilocularis metacestodes. Antimicrob Agents Chemother. 1999;43:1052–1061.
- Zhang JA, Anyarambhatla G, Ma L, et al. Development and characterization of a novel Cremophor EL free liposome-based paclitaxel (LEP-ETU) formulation . Eur J Pharm Biopharm. 2005;59:177–187.
- Castro L, Kviecinski M, Ourique F, et al. Albendazole as a promising molecule for tumor control. Redox Biol. 2016;10:90–99.
- Gabizon A, Goren D, Cohen R, et al. Development of liposomal anthracyclines: from basics to clinical applications1. J Control Release. 1998;53:275–279.
- Li S-D, Huang L. Stealth nanoparticles: high density but sheddable PEG is a key for tumor targeting. J Control Release. 2010;145:178
- Park JW, Mok H, Park TG. Physical adsorption of PEG grafted and blocked poly-L-lysine copolymers on adenovirus surface for enhanced gene transduction. J Control Release. 2010;142:238–244.
- Chang H-I, Yeh M-K. Clinical development of liposome-based drugs: formulation, characterization, and therapeutic efficacy. Int J Nanomed. 2012;7:49–60.
- Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4:95.
- Mahl D, Diendorf J, Meyer-Zaika W, et al. Possibilities and limitations of different analytical methods for the size determination of a bimodal dispersion of metallic nanoparticles. Colloid Surf A. 2011;377:386–392.
- Müller M, Mackeben S, Müller-Goymann CC. Physicochemical characterisation of liposomes with encapsulated local anaesthetics. Int J Pharm. 2004;274:139–148.
- Wang H, Zhao P, Liang X, et al. Construction of a novel cationic polymeric liposomes formed from PEGylated octadecyl‐quaternized lysine modified chitosan/cholesterol for enhancing storage stability and cellular uptake efficiency. Biotechnol Bioeng. 2010;106:952–962.
- Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. Aaps J. 2012;14:303–315.
- Yuan F, Leunig M, Huang SK, et al. Mirovascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human tumor xenograft. Cancer Res. 1994;54:3352–3356.