?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cancer incidence is still increasing due to inadequate responsive treatments. Inertness and biocompatibility of nanoparticles synthesized using plant extracts have shown therapeutic applications and make it to be a good anti-cancer candidates. This study is a recent novel spotlight that synthesized silver nanoparticle from Ficus ingens leaf extract (FILE) and studied its anti-metastatic and anti-bacterial activity. The chemical and surface analysis of the synthesized FILE silver nanoparticles (FILE-AgNPs) was studied using UV–visible, Fourier transform infrared (FTIR), X-ray diffraction (XRD), zeta sizer, atomic force microscopy (AFM) and scanning electron microscopy (SEM). Gas chromatography mass spectrometric (GC–MS) and quantitative photochemical analyses were also carried out. The antimicrobial activity of FILE-AgNPs was found to be effective with MIC of 10 µg/mL for E. coli and 20 µg/mL for S. typhi and B. cereus with significance difference. Toxicity, proliferation and anti-metastatic potential of FILE-AgNPs were studied on MDA-MB 231 cell models using tryphan blue, MTT and wound heal assay, respectively. FILE-AgNPs showed the ability to inhibit metastasis of MDA-MB 231 cells in dose-dependent manner in which 10 μg/mL and 5 μg/mL inhibit by 96% and 75%, respectively. The synthesized FILE-AgNPs are remarkable candidates for treatment of cancer cases and other cancer related cases.
Introduction
Cancer is a disease that involves abnormal cell growth and subsequent metastasis to other parts of the body [Citation1]. The metastasis is the main cause of death in majority of cancer patients. Breast cancer (BCa) is one of the most abundant malignant tumour affecting women and is the second cancer type leading to death among women accounting to about 24% of cancer related cases worldwide. The incidence of BCa is still increasing unlike other cancer types and is expected to pass two million in 2030 globally with maximal increase herein at developed countries [Citation2]. Although, there are advancements in both treatments and diagnosis strategies, BCa still maintains its position as the second most deadly disease among women globally. Due to the limitation in treatment strategies, no significant decrease in mortality rates from BCa was reported [Citation3].
Nanoscience is among the advancing area of research in the world. The materials are manipulated in such a way that it differs from the original ones in terms of shape, size and characteristics [Citation4]. Metal nanoparticles revealed special properties which include surface area as well as special surface atoms, due to their excellent physicochemical properties, which have to do with optical, magnetic, catalytic and antibacterial properties [Citation5]. Synthesis of nanoparticles more especially metallic nanoparticles are very special due to its wide application in the field of chemistry, energy and drug development [6–Citation9]. Silver nanoparticles (AgNPs) have many antimicrobial properties against different strains of microorganism, cause cell lysis on Pseudomonas aeruginosa, E. coli and Klebsiella pneumonia [Citation10].
The green synthesis of nanoparticles more especially that of silver using variety of medicinal plant extracts and sometimes using microorganisms with their application in drug development has been revealed [Citation11]. The whole plant or plant extract can be used to produce metallic nanoparticles, the reducing agent which is responsible for nanoformation is presence of large quantity in the extract than in whole plant, most of the photosynthetic methods used aqueous extract for nanoparticles synthesis. The biological synthesis is simpler than the physical or chemical method, as it does require no condition. The bioreduction activity of plant extract is higher than that of microbial culture [Citation12], AgNPs draw significant attention due to its good eco-friendly, non-pathogenic and economic method of production.
In this study, AgNPs using Ficus ingens leafs were synthesized and characterized using UV–visible, Fourier transform infrared (FTIR), X-ray diffraction (XRD), AFM and zeta sizer. Furthermore, the antimicrobial activity of FILE silver nanoparticles (FILE-AgNPs) and their effects on toxicity, proliferation and metastasis were studied on MDA-MB 231 cell models.
Materials and methods
Extract formation
Ficus ingens leaf was collected from Gaya local government area of Kano State, Nigeria. The leaf was identified and authenticated at Department of Plant Biology Bayero University Kano, Nigeria. The leaf of the plant was later washed with distilled water and dried under shed. The dried leaf was grinded into powder using mortar and pestle. The powdered was socked in methanol (1:10) for 24 h to completely extract all the active constituents. The solvent was then filtered through Whatman filter paper.
Gas chromatography mass spectrometric (GC–MS) analysis of the extract
Methanolic extracts of Ficus ingens was filtered through 0.22 µm syringe filter and 1 µL of the solution was injected into GC–MS system for analysis. The analysis was carried out using GCMS-QP2010 plus with an inbuilt WILEY library and method described by Kumar et al. [Citation13] was adopted. TRB-5 ms column operating in electron impact mode at 70 eV was used. Helium (99.999%) as carrier gas was used at a constant flow rate of 1 mL/min; injection volume of 0.5ET (split less); injection temperature of 280 °C with transfer line temperature of 300 °C. Oven temperature of the system was set at 50 °C (isothermal for 2 min), with 7 °C increase per min. Mass spectra were analysed at 70 eV at a scan interval of 0.5 s and full mass scan range from 25 m/z to 1000 m/z.
Qualitative and quantitative phytochemical analysis of the extract
Chemical tests for the screening of certain phytochemical compounds (alkaloid, flavonoid, tannins, glycosides and saponins) were performed using standard procedures [14–Citation16].
Elemental analysis
Ficus ingens leaf extract (FILE) was incubated at oven until forming dried powder. Afterwards, formed powder was pressed using hydraulic press to prepare pellet. Pellets were analysed with energy dispersive X-ray fluorescence (EDXRF) using the method [Citation17].
Synthesis of silver nanoparticles
Ficus ingens dried leaf powder (15 g) was soaked in 200 mL distilled water, allowed to stay overnight (24 h) and then filtered with Whatman No. 1 filter paper. Then, 15 mL of Ficus ingens extract was added drop wise in silver nitrate solution with constant stirring using magnetic stirrer 150 rpm at room temperature for 1 h, the mixture turn to dark ash with precipitate in the solution [Citation18]. The solution was centrifuged at 6000 rpm for 20 min for three times, the solid palates were washed with deionized water to remove the unattached molecules, and the black solid residue was further dried and stored.
Characterization of green synthesized silver nanoparticles
The optical absorbance was recorded using UV–Vis spectrophotometer (UV-2450). FTIR analysis was conducted for the structural characterization (IRPrestige 21). The surface morphology of the silver NPs was observed with atomic force microscopy (AFM, Nanomagnetics, Ankara, Turkey). The Z-average size (hydrodynamic diameter), size distribution (polydispersity index, PDI) and zeta potential (surface charge) were analysed by zeta sizer (Malvern Instruments, Model 3000 HSA, Malvern, UK) and the results were obtained by the Malvern ZS Nano software. Furthermore, the crystallinity was measured using XRD machine enhanced with Cu (copper) Kα radiation (1.54187 nm wavelength), the formation of AgNPs, structure and composition was tested using Ni (Nickel) filter with operation condition of 40 kV/20 mA in the range of 3°≤2θ ≤ 50°.
Minimum inhibitory concentration (MIC)
In this method, different concentrations of FILE-AgNPs were made (2.5, 5.0, 10.0, 20.0, 40.0, 80.0 and 120.0 µg/mL). 1.8 mL of nutrient broth was cultured with bacteria using sterile cotton swab (E. coli, B. cereus and S. typhi) similarly 0.2 mL of NP was also added, this was incubated (37 °C-24 h) and the absorbance was recorded [Citation19]. Nutrient broth only, nutrient broth with bacteria serve as controls, the test tube which did not show the growth was recorded, this was repeated in triplicates.
Antibacterial activity using disc diffusion method
Muller Hinton Agar was prepared and poured onto Petri dishes, a sterile cotton swabs were used to take bacterial solution (0.5 McFarland) and swab onto the surface of the media, the disc was finally mounted. Similarly, AgNO3 and plant extract disc was prepared by taking 100 µL from the concentrated extract by evaporating it in the oven at 45 °C for 1 h and poured onto a disc prepared from sterile filter paper, similarly, 100 µL of 1.0 M AgNO3 was poured on the disc to make a AgNO3 disc, 5 µg disc of ciprofloxacin was used as a control, the inhibition zone was recorded after incubating for 24 h at 37 °C, and all the experiments were carried out in triplicate and repeated at least three times (n = 3). p < .05 was considered significant or p > .05 insignificant and p < .0001 was considered highly significant.
Cell lines and culture medium
Strongly metastatic MDA-MB 231 human BCa cell models were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 5% foetal bovine serum (FBS), l-glutamate (2%) and penicillin/streptomycin (2%). The cells were grown to confluence at 37 °C, 5% CO2 and 95% humidity using incubator.
Toxicity assay
Cytotoxicity was evaluated by tryphan blue dye exclusion assay for the MDA-MB 231 using the method described by Fraser et al. [Citation20]. The experiment was set in 35 mm tissue culture dishes (three dishes per condition) in which 3 × 104 cells were plated per dish and in 1 mL DMEM growth media and incubated overnight in dark to settle. The cells were treated with different dosages (0, 2.5, 5, 10, 20, 40, 80 and 120 μg/mL) and 24, 48 and 72 h after the treatments, the medium in the dishes was removed followed by addition of diluted tryphan blue (0.25 mL tryphan blue dye in 0.8 mL medium). After 10 min of incubation in dark, the diluted tryphan blue was removed and 30 fields of view with at least 20 cells in each field were captured using microscope at 10× magnification and counted using ImageJ software. This procedure was repeated in three separated dishes. Data are presented as average of 3 × 30 measurements.
Measurement of cell number – MTT assay
Cellular proliferation of MDA-MB 231 was evaluated using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT assay) [Citation21]. Briefly, the cells (3 × 104) were plated in 12-well Falcon tissue plates and incubated for 24 h prior to the experiment. After the cells settled, varying concentrations (0, 2.5, 5, 10, 25, 50, 100, 150 and 200 μg/mL) of FILE-AgNPs extract were applied to the cells and incubated for 24, 48 and 72 h.
(1)
(1)
where AT is the absorbance of treated cells and AU is the absorbance of untreated cells.
Lateral motility (wound-heal) assay
The assay was performed to access the effect of the plant extract toward suppressing the movements of the cancer cells as described previously [Citation20]. Briefly, three parallel vertical lines and 15 intersecting lines were marked under the 35 mm culture dishes. MDA-MB 231 cells were plated in 35 mm dishes at 5 × 105 cells, DMEM growth media was added to make the volume up to 1 mL, the cells were spread carefully across the dishes surfaces by careful tilted of each dish back and forth as well as side by side to prevent settling of the cells on the edges of the dishes and incubated over night to settle. Three scratches along the horizontal lines were made using a sterile 200 μL pipette tips. The growth medium was replaced with fresh medium ± treatments and the wounds were photographed at 0, 24 and 48 h incubation periods using digital camera mounted on the microscope at 10× magnification. Media (±treatment) were replaced after every 24 h. ImageJ software was used to assess the initial wound and the subsequent recovery area by migrating cells. The lateral motility was calculated as motility index using the formula bellow:
(2)
(2)
where Wt represents the width of the wound at specific time (24 or 48 h) and W0 represents the initial width at zero hours (0 h).
Statistical analysis
Data are given as means ± standard errors of the mean (SEMs). Statistical comparisons were determined using unpaired Student's t-test, ANOVA followed by the Newman–Keuls post hoc were necessary using SPSS (SPSS Inc., Chicago, IL). All the experiments were carried out in triplicate and repeated at least three times (n ≥ 3). p < .05 was considered significant or p>.05 insignificant and p < .0001 was considered highly significant.
Results and discussion
Phytochemical screening of Ficus ingens methanolic extract
The phytochemical analysis of the plant extract revealed the presence of alkaloid, flavonoid, tannins, saponins and glycosides (presented in ) which could be responsible for antimetastatic as well as antimicrobial activities of the AgNPs synthesized using Ficus ingens leafs. The quantitative analysis of total phenolic and flavonoids was found to be 75.78 mg GAE/g and 52.16 mg QE/g, respectively. Some of the compounds that are present in the plant are known to have curative potential against numerous pathogens. The ability of plants to be used as medicine lies in the bioactive phytochemicals and the physiological action shown by the plant [Citation22] ().
Table 1. Phytochemical screening of Ficus ingens methanolic extracts.
Table 2. Compounds identified in methanol extracts of Ficus ingens by GC–MS.
Gas chromatography mass spectrometric analysis
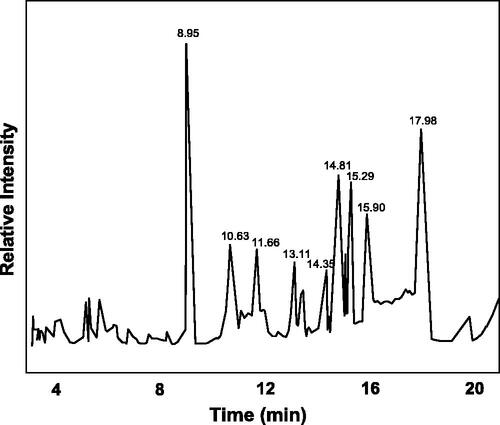
GC–MS analysis of methanol extracts of Ficus ingens leaf identified the presence of nine major compounds with their respective molecular weight and area as shown by the presence of various peak in the chromatogram (). Compounds were identified by comparison of their mass fragmentation patterns of similar compounds in WILEY library.
The major compounds present are ethanol, 1-(2-butoxyethoxy) (16.9%), cyclohexane propanol (11.6%), 2-methoxy-6-methyl pyrazine (13.8%), 11,3-dioxane (CAS) (6.8%), 2-propenoic acid octyl ester (6.31%), dihydro methyl jasmonate (7.62), 1,2-benzene dicarboxylic acid (7.31%) and 4-(bromomethyl) cyclohexane-1-ol (2.11%). Other compounds that are present in the plant extract are 2-methoxy-6-methyl pyrazine diethyl ester, cyclohexane, stearic acid, 3-oxy-4-octene, neophytadiene, pyrrolidine, 1-(1-pentenyl)-(CAS) 1-(1-pyrrolidinyl)-1-n-pentene, pentanoic acid, pentyl ester (CAS) amyl valerate, pyrrolidine, 1-(1-pentenyl)-(CAS) 1-(1-pyrrolidinyl)-1-n-pentene. Ability of plant to combat diseases or its medicinal properties has to do with the bioactive compounds present in the plants. Some of this compounds have medicinal properties and can cure many diseases including cancer. Kucuk et al. reported that 1,3-dioxane has strong ability to combat cancer and fungal related infection [Citation23]. Also, many compounds that have carboxylic functional group that are present in the extract have reported to have antibacterial and anticancer activity [Citation24].
Elemental analysis
The result revealed highest amount of Mg and Ca while Mn and Zn revealed the lowest amount as presented in . Plants usually absorbed these elements from the soil and finally integrate them into its compounds (organic) that human beings and other animals consume either from the plant fruits, flowers, stem or root [Citation25]. Majority of this trace element plays a very important role as cofactors during metabolic activities and they also have medicinal value. Similar elemental content was also observed from other plants flowers and stem bark [Citation26].
Table 3. Elemental analysis of Ficus ingens leaf in terms of mg/kg.
Silver nanoparticles characterization
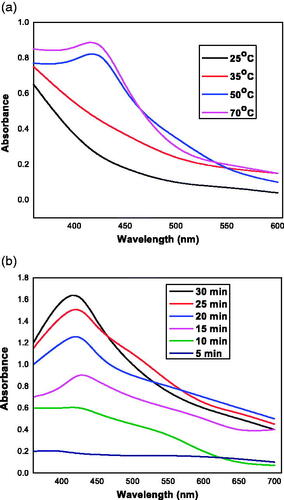
The UV–vis spectroscopy, which is the most specific and indirect approach for identifying the presence of nanoparticles, was employed to monitor the synthesis of FILE-AgNPs by considering different temperature and time intervals. Furthermore, the Ag+ from AgNO3 during the reaction tends to reduce to AgNP, and it can be monitored by observing maximum absorbance peak as well as colour change during the reaction. FILE-AgNPs was confirmed after immediate colour changed from colourless to dark ash and to brown within 48 h. Moreover, some specific parameters have to be considered during synthesis of nanoparticles, those parameters include incubation time and temperature. The incubation of the reaction mixture at different temperature (25, 35, 50 and 70 °C) lead to gradual synthesis of nanoparticle as shown in . Also, after completion of the reaction the incubation time was also monitored at 50 °C and the absorbance was recorded at different time intervals prior to the appearance of clear absorbance peak, which was used for synthesizing the FILE-AgNPs. The UV–vis spectra of FILE-AgNPs revealed broad peak at 425 nm. Debasis et al. reported that, appearance of absorption peak has to do with surface Plasmon resonance characteristics of the metallic nanoparticles that happened as a result of movement of free electrons on its surface when they are aligned in resonance and also with the wavelength of irradiated light [Citation26].
Figure 2. (a) UV–vis spectra of synthesized FILE-AgNPs at different temperatures. (b) UV–vis spectra of synthesized FILE-AgNPs at different time intervals.
Phytochemicals (like phenolic, flavonoids etc.) have been reported to play specific role in the AgNPs synthesis by enhancing the conversion of ionic appearance of AgNP to metallic [Citation27].
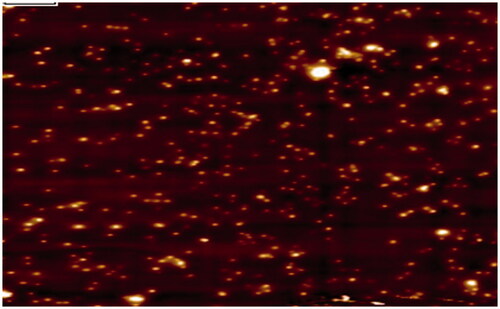
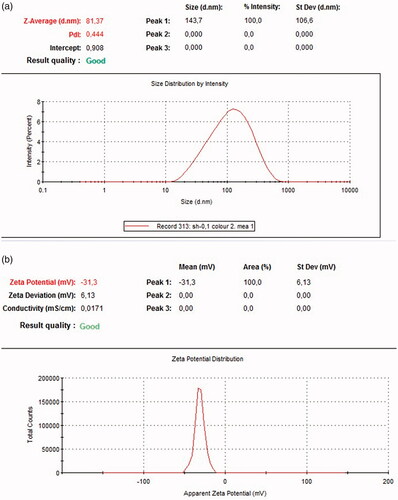
Dynamic light scattering (DLS) measurements were conducted to reveal the hydrodynamic size, heterogeneity index and zeta potential of the synthesized nanoparticle in a colloidal environment. Zeta potential measured the magnitude of electrostatic charge repulsion or attraction involving particles in a liquid medium and particles with greater than +30 mV and less than –30 mV are usually considered stable without static stabilization for colloidal dispersion. It has been reported that when particles present freely in a medium, they displayed Brownian motion which can be ascertained by changes in the intensity of scattered light within the system out of which Stokes–Einstein equation used the translational diffusion coefficient and calculated the hydrodynamic size of the particle [Citation28]. The size of the synthesized FILE-AgNPs leaf extract was 81.37 nm and the zeta potential was found to be –31 mV as shown in , respectively. The negative capacity result was likely due to the capping action of some organic compounds present in most of the extract responsible for electrostatic stabilization of the colloidal solution [Citation29]. The PDI of AgNPs was found to be 0.444 revealing that, the synthesized nanoparticles are monodispersed ().
Figure 3. (a) Dynamic light scattering image. (b) Surface zeta potential image of synthesized FILE-AgNPs.
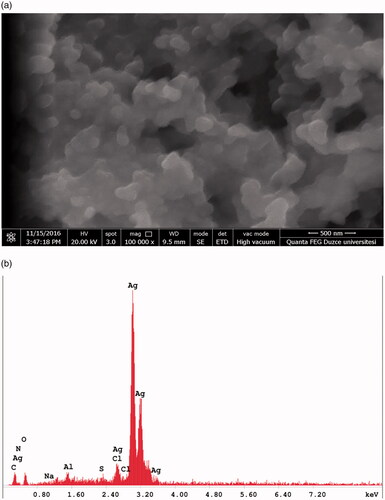
Scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis of FILE-AgNPs described the nature of the synthesized nanoparticle which is spherical in shape (). Similarly, the EDX spectra of AgNPs also revealed and confirm the establishment of AgNPs. It has been reported that, metallic AgNPs usually reveal optical peak around 3 keV [Citation30]. The major constituent is silver (78%) followed by oxygen (8.8%), carbon (4.9%), aluminium (2.6%), chlorine (2.1%), oxygen (1%) and trace of nitrogen which is less than 1%. The EDX result showed strong peak for silver together with oxygen peak which may have happened as a result of other biomolecules that are attached to the surface of the AgNPs, signifying the conversion of Ag + to Ag. This is one of the major benefits of using plant extract to synthesized nanoparticles than using chemicals.
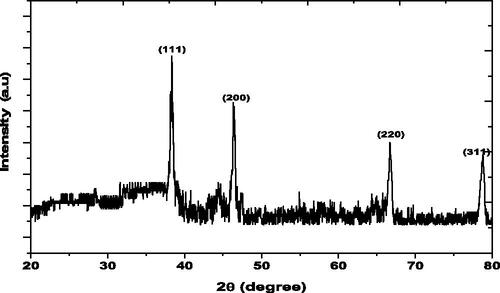
shows the XRD pattern of the synthesized FILE-AgNPs, the diffraction peaks at 2θ values of 38.33°, 46.32°, 66.72° and 78.80° match the respective (111), (200), (220) and (311) lattice planes of AgNPs, indicates lattice planes of the crystalline silver face-centred cubic structure. The results are in agreement with several studies reported the cubic nature of biologically synthesized AgNPs [Citation31].
The functional groups present in the leaf extracts were obtained from various spectrum peaks at different wavelengths (). The presence of C–Br stretch (515 cm−1) is a characteristic of alkyl halides in the dried extract, also C–H of alkynes and that of aromatic (neophytadiene) were detected at 610 and 770 cm−1, respectively. However, large visible C–N band of aliphatic amines (1070 and 1250 cm−1) and small stretch at 1450 cm−1and 1620 cm−1, was alkane (C–H) and aromatic bending. The alkyl C–H and carboxylic acid stretch (hexadecanoic acid) was observed at 2850 and 2950 cm−1, respectively. Carboxylic acid and phenol (2-ethylhexanoic acid and 2-pyrrolidinone, 1-methanol) stretch (O–H) was also observed at 3350 cm−1.
Figure 7. (a) FTIR spectra of Ficus ingens leaf extract (FILE). (b) FTIR Spectra of silver nanoparticles synthesized from Ficus ingens leaf extract (FILE-AgNPs).
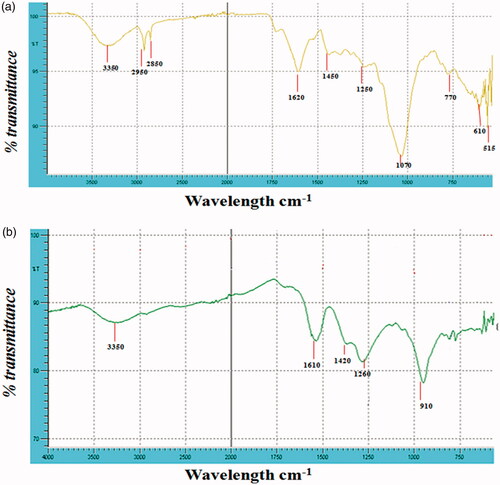
The functional groups present in the synthesized nanoparticles were obtained from various spectrum peaks at different wavelengths as shown in . At lower wavelength 500 cm−1, there was the presence of C–Br and C–Cl stretch that reveal alkyl halides. O–H bend at 910 cm−1 was the characteristic peak of carboxylic acid and C–H stretch at 1260 cm−1 revealed the presence of alkane. Also, N–O bend of nitro compound was shown around 1420 cm−1 and N–H bend of primary amines at 1610 cm−1. Finally, alcohol or phenol O–H stretch was also observed at 3350 cm−1. In similar studies, a clear diagnostics functional groups (i.e. O–H alcoholic/acid, and C–O–C) were displayed [Citation32]. Moreover, the relative shift of these peak positions and intensity distribution in the IR of the two spectra () indicates that different functional groups of the plant extract were probably involved in the AgNPs formation, and plays an important role in the capping and stabilization, which helps to avoid the AgNPs agglomeration.
Antimicrobial activity
In order to conquer the problem of drug resistance with small, cheap and limited resources, studies revealed AgNPs to be the most effective antimicrobial agent [Citation33]. In this study, the MIC of the FILE-AgNPs was carried out against E. coli, S. typhi and B. cereus and FILE-AgNPs show significant activity as shown in . MIC value of 10 µg/mL was recorded on E. coli, this corresponds with the study carried out by Patil et al. [Citation11], similarly 20 µg/mL on both S. typhi and B. cereus. Gao et al. synthesized Ag/PVP (polyvinyl pyrrolidone) nanosphere against E. coli, P. aeruginosa and S. aureus with MIC of around 10 µg/mL. F. benghalensis and A. indica bark were used to synthesize AgNPs with MIC of 12.5 µg/mL against P. aeruginosa, and E. coli and 25 µg/mL against B. subtilis. This confirms that F. ingens has significant activity against tested bacterial strains [Citation34].
Table 4. Minimum inhibitory concentration using different concentration.
The antibacterial properties of synthesized nanoparticles against three strains of bacteria (E. coli and S. typhi (Gram negative), and B. cereus (Gram positive)) were studied. E. coli, S. typhi and B. cereus had inhibition zone of 24.7 mm, 24.3 mm and 22.7 mm respectively against AgNPs, 13.0 mm, 12.0 mm and 12.3 mm respectively against silver nitrate, which are shown in . Leaf extract showed significant antibacterial activity of 15.3 mm, 14.7 mm and 14.3 mm against E. coli, S. typhi and B. cereus, respectively. Blank disc (0.0 µg/mL) was used as negative control while ciprofloxacin (5 µg) serves as positive control (). Similarly, another research indicated that increase in concentration and size has significant effect on the bacteria [Citation11]. Moreover, Morones et al. reported that, silver serves as soft acid that reacts with sulphur and phosphorus in the DNA there by destabilizing replication [Citation35].
Figure 8. Diagram showing disc diffusion method (A) E. coli, (B) B. cereus, (C) S. typhi, (1) zero control, (2) FILE-AgNPs, (3) silver nitrate, (4) plant extract and (5) ciprofloxacin antibiotic.
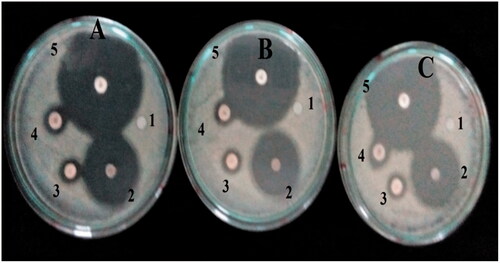
Table 5. Zone of inhibition of silver nanoparticle synthesized from F. ingens.
Anti-metastatic activity of synthesized FILE-AgNPs
The MDA-MB 231 cell line was treated with different doses (2.5, 5.0, 10.0, 20.0, 40.0, 80.0 and 120.0 µg/mL) of the synthesized FILE-AgNPs. After 24, 48 and 72 h, exposure viability of the cell decreases significantly at 80 μg/mL and above (p>.05; n≥ 3; ). But lower concentration of treatments including 2.5, 5.0, 10.0 and 20.0 μg/mL showed no significant difference when compared with untreated control cells (p>.05; n≥ 3; ). Similarly, the viability was assayed with same doses used in toxicity studies (2.5, 5.0, 10.0, 20.0, 40.0, 80.0 and 120.0 µg/mL) of the synthesized FILE-AgNPs. After 24, 48 and 72 h exposure viability of the cell decreases significantly at 80 μg/mL and above (p>.05; n≥ 3; ). But lower concentration of treatments including 2.5, 5.0, 10.0 and 20.0 μg/mL showed no significant difference when compared with untreated control cells after 24 and 48 h of treatments (p>.05; n≥ 3; ). Although, a significant difference (p<.05) was observed on cell viability with the same lower doses after 72 h of treatments indicating the effect is also time dependent.
Figure 9. (a) Effect of FILE-AgNPs on viability of MDA-MB 231 cell models at different time intervals. (b) Typical phase-contrast light-microscopy images of MDA-MB 231 cells before and after treatment with different concentrations of AgNPs. (c) Effect of AgNPs on proliferation of MDA-MB 231 cell models at different time intervals.
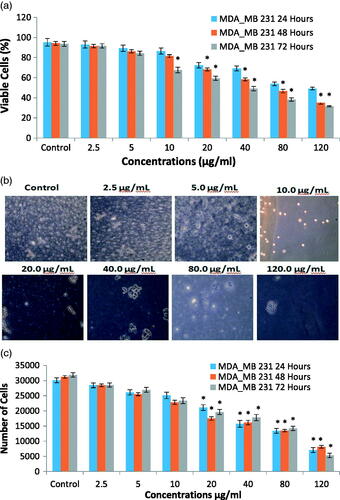
The treatment with FILE-AgNPs revealed that strongly metastatic cell decreases as the treatment time dose increases. The treatment with higher doses above 40.0 µg/mL shows some morphological changes in cells which has to do with some changes in the entire enzymatic system of the cell, permeability and the synthesis of stress enzymes popularly known as reactive oxygen species [Citation36]. The proliferation assay results of MDA-MB 231 cells after 24 and 48 h of treatments with different concentrations of NP's showed effects on cell proliferation which depend solely on the treatment concentrations. Lower doses (0, 2.5, 5.0 μg/mL) did not affect proliferation. However, 10 μg/mL and above showed significant effect on proliferation of cells. Also, the effect of the treatment was time dependent because 72 h treatment showed different results in which both the lower and higher treatment concentrations revealed effect on proliferation of cells. Considering the result obtained, the effect of the treatment on proliferation was both time and dose dependent. Some plant extract revealed effect on proliferation of MDA-MB cell models [Citation37,Citation38].
The effect of the synthesized FILE-AgNPs on lateral motility of MDA-MB 231 cell lines revealed the following motility index (0.65 ± 0.007, 0.004 ± 0.61 ± 0.006, 0.23 ± 0.005, respectively p<.05, n≥ 3, ) after 24 h incubation. And 48 h incubation after the treatment shows extreme change in lateral motility index of MDA-MB 231 ().
Figure 10. (a) Bar diagram showing motility index of MDA-MB 231 cells incubated 24 h and 48 h after treatment with FILE-AgNPs. (b) Typical phase-contrast light-microscopy images obtained from wound-heal assay obtained from wound-heal assay of MDA-MB 231 cells incubated 24 h and 48 h after treatment with synthesized FILE-AgNPs. Scale bar (50 μm).
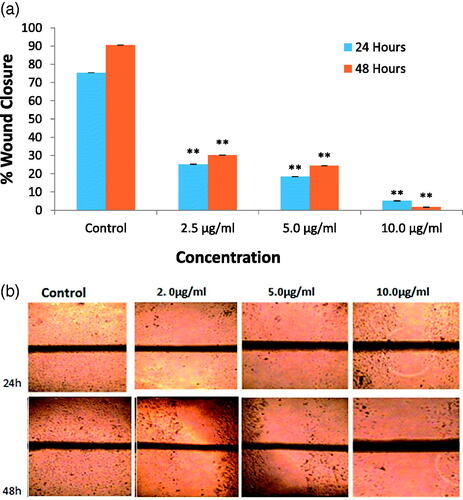
The FILE-AgNPs had shown the ability to inhibits lateral motility of MDA-MB 231 cell in dose dependent manner and the 10 μg/mL dose inhibit lateral motility of MDA-MB 231 cell by almost 96% and 5 μg/mL by 75%. The invasion and metastasis processes involved variation on how cells interact, attached to one another and the extracellular matrix [Citation1].
Conclusions
AgNPs have been synthesized using a FILE. The plant contains many phytochemicals that have ability to combat diseases and to rapidly convert Ag+ to AgNPs. Phytochemical screening analysis of the plant extract revealed the presence of alkaloid, flavonoid, tannins, saponins and glycosides which could be responsible for anti-proliferative, anti-metastatic and antimicrobial activities of the synthesized FILE-AgNPs. The results of the antibacterial activity analysed by disc diffusion method, demonstrated higher potential of synthesized FILE-AgNPs against E. coli, B. cereus and S. typhi than FILE and silver nitrate. It shows toxicity against BCa cell lines and it also revealed anti-proliferative and anti-metastatic activities against MDA-MB 231 cell models. The use of medicinal plants as antibiotics is still increasing due to the fact that, they are competent, they does not have much side effects, they are convenient and easy to handle. This study also revealed the anti-proliferative and anti-metastatic activity of the plant against BCa cell models which shows that the plants have very high potential to be future of anti-cancer drugs.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674.
- Jemal A, Bray F, Center MM, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893–1907.
- Cadaval Gonçalves AT, Costa Jobim PF, Vanacor R, et al. Increase in breast cancer mortality in Southern Brazil from 1980 to 2002. Cad Saúde Pública. 2007;23:1785–1790.
- Kavaz D, Çırak T, Bayram C, et al. Preparation of magnetic chitosan nanoparticles for diverse biomedical applications. Functional Nanoscale Mater Dev Syst. 2007;313–320. (Part of theNATO Science for Peace and Security Series B: Physics and Biophysics book series (NAPSB)).
- Essien EA, Kavaz D, Solomon MM. Olive leaves extract mediated zero-valent iron nanoparticles: synthesis, characterization, and assessment as adsorbent for nickel (II) ions in aqueous medium. Chem Eng Commun. 2018;205(11):1568–1582. 10.1080/00986445.2018.1461089.
- Kavaz D, Aigbe R, Augustine EE. Detection of pathogens in aqueous media with modified magnetic nanoscaled particles. Fresenius Environ Bull. 2017;26:761–771.
- Kavaz D, Odabas S, Denkbas EB, et al. A practical methodology for IgG purification via chitosan based magnetic nanoparticles. Digest J Nanomater Biostruct. 2012;7:1165–1177.
- Okonkwo EC, Essien EA, Akhayere E. Thermal performance analysis of a parabolic trough collector using water-based green-synthesized nanofluids. Solar Energy. 2018;170:658–670.
- Kavaz D, Odabaş S, Güven E, et al. Bleomycin loaded magnetic chitosan nanoparticles as multifunctional nanocarriers. J Bioact Compat Polym. 2010;25:305–318.
- Kumar DA, Palaninchamy V, Roopan SM. Green synthesis of silver nanoparticles using Alternanthera dentata leaf extract at room temperature and their antimicrobial activity. Spectrochim Acta A: Mol Biomol Spectrosc. 2014;127:168–171.
- Patil S, Ghosh S, Ahire M, et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int J Nanomedicine. 2012;7:483–496.
- Narayanan KB, Sakthivel N. Phytosynthesis of gold nanoparticles using leaf extract of Coleus amboinicus Lour. Mater Character. 2010;61:1232–1238.
- Kumar J, Dhar P, Tayade AB, et al. Chemical composition and biological activities of trans-Himalayan Alga Spirogyra porticalis (Muell.) Cleve. PLos One. 2015;10:2–8.
- Harbone JB. Phytochemical methods of extraction. London, UK: Cox and Wymann Ltd.; 1973. p. 66–70.
- Trease GE, Evans WC. Phytochemicals. In: Pharmacognosy. 15th ed. London: Saunders Publishers; 2002. p. 221–229.
- Sofowora A. Medicinal plant and medicine in Africa. Ibadan, Nigeria: John Wiley; 1993. p. 281–285.
- Khuder A, Sawan MK, Karjou J, et al. Determination of trace elements in Syrian medicinal plants and their infusions by energy dispersive X-ray fluorescence and total reflection X-ray fluorescence spectrometry. Spectrochim Acta B: Atom Spectrosc. 2009;64:721–725.
- Nazeruddin GM, Prasad NR, Waghmare SR, et al. Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J Alloys Compd. 2014;583:572–577.
- Singhal G, Bhavesh R, Kasariya K, et al. Biosynthesis of Silver nanoparticles using Ocimum sanctum (Tulsi) leaf extract and screening its antimicrobial activity. J Nanopart Res. 2011;13:2981–2988.
- Fraser SP, Salvador V, Manning EA, et al. Contribution of functional voltage-gated Na + channel expression to cell behaviors involved in the metastatic cascade in rat prostate cancer: I. lateral motility. J Cell Physiol. 2003;195:479–487.
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63.
- Akinmoladun AC, Ibukun EO, Afor E, et al. Phytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimum. Sci Res Essay. 2007;2:163–166.
- Kucuk HB, Yusufoglu A, Mataraci E, et al. Synthesis and biological activity of new 1,3-dioxolanes as potential antibacterial and antifungal compounds. Molecules. 2010;8:6806–6815.
- Jha AN, Prasad K. Green synthesis of silver nanoparticles using Cycas leaf. Int J Green Nanotechnol: Phys Chem. 2010;1:110–117.
- Zahid KA, Shalini S, Mohamed IS, et al. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J Biol Sci. 2015;22:322–326.
- Debasis N, Sarbani A, Pradipta RR, et al. Bark extract mediated green synthesis of silver nanoparticles: evaluation of antimicrobial activity and antiproliferative response against osteosarcoma. Mater Sci Eng. 2016;58:44–52.
- Das S, Barman S. Antidiabetic and antihyperlipidemic effects of ethanolic extracts of leaves of Punica granatum in alloxan-induced non-insulin-dependent diabetes mellitus albino rats. Indian J Pharmacol. 2012;44:219–224.
- Frisken BJ. Revisiting the method of cumulants for the analysis of dynamic light-scattering data. Appl Opt. 2001;40:4087–4091.
- Anandalakshmi K, Venugobal J, Ramasamy V. Characterization of silver nanoparticles by green synthesis method using Pedalium murex leaf extract and their antibacterial activity. Appl Nanosci. 2016;6:399–408.
- Kaviya S, Santhanalakshmi J, Viswanathan B, et al. Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79:594–598.
- Shaligram NS, Bule M, Bhambure R, et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009;44:939–943.
- Mohani N, Ahmad M, Noor Jahan N. Evaluation of phytoconstituents of three plants Acorus calamus Linn. Artemisia absinthium Linn and Bergenia himalaica Boriss by FTIR spectroscopic analysis. Pak J Pharm Sci. 2014;27:2251–2255.
- Naqvi SZH, Kiran U, Ali MI, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanomed. 2013;8:3187–3195.
- Gao Y, Chen S, Hu M, et al. Purification and characterization of a novel chlorpyrifos hydrolase from Cladosporium cladosporioides Hu-01. PLoS One. 2012;7:e38137.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353.
- Wang P, Wang X, Wang L, et al. Interaction of gold nanoparticles with proteins and cells. Sci Technol Adv Mater. 2015;16:034610.
- Lambertini E, Piva R, Khan MT, et al. Effects of extracts from Bangladeshi medicinal plants on in vitro proliferation of human breast cancer cell lines and expression of estrogen receptor alpha gene. Int J Oncol. 2004;24:419–423.
- Nayak D, Pradhan S, Ashe S, et al. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 2015;457:329–338.