Abstract
Aim
To analyze the impact of CD32a polymorphism rs1801274 on the occurrence of Kawasaki disease (KD) through the meta-analysis.
Methods
The correlation between CD32a polymorphism rs1801274 and the susceptibility to KD was appraised using summarized odds ratios (ORs) with their 95% confidence intervals (95% CIs). Besides, stratification analyses were further implemented on the basis of ethnicity and control source, respectively. Between-study heterogeneity was checked adopting chi-square-based Q test, with p < .05 as significant level. And results from Q test determined which model would be employed for OR calculation, fixed- or random-effects. Sensitivity analysis was accomplished to test the stability of final results. Potential publication bias among included studies was investigated using Begg’s funnel plot and Egger’s test. If publication bias was significant, its influence on overall estimates would be measured adopting the trim-and-fill method.
Results
CD32a polymorphism rs1801274 significantly increased KD risk in total analysis under the comparisons of AA vs. GG, AA + AG vs. GG, AA vs. GG + AG, A vs. G and AG vs. GG (OR = 2.69, 95% CI = 1.39–5.20; OR = 2.00, 95% CI = 1.23–3.26; OR = 1.90, 95% CI = 1.23–2.94; OR = 1.77, 95% CI = 1.34–2.34; OR = 1.53, 95% CI = 1.07–2.19). After stratification analysis by ethnicity, similar tendency was also observed in Caucasian and Asian subgroups under corresponding genetic models. And parallel results were replicated in population-based and other-source subgroups after stratified analysis by control source, under some contrasts.
Conclusion
CD32a polymorphism rs1801274 has strong relation to KD onset, and the presence of its A allele could elevate the disease incidence.
Introduction
Kawasaki disease (KD) is an acute pediatric rash and fever disease with unclear pathogenesis also called mucocutaneous lymph node syndrome (MCLS) [Citation1]. The disease mainly attacks patients under 5 years old, especially among those between 6 and 18 months old, with boys facing higher risk [Citation2]. Major pathological change in KD refers to systemic non-specific vasculitis, involving small and middle-sized blood vessels, especially coronary artery [Citation3,Citation4]. And coronary artery lesion represents its most serious complication, including myocardial infarction, coronary artery ectasia, coronary artery fistula and coronary artery aneurysm [Citation5,Citation6]. KD has become a primary reason for acquired heart diseases in pediatric department, and a risk factor for adult ischemic heart diseases [Citation7]. For years, this disorder has attracted increasing attentions, and been observed in the vast majority of countries or regions around the world [Citation8]. KD shows familial aggregation, and its incidence rate is dramatically more higher among children whose siblings suffer the disease and those whose parents have the disease history [Citation9]. All these evidence indicate genetic component could exert vital influences on the disease onset.
Fcγ receptor (FcγR) stands for a transmembrane glycoprotein binding to Fc fragment of IgG and belongs to the superfamily of immunoproteins [Citation10,Citation11]. One end of FcγR could combine with immune cells, while the other end with specific antibodies, thus triggering a series of functions of cellular effector molecules, like phagocytosis, antibody-dependent cell-mediated cytotoxicity, the production of super-oxygen ions, antigen presentation, the releasing of cytokines and the generation of regulatory antibodies [Citation12–15]. According to their affinity to IgG subtypes, FcγR could be categorized into FcγRI, FcγRII and FcγRIII, with the middle one showing low affinity to IgG [Citation16]. Based on its function, FcγRII could be further classified into three subtypes, A, B and C [Citation17]. FcγRIIA expresses most widely and could be observed on multiple immune cells (including natural killer cells, macrophage and neutrophils), participating in cell activation and the uptake of immune complexes [Citation18]. The gene CD32a, encoding for FcγRIIA, contains the polymorphism rs1801274 newly identified locus related to KD susceptibility, and the A allele of the polymorphism has been proposed to be risk factor for the disease onset [Citation19]. But different opinions still exist.
Consequently, an updated meta-analysis would be necessary to pool previous relevant findings for comprehensive conclusion on the relationship of CD32a polymorphism rs1801274 with KD risk.
Materials and methods
Literature search strategy
To obtained potentially relevant publications published in English or Chinese language, we systemically searched online databases of PubMeb, EMBASE, Google Scholar Web, ISI Web of Science, Cochrane Library, CNKI and Wenfang up to 31 May 2019, adopting the combination of the following key terms: “Kawasaki disease or KD or mucocutaneous lymphnode syndrome or MCLS”, “CD32 or CD32a or CDw32 or FCG2 or FCGR2 or FCGR2A1 or FcGR or IGFR2” and “polymorphism or variant or variation or mutant or mutation or SNP”. Meanwhile, other available sources have also been screened for additional papers. No restrictions were imposed on publication year, original country, ethnic group or sample size. Moreover, reference lists of enrolled reports were manually checked for potentially relevant articles missed in electronic searching.
Selection criteria
Publication encompassed in this meta-analysis must fulfil each of the following criteria: (1) conforming to case-control design; (2) involving KD susceptibility and CD32a polymorphism rs1801274; (3) providing adequate information on genotype and/or allele frequencies of the polymorphism in cases and controls; (4) using initial data set(s); and (5) recruiting humans as study participants. Papers not satisfying any one of those standards were removed from this meta-analysis; so were letters, editorials, comments, review articles and conference abstracts. As for reports covering the same group of study subjects, the one with the largest sample size was chosen; if all were based on same number of enrolled participants, the one most recently published would be finally selected.
Data extraction
From every enrolled study, two independent reviewers extracted essential information using identical data form, which mainly contained the first author’s name, publication year, original country, ethnic descent, control source, genotyping method, the numbers of cases and controls, genotype and/or allele frequencies in the two groups, and p values for Hardy–Weinberg equilibrium (HWE) in controls. When any disagreements appeared over the recorded data, they would be solved by the reviewers through discussion until reaching consensus on each item. As for studies in one paper, their data would be extracted as separated ones.
Statistical analysis
In this meta-analysis, we adopted STATA 12.0 software (Stata Corporation, College Station, TX, USA) to implement data syntheses and figure plotting. The connection between CD32a polymorphism rs1801274 and KD risk was assessed through pooling raw odds ratios (ORs) with their 95% confidence intervals (95% CIs) under the comparisons of AA vs. GG, AA + AG vs. GG, AA vs. GG + AG, A vs. G and AG vs. GG. Besides, subgroup analyses based on ethnicity and control source were also completed to further explore potential specific relationship between two sides. Chi-square-based Q test and I2 statistic were employed to examine heterogeneity between included studies, with p < .1 or I2 > 50% standing for the presence of statistical significance. Accordingly, random-effects model would be chosen for OR summary if inter-study heterogeneity was significant; otherwise, fixed-effects model was applied. When between heterogeneity was statistically significant, meta-regression analysis would be adopted to identify its potential sources. Quality assessment for included studies was performed by Reviewer Manager 5.1. Sensitivity analysis was conducted to determine the stability of final results through sequentially omitting each of the eligible studies and re-calculating overall estimates. Begg’s funnel plot and Egger’s regression analysis were both operated to inspect possible publication bias among selected studies. When the bias was statistically significant, the trim-and-fill method would be utilized to measure the influence of the bias on ultimate evaluations.
Results
Yields of literature searching and selection
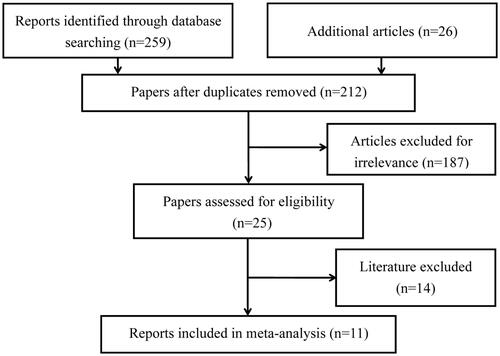
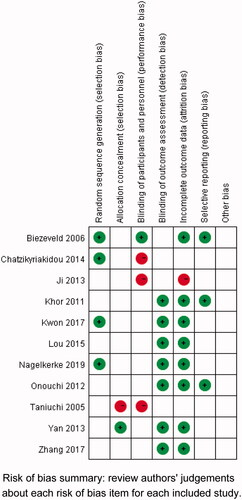
Initially, our literature search strategy yielded a total of 285 potentially relevant articles, 259 from electronic databases and 26 from other sources (). In primary examination, 73 duplicates were first removed, and additional 187 papers were then deleted for obvious irrelevance. During the full-text screening, 14 more publications were further omitted for not conforming to our inclusion criteria. As a result, 11 eligible reports were ultimately encompassed into this meta-analysis [Citation19–29], containing 17 independent studies. Of the studies, 13 were on Asian populations while only 4 on Caucasians. And the vast majority of enrolled studies recruited their control subjects from general populations. More detailed descriptions on these studies are listed in . All of the included studies had more than moderate quality ().
Table 1. Essential information on included studies in the Meta-analysis.
Data synthesis
As shown in , CD32a polymorphism rs1801274 significantly affected the susceptibility to KD in total analysis under all five genetic models of AA vs. GG (), AA + AG vs. GG, AA vs. GG + AG, A vs. G () and AG vs. GG (OR = 2.69, 95% CI = 1.39–5.20; OR = 2.00, 95% CI = 1.23–3.26; OR = 1.90, 95% CI = 1.23–2.94; OR = 1.77, 95% CI = 1.34–2.34; OR = 1.53, 95% CI = 1.07–2.19). Besides, similar results were replicated in Caucasian () and Asian () subgroups under corresponding comparisons. And so were in population-based and other-source subgroups after stratification analysis by control source ().
Figure 3. Forest plot for the association between CD32a polymorphism rs1801274 and the risk of Kawasaki disease under the contrast AA vs. GG after stratification analysis by control source.
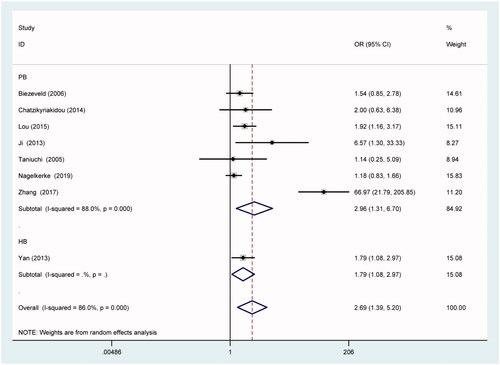
Figure 4. Forest plot for the association between CD32a polymorphism rs1801274 and the risk of Kawasaki disease under the contrast A vs. G after stratification analysis by ethnicity.
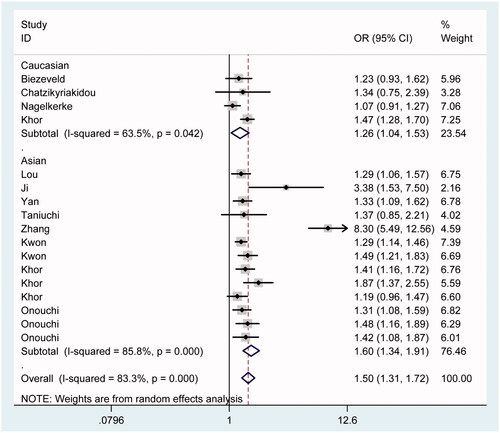
Table 2. Association between CD32a polymorphism rs1801274 and the risk of Kawasaki disease.
Heterogeneity test and sensitivity analysis
Q test revealed significant heterogeneity between eligible studies under all contrasts (p < .1 and I2 > 50%), so the random-effects model was selected to summarize overall estimates. In meta-regression analysis, we separately examined potential capability of publication year, original country, ethnic descent and control source to explain the presence of significant heterogeneity. As a result, none of them could qualitatively contribute to heterogeneity rising under any contrast, except under A vs. G comparison, the study enrolling controls from hospital could account for around half of significant heterogeneity. Due to limited information from original papers, other possible sources of significant heterogeneity failed to be discussed.
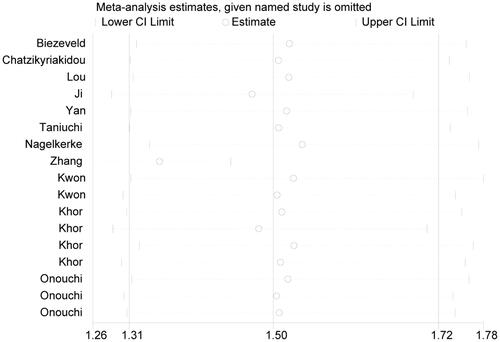
In the whole course of sensitivity analysis, we observed no substantial alterations in re-obtained estimates after deleting any one of included studies, when compared to original ones (), indicating the stability of final results in this work.
Publication bias inspection
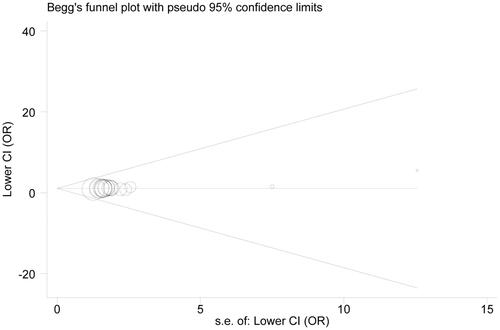
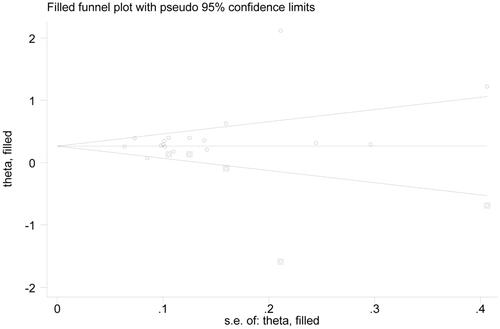
In the examination on publication bias, we uncovered its significance under the contrast A vs. G () according to asymmetrical funnel plot and statistical data from Egger’s test (p = .009). Nonetheless, even in this case, publication bias wielded no material influence on our final results, confirmed by the findings of the trim-and-fill method. Specifically, after filling 5 potentially missed papers (), re-gotten estimate still held statistical significance (OR = 1.30, 95% CI = 1.12–1.52). Consequently, our results were statistically robust to some extent.
Discussion
Among human beings, CD32a gene is located at chromosome 1 which is complicated due to the presence of copy number variations [Citation30]. In this gene, the polymorphism rs1801274, seating out of copy number variation region in chromosome 1, could lead to the transformation from histidine to arginine due to the substitution of base A by G at position coding amino acid 131 [Citation31]. And such substitution could gravely impact the capability of FcγRIIA to combine with IgG [Citation31]. As the sole receptor effectively binding to IgG2 among humans, FcγRIIA stands for a subtype of immunoglobulin receptor [Citation10]. Reportedly, FcγRIIA can efficiently combine with IgG2 when histidine appears at the polymorphism rs1801274, but fails to do so when arginine takes the place [Citation31]. Consequently, in IgG2-mediated phagocytosis, individuals carrying AA genotype would show higher efficiency than those with GG genotype. And altered genotype of the polymorphism rs1801274 could cause insufficient elimination of immune complexes whose depositions on vascular walls might further result in vascular diseases [Citation29]. Besides, earlier reports have suggested potential connection for this polymorphism with some inflammatory diseases and autoimmune diseases, such as systemic lupus erythemathosus, inflammatory bowel disease and rheumatoid arthritis [Citation32–34].
Focusing on KD incidence, previous experiments also explored its relationship with CD32a polymorphism rs1801274 but reached inconsistent findings. For example, Khor and colleagues reported that the allele A of the polymorphism rs1801274 contributed to elevated KD risk [Citation22], and similar findings have also been achieved by Onouch and colleagues [Citation19]. Besides, Lou and colleagues exhibited nominally reducing tendency for AG + GG vs. AA and GG vs. AA in their study [Citation26]. While Kwon and colleagues claimed that significant correlation of the polymorphism with KD onset only appeared in males, not among females [Citation29]. Nonetheless, some other researches detected no distinct influence for the polymorphism rs1801274 on KD occurrence at all [Citation20,Citation21,Citation25]. Given such a situation, an up-dated meta-analysis would be necessary to extract more comprehensive conclusion on this issue.
In the present meta-analysis, we enrolled relevant studies as many as possible through different ways, and our pooled statistics showed that CD32a polymorphism rs1801274 could significantly affect the susceptibility to KD. Specifically, the polymorphism enhanced the disease incidence not only in total analysis under all five comparisons of AA vs. GG, AA + GA vs. GG, AA vs. GG + GA, A vs. G and GA vs. GG but also in every subgroup under corresponding genetic models after stratification analyses by ethnicity and control source, respectively. Between our enrolled studies, significant heterogeneity was detected under all contrasts, and we failed to reveal precise sources for it, though under A vs. G comparison, the study selecting control subjects from hospital could explain about half of such heterogeneity. In sensitivity analysis, no substantial alterations have been observed in re-pooled estimates when compared to original ones, indicating fine stability of our findings. Meanwhile, although publication bias among enrolled studies was statistically significant in some cases, the trim-and-fill method ensured that the bias did not affect final estimates materially.
Despite those strengths, our meta-analysis still had its own shortcomings. First of all, considerable number of eligible studies did not offer genotype frequencies for our focused polymorphism, and we could only discuss its allele effect on KD. Second, the vast majority of the studies in our analysis were towards Asian populations, so final results might be less representative in other ethnic groups. Third, all collected papers were in either English or Chinese language, and relevant articles published in other languages might be missed. Fourth, our findings were extracted on the basis of unadjusted data and certain bias might be produced. Last but not the least, possible influences of other related elements on KD incidence were not taken into account in our meta-analysis. Consequently, our conclusion should be adopted cautiously.
All in all, the allele A and genotype AA of CD32a polymorphism rs1801274 may heighten the susceptibility to KD, especially in Asian people. In view of the limitations before listed, our findings from this meta-analysis need to be further verified by future researches containing multiple ethnic groups.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Leung AKC, Leong KF, Lam JM. Onychomadesis in a 20-month-old child with Kawasaki disease. Case Rep Pediatr. 2019;2019:3156736
- Fernandez-Cooke E, Barrios Tascon A, Sanchez-Manubens J, et al. Epidemiological and clinical features of Kawasaki disease in Spain over 5 years and risk factors for aneurysm development (2011–2016): KAWA-RACE study group. PLoS One. 2019;14:e0215665.
- Huang YH, Chen KD, Lo MH, et al. Decreased DNA methyltransferases expression is associated with coronary artery lesion formation in Kawasaki disease. Int J Med Sci. 2019;16:576–582.
- Namita U, Saddiq MH, Ahamed Z. Simultaneous development of Kawasaki disease in identical twins: a case report. J Family Med Prim Care. 2019;8: 1481–1482.
- Goh YG, Ong CC, Tan G, et al. Coronary manifestations of Kawasaki Disease in computed tomography coronary angiography. J Cardiovasc Comput Tomogr. 2018;12:275–280.
- Kuo HC. Preventing coronary artery lesions in Kawasaki disease. Biomed J. 2017;40:141–146.
- Huang FC, Kuo HC, Huang YH, et al. Anti-inflammatory effect of resveratrol in human coronary arterial endothelial cells via induction of autophagy: implication for the treatment of Kawasaki disease. BMC Pharmacol Toxicol. 2017;18:3.
- Lin MT, Wu MH. The global epidemiology of Kawasaki disease: review and future perspectives. Glob Cardiol Sci Pract. 2017;2017:e201720.
- Maggio MC, Cimaz R, Alaimo A, et al. Kawasaki disease triggered by parvovirus infection: an atypical case report of two siblings. J Med Case Rep. 2019;13:104
- Bournazos S, Ravetch JV. Fcγ receptor function and the design of vaccination strategies. Immunity. 2017;47:224–233.
- Bruggeman CW, Dekkers G, Bentlage AEH, et al. Enhanced effector functions due to antibody defucosylation depend on the effector cell Fcgamma receptor profile. JI. 2017;199:204–211.
- Acevedo OA, Diaz FE, Beals TE, et al. Contribution of Fcgamma receptor-mediated immunity to the pathogenesis caused by the human respiratory syncytial virus. Front Cell Infect Microbiol. 2019;9:75.
- Munde EO, Okeyo WA, Raballah E, et al. Association between Fcgamma receptor IIA, IIIA and IIIB genetic polymorphisms and susceptibility to severe malaria anemia in children in western Kenya. BMC Infect Dis. 2017;17:289.
- Yagi H, Takakura D, Roumenina LT, et al. Site-specific N-glycosylation analysis of soluble Fcgamma receptor IIIb in human serum. Sci Rep. 2018;8:2719.
- Chung S, Quarmby V, Gao X, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcgamma receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs. 2012;4:326–340.
- Suzuki M, Yamanoi A, Machino Y, et al. Effect of trastuzumab interchain disulfide bond cleavage on Fcgamma receptor binding and antibody-dependent tumour cell phagocytosis. J Biochem. 2016;159:67–76.
- Lu X, Peng S, Wang X, et al. Decreased expression of FcgammaRII in active Graves' disease patients. J Clin Lab Anal. 2019;33(6):e22904.
- Arman M, Krauel K. Human platelet IgG Fc receptor FcgammaRIIA in immunity and thrombosis. J Thromb Haemost. 2015;13:893–908.
- Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44:517–521.
- Taniuchi S, Masuda M, Teraguchi M, et al. Polymorphism of Fc gamma RIIa may affect the efficacy of gamma-globulin therapy in Kawasaki disease. J Clin Immunol. 2005;25:309–313.
- Biezeveld M, Geissler J, Merkus M, et al. The involvement of Fc gamma receptor gene polymorphisms in Kawasaki disease. Clin Exp Immunol. 2007;147:106–111.
- Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011;43:1241–1246.
- Ji YX, Zhang HY, Lin SX. Single nucleotide polymorphism of FCGR2A gene in Han Chinese children with Kawasaki disease. Zhongguo Dang Dai Er Ke Za Zhi 2013;15:196–200.
- Yan Y, Ma Y, Liu Y, et al. Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet. 2013;132:669–680.
- Chatzikyriakidou A, Aidinidou L, Giannopoulos A, et al. Absence of association of FCGR2A gene polymorphism rs1801274 with Kawasaki disease in Greek patients. Cardiol Young. 2015;25:681–683.
- Lou J, Zhong R, Shen N, et al. Systematic confirmation study of GWAS-identified genetic variants for Kawasaki disease in a Chinese population. Sci Rep. 2015;5:8194.
- Nagelkerke SQ, Tacke CE, Breunis WB, et al. Extensive ethnic variation and linkage disequilibrium at the FCGR2/3 locus: different genetic associations revealed in Kawasaki disease. Front Immunol. 2019;10:185.
- Zhang Y, Zhang BD, Liu YF, et al. Genetic susceptibility of ABCC4, FCGR2A and BLK polymorphisms with Kawasaki disease in children from southern China. Chinese J Lab Med. 2017;40:372–377.
- Kwon YC, Kim JJ, Yun SW, et al. Male-specific association of the FCGR2A His167Arg polymorphism with Kawasaki disease. PLoS One. 2017;12:e0184248.
- Blackburn TE, Santiago T, Burrows PD. FCRLA-A resident endoplasmic reticulum protein that associates with multiple immunoglobulin isotypes in B lineage cells. Curr Top Microbiol Immunol. 2017;408:47–65.
- Stein MM, Hrusch CL, Sperling AI, et al. Effects of an FcgammaRIIA polymorphism on leukocyte gene expression and cytokine responses to anti-CD3 and anti-CD28 antibodies. Genes Immun. 2019;20(6):462–472.
- Zhang C, Wang W, Zhang H, et al. Association of FCGR2A rs1801274 polymorphism with susceptibility to autoimmune diseases: a meta-analysis. Oncotarget. 2016;7:39436–39443.
- Li H, Jin Z, Li X, et al. Associations between single-nucleotide polymorphisms and inflammatory bowel disease-associated colorectal cancers in inflammatory bowel disease patients: a meta-analysis. Clin Transl Oncol. 2017;19:1018–1027.
- Lee YH, Bae SC, Song GG. FCGR2A, FCGR3A, FCGR3B polymorphisms and susceptibility to rheumatoid arthritis: a meta-analysis. Clin Exp Rheumatol. 2015;33:647–654.