Abstract
This study aimed to investigate the effect of Fyn gene silencing on the apoptosis of oligodendrocytes (OLs) in epileptic model in vitro and the involved mechanism. Primary oligodendrocyte pro-genitor cells (OPCs) were separated from rats and differentiated to OLs. Immunofluorescent labeling showed positive expression of A2B5 in OPCs and Olig2 in OLs, suggesting the successful separation of OPCs and OLs. Three Fyn siRNAs (si-Fyn) and Fyn siRNA negative control (NC) were transfected into OLs. Western blot showed that among three si-Fyn groups, si-Fyn3 caused the lowest Fyn expression, so si-Fyn3 was chosen for following experiment. Cells were divided into four groups: Control, Model, NC and si-Fyn. In the Model group, cells were cultured in Mg-free extracellular fluid for 3 h. The morphology of control cells was normal. However, the migration of neurons, the aggregation of cell bodies and the “grid-like” changes of neural networks were observed in the model cells. OLs apoptosis in various groups was assessed by flow cytometry. Expression of Fyn, ERK1/2 and phosphorylated ERK1/2 (p-ERK1/2) in OLs of various groups was evaluated by western blot. Compared with the Control group, the apoptotic rates, the Fyn expression and p-ERK1/2/ERK1/2 ratio in the Model and NC groups increased significantly (p < .05). However, the apoptotic rate, the Fyn expression and p-ERK1/2/ERK1/2 ratio in the si-Fyn group were remarkably smaller than those in the Model group (p < .05). In conclusion, Fyn gene silencing reduced the apoptosis of OLs through inhibiting the phosphorylation of ERK1/2 in epileptic model.
Introduction
Epilepsy is one of the serious chronic nervous system diseases with recurrent spontaneous seizures and low cure rate. At present, drug therapy for disease control is the main treatment of epilepsy. However, most antiepileptic drugs have many side effects such as cognitive impairment, psychiatric side effects and epilepsy recurrence [Citation1–3]. Furthermore, most of the present antiepileptic drugs only control the symptomatic onset of epilepsy, but cannot affect the pathological changes of epilepsy and block the progression of epilepsy [Citation1–3]. These drugs do not treat epilepsy from the source of the disease. Epilepsy has caused physical damage and psychological and mental disorders to patients, greatly reducing the survival and life quality of the patients.
Neurogliocytes are widely distributed in central nervous system (CNS), including astrocytes, oligodendrocytes (OLs) and microglial cells. They have the functions of supporting and nourishing neurons, secreting and regulating some active substances. OLs are the myelin-forming cells of CNS, which plays a distinctly important role in the formation of myelin and the transmission of information. OLs originate from the proliferative neural progenitor cells (NPCs) in the periventricular and subventricular regions of CNS. Their development process goes through several successive stages: NPCs, oligodendrocyte prepro-genitors, oligodendrocyte pro-genitor cells (OPCs), immature OLs and mature OLs [Citation4]. Only mature OLs have the ability to form myelin [Citation4]. The myelin in CNS of vertebrates is a multilamellar membrane structure formed by the OLs cytoplasm extending to and enclosing the nearby axons, which has the function of accelerating the speed of neurotransmission, insulation and nutrition. The formation and integrity of myelin ensures the accurate and efficient transmission of neural information and plays an extremely important role in the integration of central information. It is suggested that myelin formation is a significant marker of brain maturation and normal brain function [Citation5,Citation6].
Fyn is a member of the Src family of non-receptor protein tyrosine kinases. Fyn is highly expressed in OLs and plays an important role in the initial stage of myelin formation [Citation7,Citation8]. A recent report reveals that myelin thinning in Fyn deficient mice is 40–50% [Citation9]. Fyn has no extracellular and transmembrane regions. It is free in the cytoplasm or connects to the membrane by acyl groups at the amino terminal. When cells are activated by external signals related to their differentiation and development, phosphorylation of tyrosine residues of target proteins enables Fyn to transmit information within cells, consequently activating downstream signals [Citation10].
Therefore, in this study, we investigated the effect of Fyn gene silencing on the apoptosis of OLs in epileptic model in vitro and the involved mechanism, which might contribute to the study and treatment of epilepsy.
Materials and methods
Materials and animals
DNase1 was bought from Solarbio (Beijing, China). Rat fibroblast growth factor 2 (FGFb) was purchased from Novoprotein (Shanghai, China). Platelet derived growth factor α (PDGFα) was obtained from Sino Biological (Beijing, China). DMEM/F12 medium was provided by Gibco (Grand Island, NY, USA). Fetal bovine serum (FBS) was gotten from Biological Industries (Kibbutz Beit Haemek, Israel). Lipofectamine 3000 was obtained from Thermo Fisher Scientific (Waltham, MA, USA). Annexin V-FITC/PI apoptosis kit was bought from Multi Sciences (Zhejiang, China). RIPA buffer was purchased from Applygen (Beijing, China). Mouse anti-A2B5 monoclonal antibody (ab53521), mouse anti-β-actin monoclonal antibody (ab8226), rabbit anti-Fyn monoclonal antibody (ab184276), rabbit anti-extracellular signal-regulated kinase 1/2 (ERK1/2) polyclonal antibody (ab17924) and rabbit anti-phosphorylated ERK1/2 (p-ERK1/2) polyclonal antibody (ab214362) were obtained from Abcam (Cambridge, MA, USA). Rabbit anti-Olig2 polyclonal antibody (bs-11194R) was provided by Bioss (Beijing, China). Mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (TA-08), peroxidase-conjugated goat anti-mouse IgG(H + L) (ZB-2305) and peroxidase-conjugated goat anti-rabbit IgG(H + L) (ZB-2301) were gotten from ZSGB-BIO (Beijing, China).
Specific pathogen free (SPF)-grade Sprague Dawley (SD) rats within 48 h of birth were obtained from Hunan Slac JD Laboratory Animal Co., Ltd. (License No. SCXK(XIANG)2016-0002). All rats with free access to food and water were housed at 22–25 °C and 12 h/12 h light/dark cycle. Study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Nanchang University.
Induction of primary OPCs to OLs
SD rats within 48 h of birth were anesthetized. The brain was taken out and cut open along the midline. Olfactory bulb, basal nucleus, hippocampus, meninges and blood vessels were removed and the cortex was cut into 1 mm3 tissue blocks. The tissue blocks were filtered by screen mesh and digested in DNase1 (0.2 mg/mL) and 0.25% trypsin at 37 °C. After 15 min, complete medium was added to stop the digestion. The mixture was centrifuged and the pellet was resuspended in DMEM/F12 medium supplemented with 5% FBS, penicillin-streptomycin (100×), PDGFα (10 ng/mL) and FGFb (10 ng/mL) to prepare cell suspension with 5 × 106 cells/mL. The cells were seeded in culture plates and cultured at 37 °C and 5% CO2.
OPCs were separated and purified by oscillating separation and differential adhesion. PDGFα and FGFb promoting proliferation were added to obtain a large number of OPCs. The culture medium was replaced by DMEM/F12 medium supplemented with penicillin-streptomycin (100×), PDGFα (10 ng/mL) and FGFb (10 ng/mL) and cells were cultured to become mature OLs.
Immunofluorescent labeling
Petri dishes in the culture plate were climbed by cells, fixed in 4% paraformaldehyde for 15 min, washed with PBS, incubated in 0.5% Triton X-100 at room temperature for 20 min and washed with PBS three times. PBS was removed and blotted up. The petri dishes were incubated in 5% bovine serum albumin (BSA) at 37 °C for 30 min, incubated in primary antibody buffer (A2B5 antibody and Olig2 antibody) at 4 °C overnight, and incubated in secondary antibody buffer at room temperature for 30 min. The petri dishes were washed and incubated in 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 5 min in the dark. Redundant DAPI was removed by PBS rinsing. The petri dishes were mounted with 50% glycerin and microscopically examined (CKX53, Olympus, Japan).
Construction of Fyn interference vector
According to Fyn gene sequence, siRNAs were designed and synthesized by General Biosystems (Anhui) Co., Ltd. Sequences of Fyn siRNAs (si-Fyn) are shown in .
Table 1. Sequences of Fyn siRNAs.
Cell transfection
Mixture of lipofectamine 3000, plasmid and P3000 was prepared according to the manufacturer’s instruction and added into cells in FBS-free medium at 70% confluence. After culturing for 4 h, complete medium containing 20% FBS was added and cells were cultured for another 48 h for following experiments.
Model establishment and experimental grouping
Cells were divided into several groups: Control, Model, NC and si-Fyn. Normal cultured cells served as the Control. In the Model group, cells were cultured in Mg-free extracellular fluid for 3 h and then cultured in the original medium. Cells in the Model group were microscopically examined. In the NC group, cells were transfected with Fyn siRNA NC and then modeled. In the si-Fyn group, cells were transfected with Fyn siRNA and then modeled.
Western blot
Cells in various groups were collected and lysed in RIPA buffer for 30 min. The lysate was centrifuged to collect the supernatant as total protein. Protein concentration was examined using BCA kit. Subsequently, the protein was boiled for denaturation and about 30 ng of protein was loaded to perform SDS-PAGE for 1.5 h. The protein was then transferred to a membrane for 40 min by a wet method. The membrane was incubated in 2% BSA at room temperature for 1 h, incubated in primary antibody buffer at 4 °C overnight, and incubated in secondary antibody buffer at room temperature for 1–2 h. Chemiluminescence solution was added and the membrane was examined using a gel imaging system (ChemiDocTM XRS+, Bio-Rad, USA). Gray values were measured using Quantity One 4.6.2 software (Bio-Rad, USA).
Cell apoptosis
Cells (1 × 106–3 × 106) in various groups were collected, treated with Annexin V-FITC/PI apoptosis kit according to the manufacturer’s instruction, and evaluated using a flow cytometer (NovoCyte 2060 R, ACEA Biosciences, San Diego, CA, USA).
Statistical analysis
Statistical analysis was performed with analysis of variance (ANOVA) followed by a post hoc test using SPSS 19.0 software. Significant difference was defined when p values was less than 0.05.
Results
Identification of OPCs and OLs
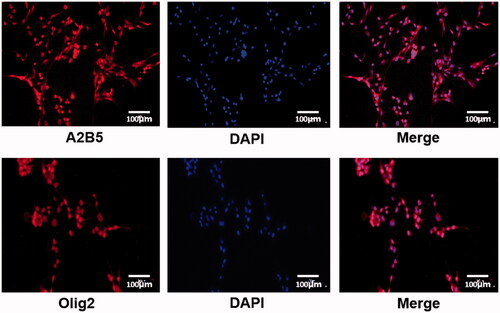
The separated OPCs and OLs were identified by immunofluorescent labeling of A2B5 and Olig2 (). Cell nuclei were stained by DAPI. Target proteins (A2B5 and Olig2) were labeled as red. Positive expression of A2B5 in OPCs and Olig2 in OLs was observed, suggesting the successful separation of OPCs and OLs.
Verification of cell transfection
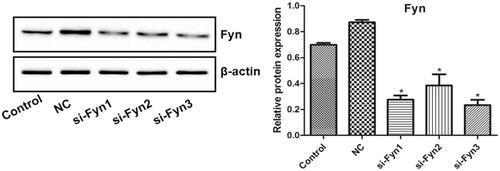
shows the Fyn expression in OLs transfected with three Fyn siRNAs (si-Fyn1/2/3) and Fyn siRNA NC which was evaluated by western blot. There was no significant difference in the Fyn expression between the Control and NC groups. Compared with the Control group, the Fyn expression decreased significantly in all three si-Fyn groups (p < .05). Among three si-Fyn groups, the Fyn expression in si-Fyn3 group seemed to be the lowest, so si-Fyn3 was chosen for following experiment.
Model establishment
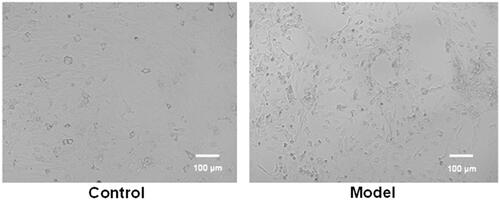
Typical images of epileptic model cells and control cells are shown in . The morphology of control cells was normal. However, the migration of neurons, the aggregation of cell bodies and the “grid-like” changes of neural networks were observed in the image of model cells. These results suggested the successful establishment of epileptic model in vitro.
Cell apoptosis
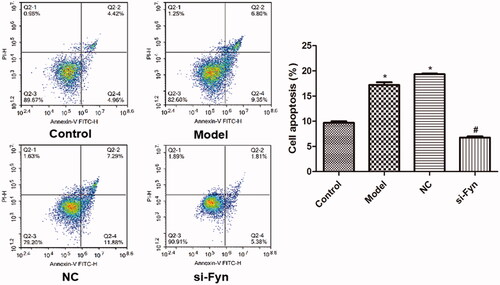
shows the apoptosis of OLs in various groups which was assessed by flow cytometry. Compared with the Control group, the apoptotic rates in the Model and NC groups increased significantly (p < .05). However, the apoptotic rate in the si-Fyn group was remarkably smaller than that in the Model group (p < .05).
Figure 4. The apoptosis of OLs in various groups which was assessed by flow cytometry. Normal cultured cells served as the Control. In the Model group, cells were cultured in Mg-free extracellular fluid for 3 h and then cultured in the original medium. In the NC group, cells were transfected with Fyn siRNA NC and then modeled. In the si-Fyn group, cells were transfected with Fyn siRNA and then modeled. *p < .05 vs. Control; #p < .05 vs. Model.
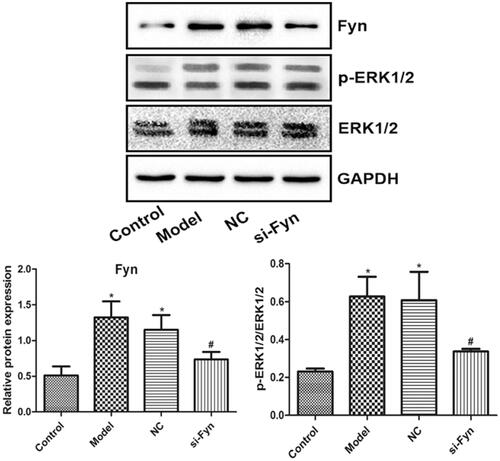
Expression of Fyn, ERK1/2 and p-ERK1/2
demonstrates the expression of Fyn, ERK1/2 and p-ERK1/2 in OLs of various groups which was evaluated by western blot. Similar blots of ERK1/2 were found among various groups. Compared with the Control group, the Fyn expression and p-ERK1/2/ERK1/2 ratio in the Model and NC groups were elevated remarkably (p < .05). However, compared with the Model group, the Fyn expression and p-ERK1/2/ERK1/2 ratio in the si-Fyn group were reduced significantly (p < .05).
Figure 5. The expression of Fyn, ERK1/2 and p-ERK1/2 in OLs of various groups which was evaluated by western blot. Normal cultured cells served as the Control. In the Model group, cells were cultured in Mg-free extracellular fluid for 3 h and then cultured in the original medium. In the NC group, cells were transfected with Fyn siRNA NC and then modeled. In the si-Fyn group, cells were transfected with Fyn siRNA and then modeled. *p < .05 vs. Control; #p < .05 vs. Model.
Discussion
The occurrence of epilepsy includes the process of susceptibility of the epileptic brain to spontaneous seizures prior to the first spontaneous seizure and the process of aggravating drug resistance [Citation11]. Various brain injuries such as brain trauma, stroke or intracranial infection induce cascading events in the brain, leading to increased susceptibility to epilepsy and spontaneous seizures. At present, it has been generally acknowledged that a specific brain injury can induce epileptic seizures after a latent period [Citation12]. OLs are distributed in both white matter and gray matter of brain, which insulate the axons of neurons, make saltatory conduction of neural information and speed up the transmission of neural information. Myelin formation is regulated by many factors [Citation13–14]. OLs need to establish close connections with neural blood vessels to maintain the formation of myelin and the integrity of axonal function. Therefore, when brain tissue is damaged and even if only a single OL is damaged, it may cause damage to multiple neuronal axons, thus affecting nerve function. In this study, OPCs were separated and identified by specific antibody A2B5, which could be used to induce differentiation to OLs. The differentiated OLs were identified by specific antibody Olig2, which could be used for following experiments.
Fyn signaling pathway plays an important role in myelin formation by activating myelin basic protein gene [Citation15]. In addition, further study on the signaling molecular pathway of Fyn deficient mice shows that Fyn tyrosine kinase initiates myelin formation by promoting tyrosine phosphorylation and phosphorylation cascade reactions of tyrosine kinase participate in signal transduction during myelin formation [Citation16]. In this study, three sequences interfering with Fyn expression were designed and results of western blot showed that si-Fyn3 had the best interference effect.
Epilepsy induced by absence of magnesium can lead to the decrease of presynaptic silent synapses and postsynaptic silent synapses [Citation17]. The reduced silent synapses are likely to activate into functional synapses and become the pathological basis of epilepsy [Citation17]. In this study, we observed the migration of neurons, the aggregation of cell bodies and the “grid-like” changes of neural networks after the establishment of epileptic cell model.
Neural cell death after epilepsy includes necrosis and apoptosis [Citation18]. A previous study shows that number of apoptotic cells has increased at 1 day after seizure and is significantly larger than that in the control group until the seventh week, suggesting that apoptosis runs through the course of epilepsy in rats [Citation19]. Another study shows that neuronal apoptosis partially participates in the formation of hippocampal sclerosis in epileptic patients [Citation19]. The results of this study revealed that apoptotic rate of OLs increased remarkably after the establishment of epileptic model and decreased significantly after the down-regulation of Fyn expression, which was consistent with the above reports [Citation19]. These results suggested that the apoptosis of OLs was regulated by Fyn expression.
ERK1/2 signaling pathway is a crucial pathway in eukaryotic cells, which plays an important role in cell growth, proliferation and differentiation [Citation20]. ERK1/2 is the key kinase of this pathway. Previous studies show that ERK1/2 participates in the proliferation of astrocytes after cerebral ischemia and brain injury [Citation21,Citation22], and it is extensively activated in epileptic hippocampus [Citation23]. Under normal circumstances, p-ERK1/2 is localized and less expressed in the brain, which may be involved in the regulation of neurogenesis, neuronal stabilization and axon formation [Citation24]. In this study, we found that the expression of Fyn and p-ERK1/2 was enhanced significantly after the establishment of epileptic model, which agreed with the activation of ERK1/2 signaling pathway by epilepsy in previous reports [Citation21–23]. However, the expression of p-ERK1/2 declined significantly when the Fyn expression was down-regulated, suggesting that the activation of ERK1/2 signal might be regulated by Fyn expression.
In conclusion, Fyn gene silencing reduced the apoptosis of OLs through inhibiting the phosphorylation of ERK1/2 in epileptic model. Regulation of OLs by specific activation of Fyn-ERK1/2 signaling pathway might play an important role in the pathogenesis of epilepsy. These results might contribute to the study and treatment of epilepsy.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
References
- Devinsky O, Marsh E, Friedman D. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–278.
- Wallace RH, Scheffer IE, Barnett S, et al. Neuronal sodium-channel alpha1-subunit mutations in generalized epilepsy with febrile seizures plus . Am J Hum Genet.. 2001;68(4):859–865.
- Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530.
- Kitley J, Waters P, Woodhall M, et al. Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol.. 2014;71(3):276–283.
- Dai AI, Akcali A, Koska S, et al. Contribution of KCNJ10 gene polymorphisms in childhood epilepsy. J Child Neurol.. 2015;30(3):296–300.
- Yamazaki Y, Fujiwara H, Kaneko K, et al. Short- and long-term functional plasticity of white matter induced by oligodendrocyte depolarization in the hippocampus. Glia. 2014;62(8):1299–1312.
- Sanz-Blasco S, Bordone MP, Damianich A, et al. The kinase Fyn as a novel intermediate in l -DOPA-induced dyskinesia in Parkinson’s disease. Mol Neurobiol. 2018;55(6):5125–5136.
- Huang Y, Li G, An L, et al. Fyn regulates multipolar-bipolar transition and neurite morphogenesis of migrating neurons in the developing neocortex. Neuroscience. 2017;352:39–51.
- Smith LM, Zhu R, Strittmatter SM. Disease-modifying benefit of Fyn blockade persists after washout in mouse Alzheimer’s model. Neuropharmacology. 2018;130:54.
- Dallari S, Macal M, Loureiro ME, et al. Src family kinases Fyn and Lyn are constitutively activated and mediate plasmacytoid dendritic cell responses. Nat Commun. 2017;8(1):14830.
- Szaflarski JP, Devinsky O. Cannabinoids and epilepsy – introduction. Epilepsy Behav. 2017;70(Pt B):277.
- Sinha N, Dauwels J, Kaiser M, et al. Predicting neurosurgical outcomes in focal epilepsy patients using computational modeling. Brain. 2017;140(2):319–332.
- Hu X, Wang JY, Gu R, et al. The relationship between the occurrence of intractable epilepsy with glial cells and myelin sheath-an experimental study. Eur Rev Med Pharmacol Sci. 2016;20(21):4516–4524.
- Nunes MC, Roy NS, Keyoung HM, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nature Med. 2003;9:439–447.
- Xie D, Shen F, He S, et al. IL-1β induces hypomyelination in the periventricular white matter through inhibition of oligodendrocyte progenitor cell maturation via FYN/MEK/ERK signaling pathway in septic neonatal rats. Glia. 2016;64(4):583–602.
- Lyu S, Han D, Li X, et al. Fyn knockdown inhibits migration and invasion in cholangiocarcinoma through the activated AMPK/mTOR signaling pathway. Oncol Lett. 2018;15:2085–2090.
- Thompson SJ, Ashley MD, Stöhr S, et al. Suppression of TNF receptor-1 signaling in an in vitro model of epileptic tolerance. Int J Physiol Pathophysiol Pharmacol. 2011;3:120–132.
- Huang LG, Zou J, Lu QC. Silencing rno-miR-155-5p in rat temporal lobe epilepsy model reduces pathophysiological features and cell apoptosis by activating Sestrin-3. Brain Res. 2018;1689:109.
- Zhang B, Zhang J, Wang W, et al. Effect of lamotrigine on epilepsy-induced cognitive impairment and hippocampal neuronal apoptosis in pentylenetetrazole-kindled animal model. Synapse. 2017;71(2):e21945.
- Ge LC, Wang HS. A commentary on “Involvement of activating ERK1/2 trough G protein coupled receptor 30 and estrogen receptor α/β in low doses of bisphenol A promoting growth of Sertoli TM4 cells”. Toxicol Lett. 2016;240(1):236–237.
- Chen M, Dai LH, Fei A, et al. Isoquercetin activates the ERK1/2-Nrf2 pathway and protects against cerebral ischemia-reperfusion injury in vivo and in vitro. Experiment Therapeutic Med. 2017;13:1353.
- Bhowmick S, D’Mello V, Abdul-Muneer PM. Synergistic inhibition of ERK1/2 and JNK, not p38, phosphorylation ameliorates neuronal damages after traumatic brain injury. Mol Neurobiol. 2019;56(2):1124–1136.
- Pilar NM, Elisabet GP, Olmo Marisol MD. Compound inhibiting activation of the enzyme ERK1/2 for use in the treatment of neurogenerative illnesses. WO2011147999. 2016.
- Hu SP, Zhao JJ, Wang WX, et al. Dexmedetomidine increases acetylation level of histone through ERK1/2 pathway in dopamine neuron. Hum Exp Toxicol. 2017;36:474–482.