Abstract
Osteoarthritis is a common type of degenerative joint disease. Inflammation-related chondrocyte senescence plays a major role in the pathogenesis of osteoarthritis. Omentin-1 is a newly identified anti-inflammatory adipokine involved in lipid metabolism. In this study, we examined the biological function of omentin-1 in cultured chondrocytes. The presence of omentin-1 potently suppresses IL-1β-induced cellular senescence as revealed by staining with senescence-associated beta-galactosidase (SA-β-Gal). At the cellular level, omentin-1 attenuates IL-1β-induced G1 phase cell-cycle arrest. Mechanistically, we demonstrate that omentin-1 reduced IL-1β-induced expression of senescent factors including caveolin-1, p21, and PAI-1 as well as p53 acetylation through ameliorating SIRT1 reduction. Notably, silencing of SIRT1 abolishes IL-1β-induced senescence along with the induction of p21 and PAI-1, suggesting that the action of omentin-1 in chondrocytes is dependent on SIRT1. Collectively, our results revealed the molecular mechanism through which the adipokine omentin-1 exerts a beneficial effect, thereby protecting chondrocytes from senescence. Thus, omentin-1 could have clinical implication in the treatment of osteoarthritis.
Introduction
Joint diseases are a major cause of disability in adults. Osteoarthritis is a common degenerative joint disease which is epidemic in the aged population [Citation1]. Aside from age, obesity is another important contributor to osteoarthritis. The traditional opinion recognises body weight as a risk factor as heavier body weight induces more stress on the joints and promotes cartilage damage. However, recent knowledge suggests that abnormal levels of regulatory adipokines in circulation or local joint tissue could be much more significant than body weight in terms of the development of osteoarthritis [Citation2,Citation3]. Adipokines are a group of active factors derived from white adipose tissue. The most common adipokines found so far include leptin, adiponectin, vaspin, and omentin, etc. and they have been shown to be involved in the modulation of many metabolic processes [Citation4]. Adipokines in joint tissue can be secreted locally or come from body fluid systematically. Among these adipokines, omentin-1 (also named intelectin-1 as it binds to lectin) is the most recently discovered adipokine, first being described in 2006, and is named after its identification in omental adipose tissue [Citation5]. Omentin-1 is a 313-amino acid protein with a molecular weight of 40 kDa. The plasma level of omentin-1 in lean subjects is high, while it is reduced in obese patients [Citation6]. Omentin-1 has been recognised as a “good” adipokine due to its anti-inflammatory property and reduced expression in metabolic and cardiovascular diseases [Citation7].
The chondrocyte is the only active cell type responsible for the synthesis of the extracellular matrix of articular cartilage in joint tissue. Chondrocytes are highly responsive to cellular inflammatory cytokine and growth factor levels. Under healthy conditions, chondrocytes synthesise adequate components of the extracellular matrix (such as collagens and proteoglycans) and maintain joint tissue homeostasis. However, “wear and tear” as seen in aged joint tissue often triggers an abnormal inflammatory response and cartilage repair. The inflammatory stimuli that are produced as a result of these processes can induce the production of matrix metalloproteinases (MMPs) by chondrocytes and reduce the expression of matrix metalloproteinase inhibitors, which in turn impairs the delicate balance between the synthesis and degradation of articular cartilage. Moreover, the high expression level of inflammatory cytokines and other factors also leads to chondrocyte senescence [Citation8]. Senescent chondrocytes lose their ability to secrete extracellular matrix components but express certain molecular markers, such as SA-β-Gal secretion, and higher amounts of caveolin-1, p21, p16, p53, and other cell cycle regulators [Citation9]. Chondrocyte senescence plays a significant role in the development of osteoarthritis [Citation1]. In our effort to seek new agents to protect against chondrocyte senescence, we have tested the effects of several adipokines in chondrocytes. Here, we report the beneficial effect of omentin-1 in the cultured chondrocytes.
Materials and methods
Cell culture and treatment experiment
We purchased chondrosarcoma SW1353 cells from ATCC. The cells were cultured in ATCC-formulated Leibovitz’s L-15m medium containing 10% FBS. For cell treatment, SW1353 cells were seeded into 6-well or 35-millimeter plates and grown to full confluence. IL-1β was dissolved in PBS-BSA solution, and omentin-1 was dissolved in DMSO solution. The fully-grown confluent cells were then stimulated with IL-1β (10 ng/mL) for 24 h. For omentin-1 co-treatment, 150 and 300 ng/mL concentrations of omentin-1 were added to the cell media for the same period of time.
Real-time PCR analysis
We extracted RNA from SW1353 cells with a commercial RNA miniprep purification kit (Qiagen). The isolation process was performed in accordance with the manufacturer’s manual. For the reverse transcription of RNA, 1 μg RNA was used to synthesise cDNA using an RT-PCR One-step kit (Bio-Rad, USA). For real-time PCR experiments, the SYBR-based real-time PCR method was employed to measure the transcripts of human caveolin-1, p21, PAI-1, and GAPDH (as a housekeeping control) on the Bio-Rad platform. The expression of each gene was normalised to GAPDH and calculated using the 2−ΔΔ CT method.
Western blot analysis
To obtain cell lysate from SW1353 cells, we used a commercial RIPA buffer supplemented with freshly prepared protease and phosphatase inhibitors. A total of 20 μg whole-cell extract from each sample was loaded and immobilised on a 10% PAGE gel. The separated protein gel was then transferred to a PVDF membrane and blocked with 5% non-fat milk in PBS, followed by incubation with the following primary antibodies: caveolin-1 (1:1000), p21 (1:2000), PAI-1 (1:2000), SIRT-1 (1:3000), Ac (k382)-p53 (1:500) and corresponding secondary antibody (1:5000). Finally, the immunoblots were visualised using the Odyssey automatic western blotting system.
Immunoprecipitation
After the indicated treatment, SW1353 cells were lysed using TNE Buffer containing protease and phosphatase inhibitors. Protein lysate was immunoprecipitated with anti-human rabbit polyclonal p53 (2381-PC-100, Trevigen) and protein-A Dynabeads W (Life Technology, USA). Immunoprecipitated products were subjected to western blot analysis with anti-acetyl-p53 at Lys382 (#2525, Cell Signalling Technology, USA).
SA-β-Gal staining
SA-β-Gal is a biomarker of cellular senescence. We purchased a cellular senescence staining kit (Sigma-Aldrich) to monitor the senescent status of SW1353 cells. In brief, the cells were seeded at a density of 5 × 104 cells/well in cell culture chambers, and the attached cells were then incubated with IL-1β-containing medium (10 ng/mL) for 24 h. To stain the cells, the chamber slides were fixed with 0.25% glutaraldehyde fixation solution for 5 min and probed with staining solution at 4 °C for 2 h. The slides were then mounted with DAPI-containing VECTOR shield oil. The digital images were captured with an LCD camera. The positive stained cells were then counted and normalised to the total cell numbers.
Flow cytometry
We assayed the cell-cycle profile using flow cytometry. Briefly, SW1353 cells under the different conditions were processed with fixation with 100% methanol, permeabilization with 0.1% Twen-20% solution, and staining with propidium iodide (PI). The cell phases were determined by capturing the fluorescence signals with software. The data from experiments performed in triplicate were collected and are presented as a bar graph.
SIRT-1 knockdown
We used a siRNA-based approach to silence SIRT-1 expression in SW1353 cells. To transfect the cells, we applied a mixture of Lipofectamine RNAi Max (Invitrogen) reagent and SIRT-1 oligos to 50% confluent SW1353 cells. The transfected cells were then grown for an additional 48–72 h.
Statistics
All the experimental data from multiple repeats in this study are presented as means ± standard derivation (SD). Group mean comparisons were tested with ANOVA. The proportional difference was tested with the Fisher test. The significance of the resulting values was determined based on a p-value of <.05.
Results
Omentin-1 ameliorates IL-1β-induced chondrocyte senescence
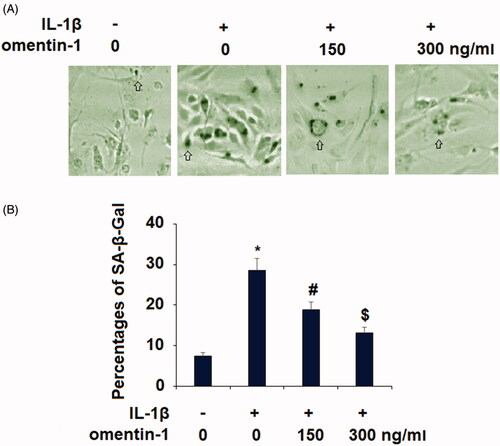
To explore the biological function of omentin-1 in chondrocytes, we tested whether omentin-1 has the potential to influence cytokine-induced chondrocyte senescence. It has been reported that 24 h treatment with extracellular toxic stimuli, including IL-1β, could induce the senescent phenotype in chondrocytes [Citation10,Citation11]. In our experiment, we measured the level of cellular senescence in IL-1β (10 ng/mL)-induced SW1535 chondrocytes with or without omentin-1 (150,300 ng/mL) for 24 h. Our results show that omentin-1 reduced the number of SA-β-Gal-positive cells (. Through quantitation of multiple areas, we found that about 7.5% of the non-treated cells were SA-β-Gal-positive, while IL-1β increased this number to about 29%. However, IL-1β induced only about 19% and 13% senescence in the presence of 100 and 300 omentin-1, respectively (.
Figure 1. Omentin-1 prevented IL-1β-induced senescence in human SW1353 cells. Cells were stimulated with IL-1β (10 ng/mL) with or without omentin-1 (150,300 ng/ml) for 24 h. (A). SA-β-Gal staining was assayed; (B) Percentages of SA-β-Gal staining positive cells (*, #, $p < .01 vs. previous group, n = 6).
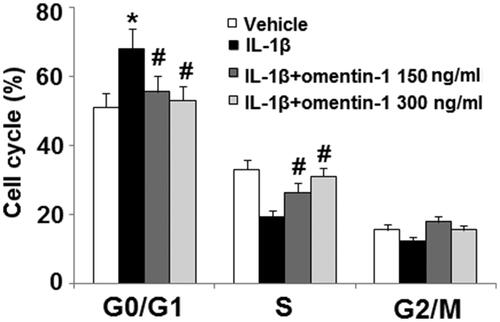
Omentin-1 rescues IL-1β-induced G1 phase cell cycle arrest
Next, we assessed the cell cycle profile via flow cytometry. Compared to non-treated cells, 24 h of IL-1β treatment induced about a 20% increase in G1 phase cell cycle arrest as well as S and G2/M phase shortening. However, the presence of omentin-1 significantly attenuated G1 phase arrest with the higher dose completely rescuing IL-1β-induced G1 arrest ().
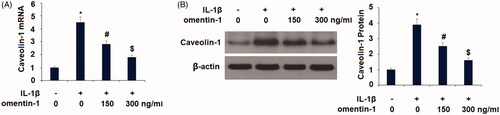
Omentin-1 suppresses IL-1β-induced caveolin-1 induction
Next, we explored the molecular mechanism of omentin-1 involved in chondrocyte senescence. Caveolin-1 is a senescence-induced protein, and we found that omentin-1 suppressed IL-1β-induced caveolin-1 expression. Compared to non-treated cells at the mRNA level, IL-1β induced more than 4-fold higher caveolin-1 transcription, but the presence of the two doses of omentin-1 significantly suppressed the induction of caveolin-1 by IL-1β, with the higher dose showing more potent suppression (. At the protein level, omentin-1 exerted a similar suppressive effect on IL-1β-induced caveolin-1 expression (.
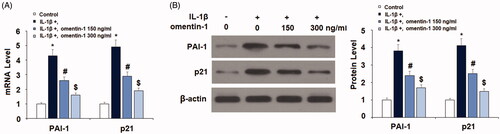
Omentin-1 inhibits IL-1β-induced PAI-1 and p21 expression
We also examined the effect of omentin-1 on the aging factors PAI-1 and p21. IL-1β induced more than 4-fold higher PAI-1 and p21 mRNA, but the addition of omentin-1 dramatically reduced PAI-1 and p21 induction at the mRNA level (. We confirmed that omentin-1 had a similar inhibitive effect on protein expression of PAI-1 and p21 (.
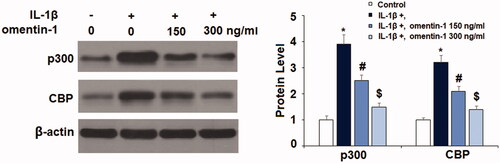
Omentin-1 suppresses IL-1β-induced K382 acetylation of p53
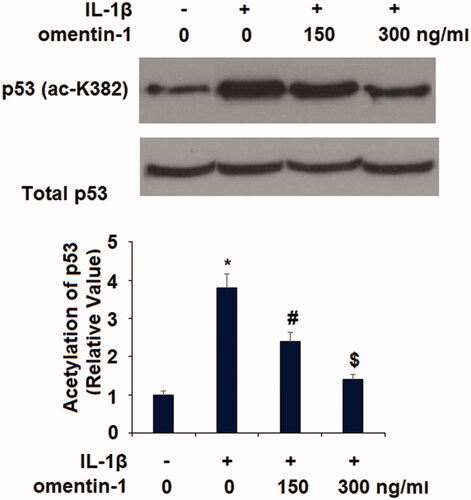
Next, we checked the influence of omentin-1 on the transcriptional factor p53. Our experiment showed that omentin-1 had an inhibitory impact on K382 acetylation of p53 (ac-K382). Treatment with IL-1β induced roughly 4-fold higher p53 acetylation, but co-treatment with omentin-1 significantly inhibited this induction (). Upregulation of p300 and CBP have been linked with K382-p53 acetylation [Citation12]. Here, we found that exposure to IL-1β remarkably upregulated the expression of p300 and CBP, which was prevented by the presence of omentin-1 in a dose-dependent manner ().
Figure 5. Omentin-1 inhibited IL-1β-induced K382 acetylation of p53 (ac-K382). Cells were treated with IL-1β (10 ng/mL) with or without omentin-1 (150,300 ng/mL) for 24 h. Acetylation of p53 was measured by immunoprecipitation with anti-human rabbit polyclonal p53 and western blot analysis with anti-acetyl-p53 at Lys382. The ratio of acetylated p53 to total p53 was quantified (*, #, $p < .01 vs. previous group, n = 5–6).
Omentin-1 recovers IL-1β-induced reduced SIRT1
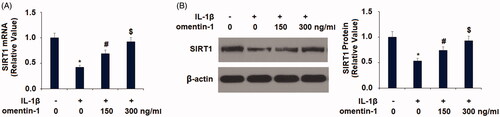
SIRT1 is one of the most well-known aging factors, so we tested whether omentin-1 would have an impact on SIRT1 expression. Our experiment showed that omentin-1 potently recovered IL-1β-mediated reduced SIRT1 expression in SW1353 cells. Compared to the non-treated control, IL-1β treatment resulted in a reduction in SIRT1 mRNA of more than half, but the addition of omentin-1 recovered most of this reduction, with the higher dose of omentin-1 almost completely rescuing the reduced expression of SIRT1 (. We confirmed the capacity of omentin-1 to rescue IL-1β-induced reduced SIRT-1 expression at the protein level (.
Silencing of SIRT1 expression abolishes the protective effect of omentin-1
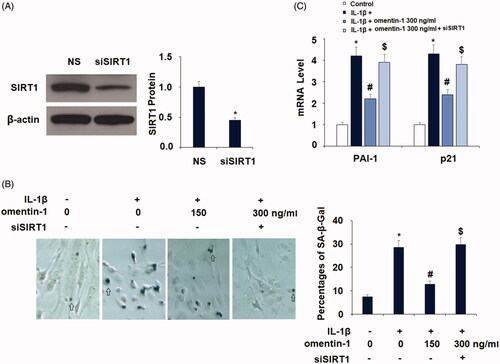
Finally, we examined whether SIRT1 expression is involved in the action of omentin-1 in chondrocytes. We transfected SIRT1-specific RNAi oligos into SW1535 cells and successfully silenced the majority of SIRT1 expression (. As described in , about 7.5% of the non-treated chondrocytes had SA-β-Gal-positive staining, and IL-1β treatment resulted in about 29% SA-β-Gal-positive staining. However, co-treatment with omentin-1 strongly suppressed IL-1β-induced SA-β-Gal-positive staining in normal SIRT1-expressing cells, but its action was completely abolished in SIRT1-silent cells (. By examining the expression of PAI-1 and p21, we confirmed that silencing of SIRT1 abolished the inhibitory effect of omentin-1 against IL-1β-induced expression of these two proteins (.
Figure 8. Knockdown of SIRT1 abolished the protective effects of omentin-1 against IL-1β-induced cellular senescence in human SW1353 cells. Cells were transfected with SIRT1 siRNA and treated with IL-1β (10 ng/mL) with or without omentin-1 (300 ng/mL) for 24 h. (A) Successful silence of SIRT1; (B) Percentages of SA-β-Gal staining positive cells; (C) mRNA of PAI-1 and p21 (*, #, $p < .01 vs. previous group, n = 5–6).
Discussion
The adipokine omentin-1 has been shown to exhibit protective roles in cardiovascular diseases, renal failure, and systematic sclerosis [Citation7,Citation13,Citation14]. Preventive measures to slow chondrocyte senescence is a highly regarded field of interest in the treatment of osteoarthritis. Based on this notion, we sought to test whether omentin-1 could exert certain beneficial effects in chondrocytes. In our in vitro cytokine-induced chondrocyte model, we showed that co-treatment with omentin-1 indeed ameliorated IL-1β-induced increased β-SA-gal activity and cell cycle arrest. We then extensively explored the involved molecular signalling mechanism through which chondrocytes can be protected from cytokine-induced senescence. At the molecular level, our study demonstrated that omentin-1 ameliorated multiple key proteins and signals involved in premature senescence. Firstly, omentin-1 repressed IL-1β-induced expression of the plasma membrane protein caveolin-1. Elevated induction of caveolin-1 is a feature of stress-induced chondrocyte senescence [Citation15,Citation16]. This result indicates that omentin-1 relieves cellular stress by modulating caveolin-1 expression. Secondly, omentin-1 suppressed IL-1β-induced expression of the cell cycle regulators p21 and PAI-1. Thirdly, omentin-1 suppressed IL-1β-induced p53 acetylation at K382. The acetylation of p53 at this site is essential in activating its role as a “guardian” [Citation17]. Both p21 and PAI-1 are recognised as important senescence mediators and signature factors [Citation18,Citation19]. p53, p21, and PAI-1 are critical regulators of cell cycle and fate; the induction of these factors can directly induce cellular senescence [Citation20]. Furthermore, p21 and PAI-1 are the direct transcriptional targets of p53. It is therefore conceivable that omentin-1-mediated repression of p21 and PAI-1 could be the result of the mitigation of p53 acetylation by omentin-1. The capacity of omentin-1 to mitigate the expression of these cell cycle regulators could account for its ameliorative effect on the G1 phase rescue. Fourthly and most importantly, we found that omentin-1 rescued IL-1β-induced reduced SIRT1 expression, but lost this protective effect in SIRT1-deficient chondrocytes, thereby indicating that the biological effect of omentin-1 is dependent on SIRT-1 expression. Acetylation of p53 is a cellular response to stress, and SIRT1 can deacetylate p53 and inhibit its activity [Citation21]. Therefore, the suppression of p53 acetylation by omentin-1 may result from its rescue of SIRT1 expression. Several studies have uncovered the roles of SIRT1 in chondrocytes. One study demonstrated that SIRT1 regulates extracellular matrix protein expression [Citation22], and another study showed that silencing of SIRT1 in chondrocytes causes osteoarthritic gene expression [Citation23]. A genetic SIRT1 chondrocyte knockout mouse model exhibited rapid development of osteoarthritis [Citation24]. Based on this evidence, we propose that omentin-1-mediated protection of SIRT1 is the central piece of this protective mechanism against cytokine-induced chondrocyte senescence. Consistently, a recent study indicated that omentin-1 suppresses several matrix metalloproteinases and alleviates degradation of extracellular matrix proteins in primary chondrocytes [Citation25]. A clinical study revealed that omentin-1 is slightly reduced in the synovial fluid of arthritis patients [Citation26]. This implies that the presence of a healthy amount of omentin-1 in synovial fluid could be critical for healthy joint function.
The usage of primary human chondrocytes is a limitation of this study for a couple of reasons. Firstly, the physiological characteristics of primary human chondrocytes are varied between donors and different isolations. Secondly, long-term cultures in vitro lead to dedifferentiation and subsequent phenotypic destabilisation of chondrocytes [Citation27]. Therefore, chondrocytic cell lines have been widely used as a substitute for primary human chondrocytes in in-vitro culture experiments. Among them, SW1353 cells have been reported to possess several features of chondrocytes. However, it should be noted that SW1353 cells have been found to express representative biomarkers of chondrocytic differentiation only scarcely. Meanwhile, SW1353 cells display some different cytokine transduction signals versus primary human chondrocytes [Citation28,Citation29]. Therefore, our results might be challenged due to the use of SW1353 cells as a substitute for primary human chondrocytes when examining the beneficial effects of omentin-1 against IL-1β-induced chondrocyte senescence. Further in vivo studies using animal models or clinical trials will be more convincing.
In summary, our study provides evidence that the adipokine omentin-1 actively exerts a protective effect against cytokine-induced chondrocyte senescence. Our study highlights the molecular mechanism of omentin-1 in inflammatory-related premature senescence, and we conclude that omentin-1 is a pleiotropic protective adipokine which provides a particular repairing mechanism to chondrocytes in the joint tissue. Thus, omentin-1 could have potential implications in the treatment of osteoarthritis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16(2):210–218.
- Scotece M, Conde J, Gómez R, et al. Beyond fat mass: exploring the role of adipokines in rheumatic disease. Sci World J. 2011;11:1932–1947.
- Richter M, Trzeciak T, Owecki M, et al. The role of adipocytokines in the pathogenesis of knee joint osteoarthritis. Int Orthop. 2015;39(6):1211–1217.
- Senolt L, Polanská M, Filková M, et al. Vaspin and omentin: new adipokines differentially regulated at the site of inflammation in rheumatoid arthritis. Ann Rheum Dis. 2010;69(7):1410–1411.
- Yang RZ, Lee MJ, Hu H, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–1261.
- de Souza Batista CM, Yang RZ, Lee MJ, et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes. 2007;56(6):1655–1661.
- Tan BK, Adya R, Randeva HS. Omentin: a novel link between inflammation, diabesity, and cardiovascular disease. Trends Cardiovasc Med. 2010;20(5):143–148.
- Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1966–1971.
- Toh WS, Brittberg M, Farr J, et al. Cellular senescence in aging and osteoarthritis. Acta Orthop. 2016;87(sup363):6–14.
- Zhao X, Wang T, Cai B, et al. MicroRNA-495 enhances chondrocyte apoptosis, senescence and promotes the progression of osteoarthritis by targeting AKT1. Am J Transl Res. 2019;11(4):2232–2244. eCollection 2019.
- Zhang D, Zhang G, Li Z, et al. Activation of the cannabinoid receptor 1 by ACEA suppresses senescence in human primary chondrocytes through sirt1 activation. Exp Biol Med (Maywood). 2018;243(5):437–443.
- Ito A, Lai CH, Zhao X, et al. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. Embo J. 2001;20(6):1331–1340.
- Miura S, Asano Y, Saigusa R, et al. Serum omentin levels: a possible contribution to vascular involvement in patients with systemic sclerosis. J Dermatol. 2015;42(5):461–466.
- Song J, Zhang H, Sun Y, et al. Omentin-1 protects renal function of mice with type 2 diabetic nephropathy via regulating miR-27a-Nrf2/Keap1 axis. Biomed Pharmacother. 2018;107:440–446.
- Dai SM, Shan ZZ, Nakamura H, et al. Catabolic stress induces features of chondrocyte senescence through overexpression of caveolin 1: possible involvement of caveolin 1-induced down-regulation of articular chondrocytes in the pathogenesis of osteoarthritis. Arthritis Rheum. 2006;54(3):818–831.
- Yudoh K, Shi Y, Karasawa R. Angiogenic growth factors inhibit chondrocyte ageing in osteoarthritis: potential involvement of catabolic stress-induced overexpression of caveolin-1 in cellular ageing. Int J Rheum Dis. 2009;12(2):90–99.
- Lee JT, Gu W. SIRT1: regulator of p53 deacetylation. Genes Cancer. 2013;4(3–4):112–117.
- Yosef R, Pilpel N, Papismadov N, et al. p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. Embo J. 2017;36(15):2280–2295.
- Vaughan DE, Rai R, Khan SS, et al. Plasminogen activator Inhibitor-1 is a marker and a mediator of senescence. Arterioscler Thromb Vasc Biol. 2017;37(8):1446–1452.
- Childs BG, Gluscevic M, Baker DJ, et al. Senescent cells: an emerging target for diseases of ageing. Nat Rev Drug Discov. 2017;16(10):718–735.
- Langley E, Pearson M, Faretta M, et al. Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. Embo J. 2002;21(10):2383–2396.
- Dvir-Ginzberg M, Gagarina V, Lee EJ, et al. Regulation of cartilage-specific gene expression in human chondrocytes by SirT1 and nicotinamide phosphoribosyltransferase. J Biol Chem. 2008;283(52):36300–36310.
- Fujita N, Matsushita T, Ishida K, et al. Potential involvement of SIRT1 in the pathogenesis of osteoarthritis through the modulation of chondrocyte gene expressions. J Orthop Res. 2011;29(4):511–515.
- Gabay O, Zaal KJ, Sanchez C, et al. Sirt1-deficient mice exhibit an altered cartilage phenotype. Joint Bone Spine. 2013;80(6):613–620.
- Li Z, Liu B, Zhao D, et al. Omentin-1 prevents cartilage matrix destruction by regulating matrix metalloproteinases. Biomed Pharmacother. 2017;92:265–269.
- Xu L, Zhu GB, Wang L, et al. Synovial fluid omentin-1 levels are inversely correlated with radiographic severity of knee osteoarthritis. J Investig Med. 2012;60(3):583–586.
- Finger F, Schörle CM, Zien A, et al. Molecular phenotyping of human chondrocyte cell lines T/C-28a2, T/C-28a4, C-28/I2. Arthritis Rheum. 2003;48(12):3395–3403.
- Gebauer M, Saas J, Sohler F, et al. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthritis Cartilage. 2005;13(8):697–708.
- Tew SR, Clegg PD, Brew CJ, et al. SOX9 transduction of a human chondrocytic cell line identifies novel genes regulated in primary human chondrocytes and in osteoarthritis. Arthritis Res Ther. 2007;9(5):R107.