?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mulberry silkworm cocoon (MSC) carbonisata has been used for the treatment of inflammatory diseases for hundreds of years; however, after years of research efforts, little information is available on its anti-inflammatory components and underlying mechanism. We developed novel carbon dots (CDs) derived from MSC carbonisata (MSC-CDs), for the first time, with an average diameter of 2.26–9.35 nm and a quantum yield (QY) of 6.32%. The MSC-CDs were prepared using a modified pyrolysis method, and no further modification and external surface passivation agent was required. With abundant surface groups, MSC-CDs showed distinct solubility and bioactivity. In this study, we innovatively used three classical experimental models of inflammation to evaluate the anti-inflammatory bioactivity of MSC-CDs. The results indicated that MSC-CDs exhibited marked anti-inflammatory bioactivity which was likely mediated by inhibition of the expression of interleukin-6 and tumour necrosis factor-α. These results suggest that MSC-CDs possess a remarkable anti-inflammatory property, which provides evidence to support further investigation of the considerable potential and effective material basis of this traditional Chinese medicine.
Introduction
Recently, carbon nanomaterials have attracted much attention from researchers worldwide [Citation1,Citation2]. As an emerging carbon nanomaterial, carbon dots (CDs) have been widely used in the field of nanobiotechnology [Citation3–5] for their dramatic properties such as excellent chemical stability, low toxicity, biocompatibility, water dispersity, and good photoluminescence. For example, CDs have been used in drug delivery [Citation6,Citation7], bioimaging [Citation8,Citation9], optical imaging, and sensing [Citation10,Citation11]. Of note, an increasing trend has been observed in research on the self-activities of CDs such as haemostatic [Citation12–14], hypoglycaemic [Citation15], peroxidase-like [Citation16], and bacteriostatic [Citation17] activities. However, in-depth investigations of CDs may be necessary, especially those used in medical- and technology-related fields.
Inflammation is recognised to not only occur during most pathological disease courses, but is also actively involved in the progression of numerous diseases [Citation18–20]. Although the medicines commonly used in the treatment of inflammation are well tolerated by some patients, they can cause severe adverse effects in others, mainly due to cardiovascular risk [Citation21] and various gastric side effects [Citation22]. Therefore, it is of great significance and value to research new and safer alternative anti-inflammatory agents with better benefits.
The description of carbonised mulberry silkworm cocoons (MSCs), a kind of charcoal-based traditional Chinese medicine (TCM) used to treat pain and bleeding, was first recorded in the Peaceful Holy Benevolent Prescriptions, the official medical book of the early Song Dynasty (960–1127 A.D.). For the next several hundred years, the carbonised MSCs were widely used for many inflammation-related diseases and conditions such as skin ulcers, fever, fatigue, thirst, and general malaise because of their pronounced bioactivities and good safety profile. However, the active ingredients and mechanisms of action of carbonised MSCs have always been ambiguous. Inspired by previous studies on the active ingredients of other charcoal drugs [Citation13–15], we focussed on CDs, which are produced during charcoal processing, but not on raw herbs.
In this study, we synthesised and identified novel MSC-derived CDs (MSC-CDs) and evaluated their cytotoxicity using the cell counting kit (CCK)-8 assay. The anti-inflammatory activity and related anti-inflammatory mechanism of MSC-CDs were preliminarily assessed using three animal models of inflammation: the ear oedema induced by single application of dimethylbenzene (DMB). The vascular permeability induced by acetic acid, and the lipopolysaccharide (LPS)-induced sepsis model.
Methods
Chemicals
Silkworm cocoons harvested in the genuine producing area of Zhejiang province were obtained from Qiancao Chinese Herbal Medicine Co. (Beijing, China). Dexamethasone (DXM) and LPS from Escherichia coli were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Acetic acid was purchased from Thermo Fisher Scientific Co., Ltd. (Shanghai, China). DMB and other analytical-grade chemical reagents were obtained from Sinopharm Chemical Reagents Beijing Co., Ltd. (Beijing, China). Dialysis bags of 1,000 Da molecular weight cut-off (MWCO) were purchased from Beijing Ruida Henghui Technology Development Co., Ltd. (Beijing, China).
Animals
A total of 36 female C57 black mice and 66 female Kunming mice (weighing 30.0 ± 3.0 g) were purchased from Si Beifu Biotechnology Co. Ltd. (Beijing, China). The animals were housed in a room maintained at 24.0 ± 1.0 °C with 55–65% humidity and on a 12-h light/dark cycle, with free access to water and food. All the animal procedures were in accordance with the Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Committee of Ethics of Animal Experimentation of the Beijing University of Chinese Medicine.
Preparation of MSC-CDs
The MSC-CDs were synthesised using the high-temperature pyrolysis method with some modifications in a muffle furnace [Citation23,Citation24] (TL0612, Beijing Zhong Ke Aobo Technology Co., Ltd., Beijing, China). Crucibles containing 150 g MSCs were covered with aluminium foil paper and the lids to create an oxygen-deficient environment, and then they were placed into the muffle furnace for calcination at 350 for 1 h °C. After cooling to room temperature, the carbonised MSCs in the crucibles were powdered and extracted with 4500 mL double-distilled water (1.5 h, 95 °C) three times. The insoluble residue in extract was filtered using a microfiltration membrane (0.22 µm aperture), and the clear brown rude extract obtained was concentrated using rotary evaporation. The concentrated solution obtained was collected and placed in dialysis bags constructed from a Spectrum Laboratories cellulose ester dialysis membrane tube (1000 MWCO, Rancho Dominguez, CA, USA), dialysed in distilled water for 3 days, and the water was changed every 4 h. Then, the MSC-CDs solution was collected and lyophilised in a freeze-dryer (LGJ-10C, Four-ring Science Instrument Plant Beijing Co., Ltd, Beijing, China). Then, the dried powder was weighed and diluted with normal saline to obtain different concentrations of 70, 35, and 17.5 mg/mL (high-, medium- and low-dose, respectively) before use. The preparation process for the MSC-CDs is shown in .
Characterisation of MSC-CDs
The morphology, size, and microstructure of the resultant MSC-CDs were characterised using transmission electron microscopy (TEM, Tecnai G2 20, FEI Co., Hillsboro, OR, USA), operated at an accelerated voltage of 200 kV. The structural details and atomic lattice fringes of the CDs were examined using a high-resolution TEM (HRTEM) system (JEN-1230, Japan Electron Optics Laboratory, Tokyo, Japan). The spectral properties of the CDs were studied using ultraviolet-visible spectroscopy (UV-Vis, CECIL, Cambridge, UK) and fluorescence spectroscopy (F-4500, Tokyo, Japan) in a standard quartz cuvette. In addition, the Fourier transform infra-red (FTIR, Thermo Fisher, Fremont, CA) spectrum was recorded to identify the organic functional groups in the CDs within a spectral window of 400–4000 cm−1. The surface composition and elemental analysis of the CDs were recorded using X-ray photoelectron spectroscopy (XPS; ESCALAB 250Xi, Thermo Fisher Scientific, Fremont, CA, USA) using a mono X-ray source Al Kalpha 150 W (200 eV for the survey, 30 eV for HR scans). X-ray diffraction (XRD, D8-Advanced X-ray diffractometer, Bruker AXS, Karlsruhe, Germany) was performed with Cu K alpha radiation (wavelength [λ] = 1.5418 Å).
QY of MSC-CDs
The fluorescence QY of the MSC-CDs was measured using quinine sulphate (% QY was 54 in 0.1 M sulphuric acid [H2SO4] solution) as a standard sample and calculated using the following equation [Citation25].
where Q represents the QY, I is the integrated area under the emission spectrum, A is the absorbance at 378 nm wavelength, and η is refractive index of the solvent. The subscript ‘CDs’ and ‘R’ refer to MSC-CDs and standard, respectively. To minimise the reabsorption effect, the AR and ACDs were maintained below 0.05.
In vitro cytotoxicity: CCK-8 assay
The cytotoxicity profiles of the MSC-CDs were studied in RAW 264.7 cells using a CCK-8 assay [Citation26]. The cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 20% foetal bovine serum in a humidified 5% CO2 atmosphere at 37 °C. The RAW 264.7 cells were counted and seeded in 96-well plates at a density of 1 × 105 cells in 100 μL medium per well. After incubation for 24 h, the original medium in each well was discarded and the RAW 264.7 cells were incubated with CDs at concentrations of 10000, 5000, 2500, 1250, 625, 312.5, 156.25, 17.13, 39.07, and 19.53 μg/mL, and then re-incubated for a further 24 h to evaluate the cytotoxicity of the CDs. The cells were washed with phosphate-buffered saline (PBS) to remove all cell culture medium in the 96-well plates, followed by the addition of 10 μL per well CCK-8 solution, and then incubation for an additional 4 h. A microplate reader (Biotek, VT, USA) was used to detect the optical density of each well at a wavelength of 450 nm. The relative cell viability was calculated using the following formula:
Abssample and Abscontrol represent the A450 of the experimental and control groups, respectively. The experiments were independently performed in triplicate.
Anti-inflammatory effects of MSC-CDs
To investigate the anti-inflammatory effects of MSC-CDs, three mouse models of inflammation were used: ear oedema induced by a single application of DMB, vascular permeability induced by acetic acid, and an LPS-induced sepsis. The mice were divided into five or six groups (n = 6), and drugs other than DMB were administered to the groups intraperitoneally (i.p.) as follows: control, received normal saline (NS), model, treated with phlogistic agents (DMB, acetic acid, or LPS), positive control (phlogistic agents + DXM, 5 mg/kg), and high-, medium-, and low-dose MSC-CDs (phlogistic agents + MSC-CDs, 1.40, 0.70, and 0.35 mg/kg, respectively).
The ear oedema essay was performed according to a previously reported method [Citation27,Citation28] with slight modifications in Kunming mice. After a 1-h pre-treatment, 20 μL DMB was topically applied on the right ear (10 μL each on the anterior and posterior surfaces). Then, 20 min later, the mice were euthanised and 7-mm diameter circular sections were excised from both the right and left ears using a metallic leather punch and weighed. The percentage inflammation was calculated using the following formula:
MOR and MOL are the mass (g) of the circular section from the right and left ears, respectively.
The peritoneal vascular permeability test in Kunming mice [Citation29]: 1 h after pre-treatment, 0.5% Evans blue dye was injected into the caudal vein (50 mg/kg). The dye injection was immediately followed by an i.p. injection of acetic acid (0.6%, 0.2 mL). The animals were euthanized after 20 min, the peritoneal cavity was lavaged with 6 mL NS, and the fluid was centrifuged for 15 min at 3500 rpm. The absorbance of the cell-free lavage fluid was measured at 650 nm.
LPS-induced sepsis in C57 mice [Citation30] was also performed 1 h after pre-treatment with the test substance. LPS was diluted in pyrogen-free NS and injected i.p. into the mice (0.25 mg/mL, 5 mg/kg). Then, 6 h post injection of LPS, blood was collected into tubes without anticoagulant via retro-orbital bleeding, and the samples were allowed to clot overnight at 4 °C before centrifugation for 10 min at approximately 3000 rpm. The serum levels of tumour necrosis factor (TNF)-α [Citation31] and interleukin (IL)-6 [Citation32], which are primarily involved in promoting inflammatory processes and play an important role in sepsis, were assessed. The enzyme-linked immunosorbent assay kits were calibrated using commercial cytokine standards and used according to the manufacturer's instructions (Cloud-Clone, USA).
After euthanasia, the mouse lungs, small intestines, and spleens were harvested for pathological examination. The organ tissues were immediately fixed in 10% formaldehyde and then immersed successively for 30 min into each of the following solvents: ethanol (50%), ethanol (60%), ethanol (70%), ethanol (80%), ethanol (96%), ethanol (100%) and xylol (100%). After drying, the biological samples were embedded in paraffin (60 °C), cut into sections (4 μm thick), and stained with haematoxylin and eosin. The tissue sections were observed under a microscope (Olympus IX73P1F Tokyo, Japan), photographed, and processed using the charge coupled device (Qimaging Q41305, Canada) and an imaging software (Q-Capture Pro 7.lik).
Statistical analysis
All data were analysed using the statistical package for the social sciences (SPSS, version 20.0). The normally distributed data and homogeneous variances were expressed as the mean ± standard deviation (SD). Multiple comparisons were performed using a one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test.
The minimum significance level was set at a p = .05 for all analyses. Significance levels of p < .01 and p < .001 were also reported.
Results
Characterisation of MSC-CDs
The MSC-CDs extracted from the MSC solution were characterised in the present study. As shown in , the TEM images revealed that the MSC-CDs were nearly spherical and well separated from each other with a size distribution in the range of 2.26–9.35 nm. Furthermore, the HRTEM showed that the CDs had a lattice spacing of 0.295 nm (), which was approximately equal to the measurement results of the XRD (0.335 nm). The UV-Vis absorption spectrum () of the aqueous MSC-CDs solution was broad. The photoluminescence spectra of the MSC-CDs in aqueous solutions () exhibited the strongest emission and excitation at approximately 459 and 378 nm, respectively. The QY of the MSC-CDs was determined to be 6.32% using quinine sulphate as a reference QY of CDs in aqueous solution.
Figure 2. Characteristics of mulberry silkworm cocoon-derived carbon dots (MSC-CDs). (A) Transmission electron microscopy (TEM) image. (B) Histogram depicting particle size distribution. (C) High-resolution TEM (HRTEM) image.
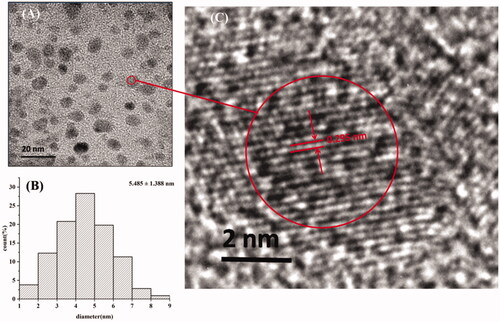
Figure 3. Characterisation of mulberry silkworm cocoon-derived carbon dots (MSC-CDs). (A) Ultraviolet-visible (UV–Vis) and (B) fluorescent spectra. EM, emission; EX, excitation. (C) X-ray diffraction (XRD) pattern. (D) Fourier transform infra-red (FTIR) spectrum.
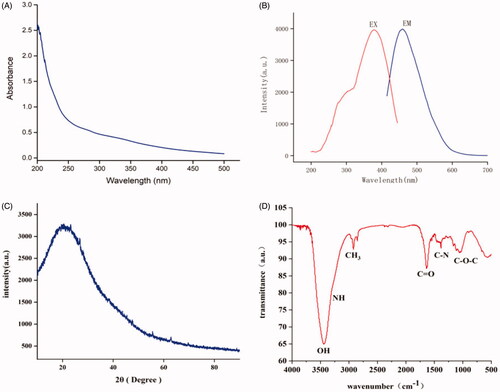
To determine the organic functional groups present on the surface of the MSC-CDs, they were further analysed using FTIR (. The typical absorption peaks at 3440 and 3273 cm−1 represented stretching vibrations of the O–H and N–H of amino groups, respectively. The peaks at 2923 and 2858 cm−1 corresponded to C–H stretching vibration, while a characteristic peak of C=O stretching vibrations was observed at 1635 cm−1. The peak located at 1384 cm−1 could be attributed to stretching vibrations of C–N, and the peak at 1061 cm−1 was due to the asymmetric and symmetric stretching vibrations of C–O–C.
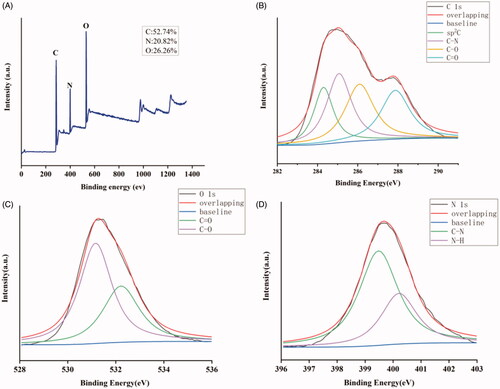
The XPS measurements () performed to investigate the elemental composition of the MSC-CDs indicated they were mainly composed of C, O, and N. It depicted the full-scan XPS spectrum of MSC-CDs from which we observed three peaks located at 285.08, 400.08, and 531.08 eV corresponding to C 1 s, N 1 s, and O 1 s, respectively in the MSC-CDs. In addition, MSC-CDs contained 52.74% carbon, 26.26% oxygen, and 20.82% nitrogen according to the XPS results. The HR XPS spectrum of C 1 s () was resolved into four peaks centred at 284.3 (C = C/C–C), 285.07 (C–N), 286.09 (C–O), and 287.87 (C = O) eV. The high-resolution O 1 s spectrum () was divided into two peaks located at 531.16 eV and 532.22 eV, which were assigned to C–O and C=O bonds, respectively. The N 1 s spectrum () was divided into two peaks at 399.48 and 400.21 eV, which were assigned to C–N and N–H bonds, respectively.
In vitro cytotoxicity (CCK-8 assay)
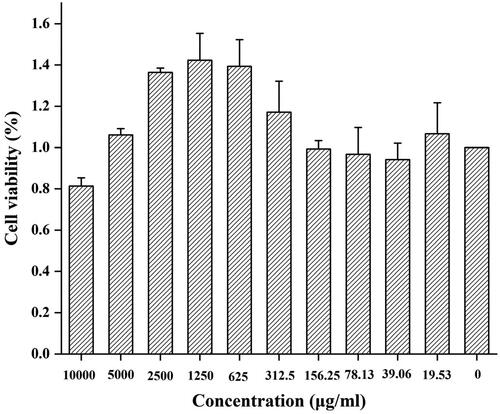
The toxicity of CDs has always been of considerable concern in their biological application. shows the cell viability of RAW 264.7 cells treated with MSC-CDs at 10 concentrations ranging from 19.53 to 10,000 μg/mL for 24 h. The MSC-CDs had no effect on RAW 264.7 cell growth at concentrations up to ∼165.25 μg/mL. The cell proliferation was increased when the CDs concentration increased from 312.5 to 5,000 μg/mL. We speculated that this was likely caused by hormesis [Citation33]. RAW 264.7 cell viability gradually decreased as the CDs concentration increased close to 10,000 μg/mL. Overall, these observations indicated that the CDs had no significant cytotoxicity at concentrations <5000 μg/mL.
Effect of MSC-CDs on mouse ear oedema
shows that the mouse ear tissue was swollen following exposure to DMB to establish the ear oedema model. The percentage inflammation of the MSC-CD-treated groups was compared with that of the NS control 20 min after the application of the DMB. The percentage inflammation was significantly lower in groups treated with MSC-CDs at doses of 1.40 (0.74 ± 0.09%) and 0.70 mg/kg (0.74 ± 0.12%) than it was in the NS control group (0.97 ± 0.15%, p < .01). There was no statistically significant difference between the group treated with 0.35 mg/kg MSC-CDs and the model control group. Moreover, there was no statistically significant difference between the groups treated with 1.40 or 0.7 mg/kg MSC-CDs and the DXM group (0.76 ± 0.15%).
Figure 6. (A) 1. Flow diagram of vascular permeability induced by acetic acid and representative images of lavage fluid from NS, Acetic and H group. (A) 2. Effect of mulberry silkworm cocoon-derived carbon dots (MSC-CDs) on acetic acid-induced vascular permeability in mice. MSC-CDs decreased plasma extravasation induced by acetic acid. ***p < .001, **p < .01, and *p < .05 compared with the acetic group; ###p < .001, compared with the NS group (all n = 6). (B) 1. Representative images of ears from normal, DMB, and H groups. (B) 2. Effect of MSC-CDs on DMB-induced ear oedema in mice. MSC-CDs decreased the percentage inflammation of mouse ears. **p < .01 compared with DMB group (n = 6). DMB, dimethylbenzene; H, M, L, high-, medium-, and low-dose MSC-CDs.
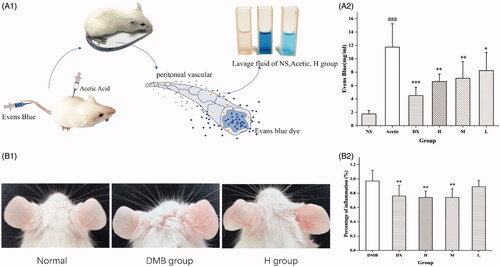
MSC-CDs decreased acetic acid-induced plasma extravasation
The anti-inflammatory effect of MSC-CDs was further assayed in the peritoneal capillary permeability test in mice. shows that acetic acid administration increased (11.75 ± 3.59 mg/mL) vascular permeability more than NS (control group, 1.75 ± 5.20 mg/mL). The plasma extravasation of the groups pre-treated with DXM (5 mg/kg i.p.) and the MSC-CDs (1.4, 0.7, and 0.35 mg/kg i.p.) was decreased more (4.51 ± 1.22 [p < .001], 6.59 ± 1.13 [p < .01], 7.08 ± 2.50 [p < .01], and 8.23 ± 2.72 [p < .05] mg/mL, respectively) than that of the model group (11.75 ± 3.59 mg/mL) was. Furthermore, no significant difference was observed between the MSC-CDs (1.4 and 0.7 mg/kg) and DXM groups.
Effects of MSC-CDs on LPS-induced sepsis model
We investigated the expression of cytokines in serum as an indicator of immune activation in response to systemic infection. showed that the LPS challenge increased serum levels of the pro-inflammatory cytokines IL-6 and TNF-α (21,562.86 ± 4011.36 and 217.86 ± 43.85 pg/mL, respectively, both p < .001) compared to that of the NS group (1676.10 ± 271.36 and 11.18 ± 4.84 pg/mL, respectively). The MSC-CDs inhibited both IL-6 and TNF-α production. The 1.4 and 0.7 mg/mL-treated MSC-CDs groups showed comparable IL-6 values to that of the DXM group. The production of TNF-α was inhibited in all groups treated with DXM and different concentrations of the MSC-CDs, and no significant difference was observed between groups.
Figure 7. Effects of mulberry silkworm cocoon-derived carbon dots (MSC-CDs) on cytokine levels and pathological features in mice. Effect on (A) IL-6 and (B) TNF-α. Effect on histopathological damage of (C) lungs, (D) small intestine, (E) spleen. Groups (n = 6 each): normal control (NS), model (LPS), DX (0.67 KU/kg), and different doses of MSC-CDs (1.4, 0.7, and 0.35 mg/kg). *p < .05; **p < .01, and ***p < .001 compared with model group; ##p < .01 and ###p < .001, compared with NS group.
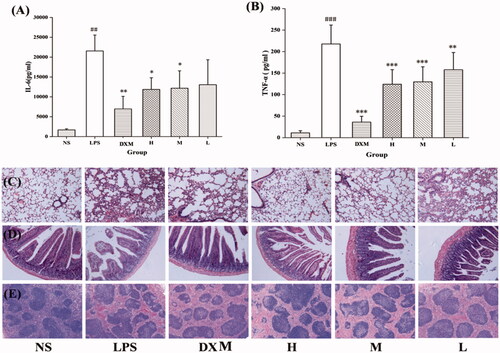
No obvious pathological change was observed in tissues of the NS group, whereas the lung injury in the LPS group was characterised by thickening of the alveolar septa and haemorrhagic areas. Compared with those in the LPS group, those treated with DXM and MSC-CDs (1.4 and 0.7 mg/kg) showed attenuated lung injury. The small intestinal villi were obviously shortened and alterative pathological changes were observed to different degrees in the mucous membrane of the LPS group. However, the treated mice showed an improvement of these signs. Haemorrhage and hemosiderin deposition were observed in the spleens of the LPS group, which showed similar improvement in the pathological examination of the treated groups.
Discussion
CDs, an emerging novel nanomaterial with particle size <10 nm, are a popular research topic because of their considerable benefits such as ease of functionalization, high biocompatibility, hypotoxicity, and excellent physicochemical and photochemical stability [Citation34]. However, the main methods for preparing CDs including pyrolysis, microwave-mediated synthesis, chemical oxidation, and hydrothermal treatment, are not ideal. In particular, large-scale production is challenging because of the large quantities of the strong acids required and the complex process. From the environmental conservation point of view, the modified pyrolysis method, which has the advantage of cheap instrumentation, environmental friendliness, and ease of manipulation, would be a conducive strategy to further investigate the bioactivities of CDs. Moreover, the abundant carbon, nitrogen, and oxygen levels of MSC make it suitable for use as a carbon source to prepare CDs. Therefore, no further modification or external surface passivation agent was required. Consequently, we used RAW cells for the cytotoxicity evaluation of MSC-CDs using the CCK-8 assay and the result demonstrated no cytotoxicity at concentrations up to 5000 μg/mL. This provide possibilities for the further development of MSC-CDs in biological fields.
In this study, novel and eco-friendly MSC-CDs with an average diameter of 2.26–9.35 nm were prepared, for the first time, using the modified pyrolysis method. Furthermore, the MSC-CDs were characterised using TEM, HRTEM, FI-IR, UV-vis spectroscopy, fluorescence spectroscopy, XRD, and XPS. The QY of the MSC-CDs in this study was 6.32%, which is comparable to that obtained in our previous research study [Citation23–24] and that reported by other related previous studies [Citation35,Citation36].
The anti-inflammatory activity of the MSC-CDs was first confirmed using the xylene-induced ear oedema and acetic acid-induced vascular permeability mouse models. These are two classical animal models used primarily for evaluating the anti-inflammatory potential of candidate agents [Citation37]. The high- and medium-dose MSC-CDs dramatically suppressed the formation of xylene-induced oedema in mice. Similarly, administration of MSC-CDs markedly suppressed the acetic acid-induced Evans blue dye leakage into the peritoneal cavity in a concentration-dependent manner. The LPS-induced experimental sepsis mice model closely resembles sepsis in humans and, therefore, we used it as a systemic inflammatory response model to explore the effect of the MSC-CDs on sepsis. The results indicated that the MSC-CDs significantly and dose-dependently decreased the plasma levels of IL-6 and TNF-α, showing a protective phenotype in the pathology. IL-6 and TNF-α are key pro-inflammatory cytokines of the inflammatory reaction in acute sepsis that act as endogenous pyrogens, up-regulate the activation of secondary effector cells, and are involved in a numerous complex immunological interactions and signalling cascades [Citation38]. Cytokine release is an early event in sepsis pathogenesis and an increase in pro-inflammatory cytokines has been shown to correlate with clinical features such as multiple organ dysfunction and mortality [Citation39]. MSC-CDs exhibited protective effects on the organ damage caused by acute organic phosphorus, and the underlying mechanism might involve marked attenuation of IL-6 and TNF-α release.
This study provided evidence supporting the safety of MSC-CDs as an effective treatment of inflammation-related diseases associated with vascular endothelial barrier leakage and cytokine release. Therefore, MSC-CDs have significant prospects for use as an anti-inflammatory drug.
Conclusions
In summary, a novel CDs formulation derived from charcoal MSC was identified and purified. In addition, pharmacodynamic studies in three animal models showed excellent anti-inflammatory activity, which may be associated with the inhibition of IL-6 and TNF-α expression. These findings are indicative of the bright prospects of MSC-CDs as a new anti-inflammatory drug. In addition, these results not only provide a new research focus for exploring the material foundation and properties of charcoal TCM but also new insights into the potential biomedical and medical applications of CDs.
Disclosure statement
The authors have declared that no competing interests exist.
Additional information
Funding
References
- Kenry, Lee WC, Loh KP, et al. When stem cells meet graphene: Opportunities and challenges in regenerative medicine. Biomaterials. 2017;155:236–250.
- Rasheed PA, Sandhyarani N. Carbon nanostructures as immobilization platform for DNA: A review on current progress in electrochemical DNA sensors. Biosens Bioelectron. 2017;97:226.
- Jaleel JA, Pramod K. Artful and multifaceted applications of carbon dot in biomedicine. J Control Release. 2018;269:302–321.
- Zhang X, Jiang M, Niu N, et al. Review of natural product-derived carbon dots: from natural products to functional materials. Chemsuschem. 2018;11(1):11–24.
- Baker SN, Baker GA. Luminescent carbon nanodots: emergent nanolights. Angew Chem Int Edit. 2010;49(38):6726–6744.
- Zheng M, Liu S, Li J, et al. Integrating oxaliplatin with highly luminescent carbon dots: an unprecedented theranostic agent for personalized medicine. Adv Mater. 2014;26(21):3554.
- Gao N, Yang W, Nie H, et al. Turn-on theranostic fluorescent nanoprobe by electrostatic self-assembly of carbon dots with doxorubicin for targeted cancer cell imaging, in vivo hyaluronidase analysis, and targeted drug delivery. Biosens Bioelectron. 2017;96:300–307.
- Ding C, Zhu A, Tian Y. Functional surface engineering of C-dots for fluorescent biosensing and in vivo bioimaging. Acc Chem Res. 2014;47(1):20.
- Min Z, Ruan SB, Liu S, et al. Self-targeting fluorescent carbon dots for diagnosis of brain cancer cells. Acs Nano. 2015;9(11):11455.
- Li H, et al. Yellow-Emissive carbon dots based optical sensing platform: cell imaging and analytical applications for biocatalytic reactions. Acs Appl Mater Interfaces. 2018;10(9):7739–7744.
- Han C, et al. Highly fluorescent carbon dots as selective and sensitive “on-off-on” probes for iron(III) ion and apoferritin detection and imaging in living cells. Biosens Bioelectron. 2016;83:229–236.
- Zhang M, Zhao Y, Cheng JJ, et al. Novel carbon dots derived from Schizonepetae Herba Carbonisata and investigation of their haemostatic efficacy. Artif Cell Nanomed B.2018;46(8):1562–1571.
- Wang Y, Kong H, Liu X, et al. Novel carbon dots derived from Cirsii Japonici Herba carbonisata and their haemostatic effect. J Biomed Nanotechnol. 2018;14(9):1635.
- Yan X, Zhao Y, Luo J, et al. Hemostatic bioactivity of novel Pollen Typhae Carbonisata-derived carbon quantum dots. J Nanobiotechnol. 2017;15(1):60.
- Sun Z, Lu F, Cheng J, et al. Hypoglycemic bioactivity of novel Eco-Friendly carbon dots derived from traditional Chinese medicine. J Biomed Nanotechnol. 2018;14(12):2146–2155.
- Yang L, Liu X, Lu Q, et al. Catalytic and peroxidase-like activity of carbon based-AuPd bimetallic nanocomposite produced using carbon dots as the reductant. Anal Chim Acta. 2016;930:23–30.
- Das P, Ganguly S, Bose M, et al. Zinc and nitrogen ornamented bluish white luminescent carbon dots for engrossing bacteriostatic activity and Fenton based bio-sensor. Mat Sci Eng C. 2018;(88):115-129.
- Bak E, Yang HK, Hwang JM. Validity of the worth 4 dot test in patients with red-green color vision defect. Optom Vis Sci. 2017;94(5):626–629.
- Lasselin J, Lekander M, Axelsson J, et al. Sex differences in how inflammation affects behavior: what we can learn from experimental inflammatory models in humans. Front Neuroendocrin. 2018;50:91.
- Sung J, Ho C, Wang Y. Preventive mechanism of bioactive dietary foods on obesity-related inflammation and diseases. Food Funct. 2018;9(12):6082-6096.
- Tacconelli S, et al. Nonsteroidal anti-inflammatory drugs and cardiovascular safety - translating pharmacological data into clinical readouts. Expert Opin Drug Saf. 2017;16(7):791-807..
- Narsinghani T, Sharma R. Lead optimization on conventional non-steroidal anti-inflammatory drugs: an approach to reduce gastrointestinal toxicity. Chem Biol Drug Des. 2014;84(1):1–23.
- Liu X, Wang Y, Yan X, et al. Novel Phellodendri Cortex (Huang Bo)-derived carbon dots and their hemostatic effect. Nanomedicine. 2018;13(4):391–405.
- Zhao Y, Zhang Y, Liu X, et al. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci Rep. 2017;7(1):4452.
- Zhang J, Yu SH. Highly photoluminescent silicon nanocrystals for rapid, label-free and recyclable detection of mercuric ions. Nanoscale. 2014;6(8):4096–4101.
- Wang Y, Dai CC, Zhou C,et al. Benzotriazole enhances cell invasive potency in endometrial carcinoma through CTBP1-Mediated Epithelial-Mesenchymal transition. Cell Physiol Biochem. 2017;44(6):2357.
- Urgard E, Lorents A, Klaas M, et al. Pre-administration of PepFect6-microRNA-146a nanocomplexes inhibits inflammatory responses in keratinocytes and in a mouse model of irritant contact dermatitis. J Control Release. 2016;235:195–204.
- Cai C, Chen Y, Zhong S, et al. Synergistic effect of compounds from a Chinese herb: compatibility and dose optimization of compounds from N-Butanol extract of ipomoea stolonifera. Sci Rep. 2016;6(1):27014.
- de Almeida AAC, Silva RO, Nicolau LAD, et al. Physio-pharmacological investigations about the Anti-inflammatory and antinociceptive efficacy of (+)-Limonene epoxide. Inflammation. 2017;40(2):511–522.
- Li C-Y, Suzuki K, Hung Y-L, et al. Aloe metabolites prevent LPS-Induced sepsis and inflammatory response by inhibiting Mitogen-Activated protein kinase activation. Am J Chin Med. 2017;45(04):847–861.
- Kothari N, Bogra J, Abbas H, et al. Tumor Necrosis Factor gene polymorphism results in high TNF level in sepsis and septic shock. Cytokine. 2013;61(2):676–681.
- Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970.
- Calabrese EJ. Hormesis: why it is important to toxicology and toxicologists. Environ Toxicol Chem. 2010;27(7):1451–1474.
- Durán N, Simões MB, de Moraes ACM, et al. Nanobiotechnology of carbon dots: a review. J Biomed Nanotechnol. 2016;12(7):1323–1347.
- Pacquiao MR, de Luna MDG, Thongsai N, et al. Highly fluorescent carbon dots from enokitake mushroom as multi-faceted optical nanomaterials for Cr6+ and VOC detection and imaging applications. Appl Surf Sci. 2018;453:192–203.
- Bhamore JR, Jha S, Singhal RK, et al. Facile green synthesis of carbon dots from Pyrus pyrifolia fruit for assaying of Al3+ ion via chelation enhanced fluorescence mechanism. J Mol Liq. 2018; 264:9–16.
- Zhang Z-B, Luo D-D, Xie J-H, et al. Curcumin’s metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effects in vivo through suppression of TAK1-NF-κB pathway. Front Pharmacol. 2018;9(1181).
- Matsumoto H, Ogura H, Shimizu K, et al. The clinical importance of a cytokine network in the acute phase of sepsis. Sci Rep. 2018;8.
- Kosovrasti VY, Lukashev D, Nechev LV, et al. Novel RNA interference-based therapies for sepsis. Expert Opin Biol Ther. 2014;14(4):419–435.