Abstract
Atherosclerosis is a potentially life-threatening cardiovascular disease characterized by chronic endothelial inflammation and the formation of atherosclerotic lesions. Circulating ox-LDL is known to induce atherosclerosis by triggering oxidative stress, the expression of inflammatory mediators and adhesion molecules, as well as downregulating the atheroprotective transcriptional factor KLF2. Aloperine is an alkaloid compound isolated from the plant Sophora alopecuroides. Here, we employed various experimental methods to determine the effects of aloperine on ox-LDL-induced markers of atherosclerosis. DHE staining revealed that aloperine may restore the oxidant/antioxidant balance in HUVECs by reducing the level of ROS and rescuing the reduction in NOQ-1 and GCLC induced by ox-LDL. Aloperine treatment reduced ox-LDL-induced expression of IL-6, MCP-1, VCAM-1, and E-selectin and rescued the reduction in KLF2. Aloperine also downregulated ox-LDL-induced expression of the LOX-1. We also demonstrate that aloperine improved cell viability and inhibited the adhesion of U937 monocytes to HUVECs. Finally, we demonstrate that the effects of aloperine are mediated through the rescue of KLF2 expression via suppression of the phosphorylation of p53 protein. Together, our results implicate the potential of aloperine as a safe and effective antiatherosclerosis treatment.
Introduction
Atherosclerosis is a common but life-threatening cardiovascular disease primarily characterized by narrowing of the arteries [Citation1]. While the pathology of atherosclerosis is complicated, dysfunction of the cells that comprise the endothelium is known to be a key event in the initiation of the disease. The endothelium lines the inside of the vasculature and regulates cardiovascular functions, including blood circulation, vascular tone, and platelet aggregation, thereby maintaining vascular homeostasis and blood pressure [Citation2]. Endothelial cells accomplish this task by releasing various signalling molecules to induce or modulate the expression of pro- or anti-inflammatory factors [Citation3]. Additionally, endothelial cells play a role in regulating the oxidant/antioxidant balance by producing ROS as well as antioxidants such as NAD(P)H dehydrogenase quinone (NQO)-1 and glutamate-cysteine ligase catalytic subunit (GCLC) [Citation4–6]. Ox-LDL is a type of cholesterol that is widely linked with pathogenesis of atherosclerosis. Ox-LDL is able to increase the expression of inflammatory factors and induce monocyte recruitment to the subendothelial space. LOX-1 is a scavenger receptor through which macrophages take up ox-LDL [Citation7]. Overexpression of LOX-1 is associated with the accelerated formation of atherosclerotic lesions and increased inflammation, which has been demonstrated in a LOX-1 knockout mouse model [Citation8].
IL-6 and MCP-1 are proinflammatory factors that play a critical role in atherogenesis. Increased IL-6 has long been documented in atherosclerotic lesions and is secreted in response to ox-LDL exposure [Citation9]. Additionally, IL-6 is recognized as a key contributor to atherogenesis and plasma levels of IL-6 are an independent indicator of atherosclerosis and mortality in patients with HIV [Citation10,Citation11]. MCP-1 drives inflammation and lesion formation by recruiting monocytes to the subendothelial space [Citation12]. The attachment of monocytes to endothelial cells is driven by cellular adhesion molecules, including VCAM-1 and E-selectin. These adhesion molecules are strongly expressed in areas of disturbed flow such as bifurcations and curvatures. VCAM-1 and E-selectin induce monocytes to roll along and adhere to the arterial wall, at which point they can invade the intima, giving rise to the formation of foam cells and increasing intima-media thickness, inflammation, and oxidative stress [Citation13,Citation14]. Specific inhibition of adhesion molecules is considered an effective treatment strategy against atherosclerosis [Citation15]. KLF2 is an important protective transcriptional factor that can mitigate the functions of endothelial cells [Citation16]. KLF2 is upregulated at areas exposed to laminar shear stress, but in areas of disturbed flow, KLF2 expression is suppressed. Additionally, hyperlipidemic conditions inhibit KLF2 expression, thereby lowering a major natural defence mechanism against the formation of atherosclerotic plaques [Citation17]. Rescue of KLF2 expression is able to mediate the anti-inflammatory and anti-cellular adhesion effects of montelukast against ox-LDL-induced atherogenesis [Citation18].
Aloperine is a naturally occurring lupine alkaloid derived from the Sophora alopecuroides plant that has demonstrated anti-inflammatory and anti-cancer capacities in both in vivo and in vitro experiments [Citation19]. Sophora alopecuroides L. contains various quinolizidine alkaloids, flavanones, flavonoids, flavonostilbenes, and other components, but the quinolizidine alkaloids, including aloperine, are considered the main bioactive constituents [Citation20,Citation21]. Sophora alopecuroides L. has a history of use as a traditional Chinese and Uighur anti-inflammatory medication spanning thousands of years [Citation21,Citation22]. Here, we examined the effects of aloperine on ox-LDL-induced markers of atherogenesis in HUVECs. To our knowledge, this study is the first to explore the potential of aloperine against atherosclerosis. Our findings demonstrate a promising protective capacity of aloperine to downregulate oxidative stress and endothelial inflammation. We further demonstrate the involvement of KLF2 in mediating the effects of aloperine.
Materials and methods
Cell culture and treatment
HUVECs were from Lonza, Switzerland; human U937 monocytes were ATCC, USA. HUVECs were cultured in 2% EGM2 endothelial media (Lonza, Switzerland). U937 monocytes were cultured in 10% foetal serum (Gibco, USA) supplemented with RPMI-1640 media (Thermo Fisher Scientific, USA). For all experiments, cells were stimulated with 100 µg/mL ox-LDL (Solarbio Life Sciences, China) [Citation23] with or without 50 or 100 µM [Citation24] aloperine (Sigma-Aldrich, USA).
Dihydroethidium (DHE) staining
After stimulation, HUVECs were loaded with 1 μM DHE (Abcam, USA) and maintained for 15 min at 37 °C. The intracellular levels of ROS were determined using a fluorescent microscope. The software Image J was used to quantify the levels of intracellular ROS. We first defined regions of interest (ROI) in the DHE fluorescent images. The cell number was then determined. The integrated density value (IDV) in the ROI was quantified. Intracellular ROS = IDV/average cell number.
Real-time PCR
After stimulation, HUVECs were harvested and total RNA was isolated using Qiazol (Qiagen, Germany). 1 µg isolated RNA was applied to generate cDNA via RT-PCR. Then, cDNA was used to determine the expression of the target genes using SYBR Green Master Mix (Thermo Fisher Scientific, USA). The expression levels of the target genes were calculated by normalizing to GAPDH with the 2−ΔΔCt method. The following primers were used: human IL-6: For: 5′-GGTACATCCTCGACGGCATCT-3′, Rev: 5′-GTGCCTCTTTGCTGCTTTCAC-3′; human MCP-1: For: 5′-ATGCAATCAATGCCCCAGTC-3′, Rev: 5′-TGCAGATTCTTGGGTTGTGG-3′; human VCAM-1: For, 5′-TGTTTGCAGCTTCTCAAGCTTTT-3′, Rev, 5′-GATGTGGTCCCCTCATTCGT-3′; human E-selectin: For, 5’-GCC TGCAATGTGGTTGAGTG-3′, Rev, 5′-ACGAACCCATTGGCTGGATT-3′; human KLF2: For, 5′-TGCCATCTGTGCGATCGT-3′, 5′-GGCTACATGTGCCGTTTCATG-3′; human LOX1: For, 5′-CTGGCTGCTGCCACTCTA-3′, Rev: 5′-TTGCTTGCTCTTGTGTTAGGA-3′; human GAPDH: For:5′-TCTCCTCTGACTTCAACAGCGACA-3′, Rev, 5′-CCCTGTTGCTGTAGCCAAATTCGT-3′.
Western blot analysis
After stimulation, HUVECs were lysed using cell lysis buffer (Cell Signalling Technology, USA) supplemented with protease inhibitors. Equal amounts of cell lysates (20 μg) were separated via 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad, USA) for analysis. The membranes were blocked with 5% non-fat dry milk and then probed overnight with primary antibodies and HRP-conjugated anti-IgG at 4 °C. An ECL kit (Millipore, USA) was used to detect the blots. Western blot bands were scanned and saved. The expression levels of target proteins were quantified using the software Image J. First, we selected target bands on the film. Then, the background was subtracted, and signal intensities were calculated and exported for statistical analysis.
ELISA
After stimulation, the cell culture supernatant was collected, and the secretion of IL-6 and MCP-1 was measured using commercial kits in accordance with the manufacturer’s instructions. The following ELISA kits were used: IL-6 (#D6050, R&D Systems); MCP-1 (#DCP00, R&D Systems).
Attachment of U937 monocytes to HUVECs
Human U937 monocytes were labelled with calcein-AM (Abcam, USA) for 20 min at 37 °C. Then, 5 × 105 U937 cells were added to culture media containing 1 × 105 HUVECs for 2 h followed by washing 3 times with PBS. The number of attachment green cells was calculated.
Statistical analysis
SPSS Statistical Analysis Package software was used to perform statistical analysis. The statistical significance of differences was calculated using ANOVA. Results are expressed as means ± SEM. A p value of less than .05 was considered to denote a statistically significant difference.
Results
Aloperine reduces ox-LDL-induced oxidative stress
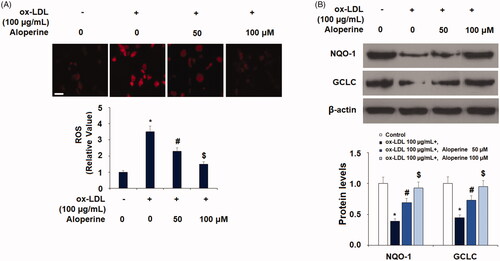
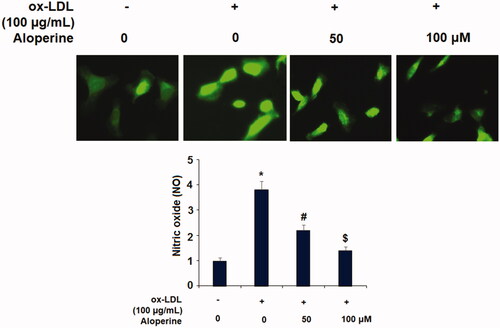
Oxidative stress is an early pathology in atherosclerosis. To determine the effect of aloperine treatment against oxidative stress, HUVECs were exposed to 100 µg/mL ox-LDL with or without 50 and 100 µM aloperine for 24 h. The production of ROS was measured using dihydroethidium (DHE) staining. As shown in , ox-LDL increased the production of ROS to 3.5-fold, which was decreased by aloperine to 2.3- and 1.5-fold. A shown in , the results indicate the ox-LDL alone decreased the expression of these two antioxidants to only 39% and 45%, respectively. However, treatment with the two doses of aloperine rescued the level of NQO-1 to 69% and 93%, and that of GCLC to 73% and 95%, thereby demonstrating a significant dose-dependent antioxidant capacity of aloperine.
Figure 1. Aloperine ameliorated ox-LDL-induced oxidative stress in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). Intracellular ROS. Scale bar, 100 μm; (B). Expression of anti-oxidative factors NQO-1 and GCLC (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
Aloperine reduces ox-LDL-induced chemokine expression
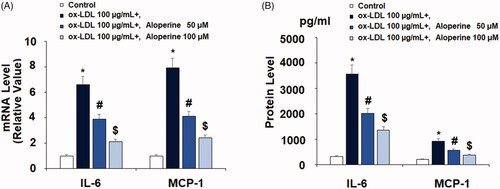
The mRNA level of IL-6 and MCP-1 was increased to 6.6- and 7.9-fold by ox-LDL, respectively, upon exposure to 100 µg/mL ox-LDL alone for 24 h. However, the addition of 50 and 100 µM aloperine reduced IL-6 to only 3.9- and 2.1-fold, and that of MCP-1 to only 4.1- and 2.4-fold. The results of ELISA in demonstrate that ox-LDL increased the concentration of IL-6 protein from 321.5 to 3561.2 pg/ml and of MCP-1 protein from 203.9 to 926.7 pg/ml. However, the two doses of aloperine reduced the protein levels of IL-6 to 2012.8 and 1358.2 pg/ml, and of MCP-1 to 572.5 and 387.1 pg/ml in a dose-dependent manner. Thus, aloperine shows potential as an anti-inflammatory treatment.
Figure 2. Aloperine reduced ox-LDL-induced expression and secretions of pro-inflammatory cytokines in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). mRNA of IL-6 and MCP-1; (B). Protein of IL-6 and MCP-1 (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
Aloperine rescues cell viability
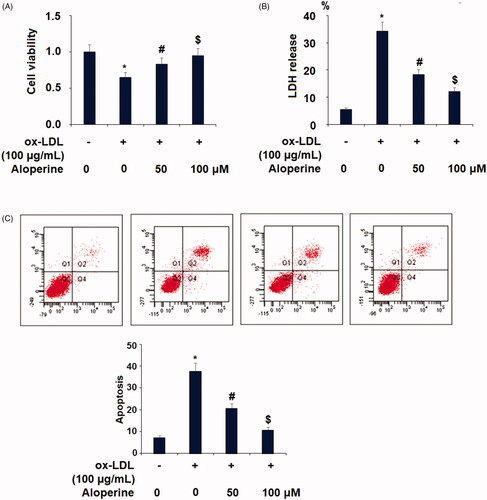
Next, we assayed whether aloperine can prevent ox-LDL-induced HUVECs death. Exposure to ox-LDL alone reduced cell viability by 35%, which was rescued to only 17% and 5% reductions by the two respective doses of aloperine (). We also measured the release of LDH, a byproduct of cell death. As shown in , the baseline level of LDH release was 5.6%, which was increased to 34.2% in the presence of ox-LDL alone and reduced to only 18.3% and 12.2% by the two respective doses of aloperine. Additionally, cell apoptosis of HUVECs was measured using the flow cytometer method. Our results indicate that ox-LDL treatment increased the apoptotic rate of HUVECs from 7.4% to 37.5%, which was reduced to 20.8% and 10.7% by co-stimulation with 50 and 100 µM aloperine (. These results demonstrate a protective effect of aloperine against ox-LDL- induced apoptosis of endothelial cells.
Figure 3. Aloperine prevented ox-LDL-induced reduction of cell viability and lactate dehydrogenase (LDH) release. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). Cell viability; (B). LDH release (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
Aloperine prevents ox-LDL-induced attachment of monocytes to endothelial cells
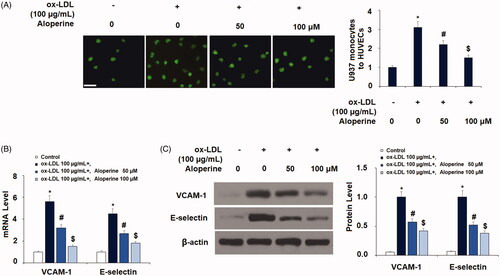
To determine the potential of aloperine as a treatment against atherosclerosis, we next measured the effects of aloperine on ox-LDL-induced attachment of monocytes to endothelial cells. Briefly, HUVECs were incubated with U937 monocytes to ox-LDL in the presence or absence of 50 and 100 µM aloperine for 24 h. As shown in , exposure to ox-LDL alone increased the number of monocytes adhered to endothelial cells 3.1-fold, which was reduced by aloperine to only 2.2- and 1.4-fold. We further investigated the involvement of two key adhesion molecules: VCAM-1 and E-selectin. As shown in , the results of real-time PCR analysis revealed that ox-LDL increased the mRNA expression of VCAM-1 to 5.6-fold, which was reduced to only 3.2- and 1.5-fold by aloperine. Similarly, the mRNA expression of E-selectin was increased to 4.5-fold by ox-LDL alone, while the two doses of aloperine reduced E-selectin expression to only 2.7- and 1.8-fold, respectively. As shown in , VCAM-1 and E-selectin were increased by ox-LDL, but the two doses of aloperine reduced the protein expression of VCAM-1 by 43% and 58%, and that of E-selectin by 48% and 62%, respectively.
Figure 4. Aloperine inhibited ox-LDL-induced attachment of human U937 monocytes to HUVECs and the expression of VCAM-1 and E-selectin. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). Attachment of U937 monocytes to HUVECs was measured. Scale bar, 100 μm; (B). mRNA of VCAM-1 and E-selectin; (C). Protein of VCAM-1 and E-selectin (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
Aloperine reduces ox-LDL-induced expression of LOX-1
To further elucidate the mechanisms through which aloperine exerts its anti-atherosclerotic effects, we measured the expression of the ox-LDL scavenging receptor LOX-1. As shown in , the results of real-time PCR analysis revealed that exposure to ox-LDL increased the mRNA expression of LOX-1 to 3.8-fold, which was reduced by the two doses of aloperine to only 2.2- and 1.4-fold. Concordantly, the protein expression of LOX-1 was increased to 3.3-fold by ox-LDL alone, while the two doses of aloperine reduced LOX-1 protein expression to only 2.4- and 1.5-fold ().
Figure 5. Aloperine reduced ox-LDL-induced expression of LOX-1 in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 6 h. (A). mRNA of LOX-1; (B). Protein of LOX-1 (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
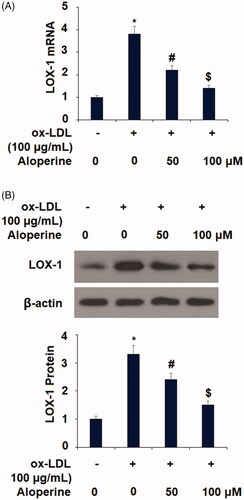
Aloperine rescues KLF2 expression
Finally, we investigated the potential mechanism behind the atheroprotective effects of aloperine observed in our experiments. KLF2 is an important protective transcriptional factor against the development of atherosclerosis. Thus, we measured the influence of aloperine on ox-LDL-induced reduced expression of KLF2. As shown in , ox-LDL decreased the mRNA expression of KLF2 by 57%, which was reduced to only 28% and 7% reductions by the two doses of aloperine, respectively. The results in demonstrate a similar effect at the protein level, with ox-LDL reducing KLF2 protein expression by 47%, which was rescued to reductions of only 25% and 5% by aloperine. KLF2 is a potent inducer of eNOS [Citation25], which plays a pivotal role in regulating endothelial function. Therefore, we examined the effects of Aloperine on the expression of eNOS. As expected, Aloperine restored ox-LDL-induced reduced eNOS expression at both the mRNA () and protein levels (.
Figure 6. Aloperine restored ox-LDL-induced reduction of KLF2 in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). mRNA of KLF2; (B). Protein of KLF2 (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
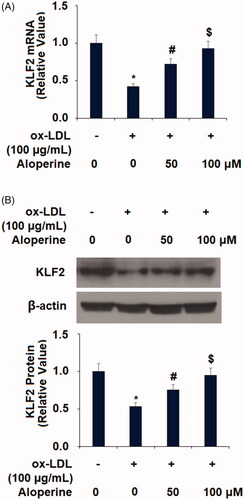
Figure 7. Aloperine restored ox-LDL-induced reduced eNOS expression in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 24 h. (A). mRNA of eNOS; (B). Protein of eNOS (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
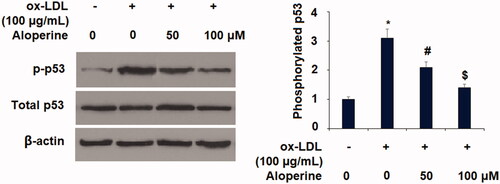
Finally, we determined the involvement of p53 protein in the mechanism behind the aloperine-mediated rescue of KLF2 expression as p53 protein is known to negatively regulate the expression of KLF2 [Citation26]. As shown in , ox-LDL induced a 3.1-fold increase in the level of phosphorylated p53, which was reduced to 2.1- and 1.4-fold by aloperine. Thus, the beneficial effects of aloperine may be mediated through rescue of KLF2 expression via inhibition of the phosphorylation of p53.
Figure 8. Aloperine mitigated ox-LDL-induced activation of p53 in HUVECs. Cells were cultured with ox-LDL (100 µg/mL) with or without aloperine (50,100 μM) for 2 h. Phosphorylated and total levels of p53 were assayed (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group; $, p < .01 vs. ox-LDL + 50 μM aloperine group).
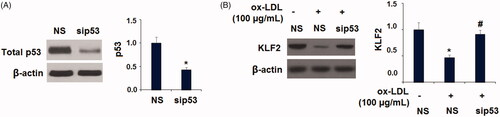
To further investigate the possible involvement of LOX-1 in mediating the activation of p53 and reduction of KLF2, a neutralizing antibody and a chemical inhibitor of LOX-1 were added. Western blot results show similar results with Aloperine, indicating that the presence of LOX-1 neutralizing antibody or inhibitor prevented ox-LDL-induced activation of p53 and the reduction of KLF2 (). Interestingly, we found that silencing of p53 by transfection with p53 siRNA abolished the ox-LDL-induced reduction of KLF2 (). These findings suggest that LOX-1 and p53 play pivotal roles in regulating the expression of KLF2 in HUVECs.
Figure 9. Blockage of LOX-1 abolished the ox-LDL-induced activation of p53 and reduction of KLF2 in HUVECs. Cells were treated with ox-LDL (100 µg/mL) with or without LOX-1 neutralizing antibody (LOX-1 Ab) and LOX-1 inhibitor Poly(I) for 24 h. The levels of p-p53, total p53, and KLF2 were measured (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group).
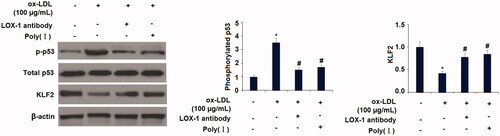
Figure 10. Silencing of p53 abolished the ox-LDL-induced reduction of KLF2 in HUVECs. Cells were transfected with p53 siRNA for 24 h, followed by stimulation with ox-LDL (100 µg/mL) for another 24 h; (A). The successful knockdown of p53; (B). Expression of KLF2 (*, p < .01 vs. vehicle group; #, p < .01 vs. ox-LDL treatment group).
Discussion
Atherosclerosis is a potentially life-threatening disease that often presents without symptoms in the early stages. Therapies that hinder or prevent the development of atherosclerosis are of great clinical value. Aloperine was originally isolated from the leaves of S. alopecuroides L. [Citation27], but there has since been little research on its pharmacological activities. Recent studies have demonstrated beneficial effects of aloperine in a wide range of diseases and cell types. For example, aloperine has been demonstrated to exert potent antiviral activities. A 2016 study proposed aloperine and its derivatives as a new class of HIV-1 inhibitors [Citation28] while a more recent study proposed the same as a new class of hepatitis C virus inhibitors [Citation29]. Aloperine exerts a significant antioxidant effect by downregulating the production of ROS and increasing enzymatic activity and total antioxidant capacity in neuronal cells [Citation30]. Another study demonstrated an anti-oxidative stress effect in an Alzheimer’s model, where aloperine suppressed ROS and 4-hydroxy-2-nonenal production while increasing mitochondrial membrane potential and adenosine triphosphate production [Citation31]. Concordantly, our findings show that aloperine inhibits the production of ROS in HUVECs exposed to ox-LDL insult. Additionally, we demonstrate that aloperine enhanced the release of NQP-1 and GCLC (). As oxidative stress is a major factor in endothelial dysfunction and atherosclerotic lesion formation, our findings suggest that aloperine may help prevent atherosclerosis by ameliorating ox-LDL-induced oxidative stress.
In the presence of high levels of circulating ox-LDL, endothelial cells secrete IL-6 and MCP-1. Recent research has emphasized the causal role of IL-6 signalling in atherosclerosis [Citation32]. A previous study using an allergic dermatitis mouse model showed that aloperine treatment could reduce the expression of IL-6 as well as that of IL-1β and tumour necrosis factor-α, two other noteworthy cytokines involved in atherogenesis [Citation33]. This research supports our finding that aloperine could significantly reduce ox-LDL-induced expression of IL-6 in HUVECs. Aloperine can ameliorate the increase in expression of the key chemokine MCP-1. To our knowledge, this study is the first to demonstrate the inhibitory effect of aloperine on MCP-1 expression. Reducing the expression of chemokines is considered an attractive therapeutic strategy against the progression of atherosclerosis [Citation34]. To further support the potential role of aloperine against atherosclerosis, we demonstrated that aloperine treatment significantly rescued HUVECs from ox-LDL-induced apoptosis.
LOX-1 is the main scavenger receptor for ox-LDL and a positive feedback loop involving LOX-1 and ox-LDL exists [Citation35]. LOX-1 plays an important role in regulating the formation of foam cells, contributing to atherogenesis by promoting the release of proinflammatory cytokines in the early stages as well as that of matrix metalloproteinases, which contribute to plaque rupture and clot formation, in the later stages. Reducing the number of foam cells is able to exert protective effects at all stages of the disease [Citation36]. Our findings show that aloperine significantly reduced the expression of LOX-1 on HUVECs induced by ox-LDL. Furthermore, we demonstrate that aloperine reduced the attachment of monocytes to endothelial cells by reducing VCAM-1 and E-selectin. Numerous contemporary studies have suggested inhibiting the attachment of monocytes to endothelial cells via downregulation of the expression of adhesion molecules as a way to combat atherosclerosis [Citation37–39]. Thus, our finding suggests a novel role of aloperine in this key event in atherogenesis.
Lastly, we found that the effects of aloperine are mediated through rescue of the protective KLF2 zinc-finger transcriptional factor via suppressing the phosphorylation of p53 protein. The role of KLF2 is rather unique, as this factor responds to mechanical forces (shear stress) as well as signalling molecules such as ox-LDL [Citation40,Citation41]. KLF2 is known to induce vasoprotective effects in endothelial cells, including anti-inflammation, antithrombosis, anti-oxidative stress, and anti-proliferation. Modulating the expression of KLF2 is considered a promising treatment strategy against atherosclerosis and other cardiovascular diseases [Citation42]. Here, we show that aloperine remarkably rescues KLF2 expression from the reduction induced by ox-LDL. We further demonstrate that this effect was mediated through inhibition of the phosphorylation of p53. Taken together, our findings provide evidence of aloperine as a safe and effective treatment against atherosclerosis. Aloperine not only ameliorated oxidative stress and the expression of IL-6 and MCP-1 but also significantly inhibited the expression of LOX-1. The results of our cell viability assay show that aloperine can prevent endothelial cell apoptosis induced by ox-LDL. The ability of aloperine to rescue KLF2 expression indicates a valuable vasoprotective effect. Further research using animal models is warranted to elucidate whether these effects are observable in vivo.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gimbrone MA, Jr, García-Cardeña G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ Res. 2016;118(4):620–636.
- Daiber A, Steven S, Weber A, et al. Targeting vascular (endothelial) dysfunction. Br J Pharmacol. 2017;174(12):1591–1619.
- Tousoulis D, Oikonomou E, Economou EK, et al. Inflammatory cytokines in atherosclerosis: current therapeutic approaches. Eur Heart J. 2016;37(22):1723–1732.
- Kattoor AJ, Pothineni NV, Palagiri D, et al. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42.
- Siegel D, Ross D. Immunodetection of NAD (P) H: quinone oxidoreductase 1 (NQO1) in human tissues. Free Radic Biol Med. 2000;29(3–4):246–253.
- Kanikarla-Marie P, Jain SK. 1, 25 (OH) 2D3 inhibits oxidative stress and monocyte adhesion by mediating the upregulation of GCLC and GSH in endothelial cells treated with acetoacetate (ketosis). J Steroid Biochem Mol Biol. 2016;159:94–101.
- Mitra S, Goyal T, Mehta JL. Oxidized LDL, LOX-1 and atherosclerosis. Cardiovasc Drugs Ther. 2011;25(5):419.
- Pirillo A, Norata GD, Catapano AL. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:1.
- Seino Y, Ikeda U, Ikeda M, et al. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994;6(1):87–91.
- Kasashima S, Kawashima A, Zen Y, et al. Upregulated interleukins (IL-6, IL-10, and IL-13) in immunoglobulin G4-related aortic aneurysm patients. J Vasc Surg. 2018;67(4):1248–1262.
- Hsu DC, Yi Fei MA, Sophia HU, et al. Plasma IL-6 levels are independently associated with atherosclerosis and mortality in HIV-infected individuals on suppressive ART. AIDS (London, England). 2016;30(13):2065.
- Drechsler M, Duchene J, Soehnlein O. Chemokines control mobilization, recruitment, and fate of monocytes in atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(5):1050–1055.
- Masseau I, Bowles DK. Carotid endothelial VCAM-1 is an early marker of carotid atherosclerosis and predicts coronary artery disease in swine. JBiSE. 2015;08 (11):767.
- Tsoref O, Amit U, Landa N, et al. E-selectin-targeted novel nano polymers stabilize atherosclerotic plaque and improve cardiac remodeling. Circulation. 2016;134(suppl_1):A14013.
- Khodabandehlou K, Masehi-Lano JJ, Poon C, et al. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp Biol Med (Maywood). 2017;242(8):799–812.
- Wang Y, Sun M, Wang Z, et al. Omentin-1 ameliorates the attachment of the leukocyte THP-1 cells to HUVECs by targeting the transcriptional factor KLF2. Biochem Biophys Res Commun. 2018;498(1):152–156.
- SenBanerjee S, Lin Z, Atkins GB, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199(10):1305–1315.
- Di X, Tang X, Di X. Montelukast inhibits oxidized low-density lipoproteins (ox-LDL) induced vascular endothelial attachment: an implication for the treatment of atherosclerosis. Biochem Biophys Res Commun. 2017;486(1):58–62.
- Wang H, Yang S, Zhou H, et al. Aloperine executes antitumor effects against multiple myeloma through dual apoptotic mechanisms. J Hematol Oncol. 2015;8(1):26.
- Iinuma M, Ohyama M, Tanaka T. Six flavonostilbenes and a flavanone in roots of Sophora alopecuroides. Phytochemistry. 1995;38(2):519–525.
- Wang H, Guo S, Qian D, et al. Comparative analysis of quinolizidine alkaloids from different parts of Sophora alopecuroides seeds by UPLC–MS/MS. J Pharm Biomed Anal. 2012;67:16–21.
- Guo C, Yang L, Wan CX, et al. Anti-neuroinflammatory effect of sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine. 2016;23(13):1629–1637.
- Ma S, Bai Z, Wu H, et al. The DPP-4 inhibitor saxagliptin ameliorates ox-LDL-induced endothelial dysfunction by regulating AP-1 and NF-κB. Eur J Pharmacol. 2019; 851:186–193.
- Zhao J, Zhang G, Li M, et al. Neuro-protective effects of aloperine in an Alzheimer's disease cellular model. Biomed Pharmacother. 2018;108:137–143.
- Lin Z, Kumar A, SenBanerjee S, et al. Kruppel-like factor 2 (KLF2) regulates endothelial thrombotic function. Circ Res. 2005; 96(5):e48–57.
- Kumar A, Kim CS, Hoffman TA, et al. p53 Impairs endothelial function by transcriptionally repressing Kruppel-like factor 2. Arterioscler Thromb Vasc Biol. 2011;31(1):133–141.
- Tolkachev ON, Monakhova TE, Sheichenko VI, et al. Alkaloids of a new type from Sophora alopecuroides L. Chem Nat Compd. 1975;11(1):29–34.
- Dang Z, Zhu L, Lai W, et al. Aloperine and its derivatives as a new class of HIV-1 entry inhibitors. ACS Med Chem Lett. 2016;7(3):240–244.
- Zhang X, Lv XQ, Tang S, et al. Discovery and evolution of aloperine derivatives as a new family of HCV inhibitors with novel mechanism. Eur J Med Chem. 2018;143:1053–1065.
- Ma NT, Zhou R, Chang RY, et al. Protective effects of aloperine on neonatal rat primary cultured hippocampal neurons injured by oxygen–glucose deprivation and reperfusion. J Nat Med. 2015;69(4):575–583.
- Zhao J, Zhang G, Li M, et al. Neuro-protective effects of aloperine in an Alzheimer’s disease cellular model. Biomed Pharm. 2018;108:137–143.
- Libby P, Rocha VZ. All roads lead to IL-6: a central hub of cardiometabolic signaling. Int J Cardiol. 2018;259:213–215.
- Yuan XY, Liu W, Zhang P, et al. Effects and mechanisms of aloperine on 2, 4-dinitrofluorobenzene-induced allergic contact dermatitis in BALB/c mice. Eur J Pharmacol. 2010;629(1-3):147–152.
- Hartmann P, Schober A, Weber C. Chemokines and microRNAs in atherosclerosis. Cell Mol Life Sci. 2015;72(17):3253–3266.
- Chistiakov DA, Orekhov AN, Bobryshev YV. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell Physiol Biochem. 2016;38(5):1851–1859.
- Childs BG, Baker DJ, Wijshake T, et al. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354(6311):472–477.
- Yang L, Liu J, Li Y, et al. Bornyl acetate suppresses ox-LDL-induced attachment of THP-1 monocytes to endothelial cells. Biomed Pharmacother. 2018;103:234–239.
- Zhang Z, Yang C, Dai X, et al. Inhibitory effect of trans-caryophyllene (TC) on leukocyte-endothelial attachment. Toxicol Appl Pharmacol. 2017;329:326–333.
- Liu S, Pan S, Tan J, et al. Oxytocin inhibits ox-LDL-induced adhesion of monocytic THP-1 cells to human brain microvascular endothelial cells. Toxicol Appl Pharmacol. 2017;337:104–110.
- Sathanoori R, Rosi F, Gu BJ, et al. Shear stress modulates endothelial KLF2 through activation of P2X4. Purinergic Signal. 2015;11(1):139–153.
- Zhu Y, Li H, Wang X. Lunasin abrogates monocytes to endothelial cells. Mol Immunol. 2017;92:146–150.
- Xu Y, Xu S, Liu P, et al. Suberanilohydroxamic acid as a pharmacological Kruppel-like factor 2 activator that represses vascular inflammation and atherosclerosis. JAHA. 2017;6(12):e007134.
