Abstract
Our previous study found that IL33 repressed the growth of pulmonary adenocarcinoma (PA) via regulation of dendritic cells (DCs). However, the molecular mechanism of DCs in PA is still unclear. The present work showed that CYLD−/− mice have a shorter survival rate of PA, and knockout CYLD in DCs also repress the progression of PA in mice. Subsequently, we found that decreased expression and reduced the nuclear translocation of NF-κB signalling was observed in CYLD knockout DCs, and inhibiting NF-κB pathway repressed DCs-induced proliferation and function of CD4+ T cells. These results indicated that CYLD function as a tumour suppresser in PA via regulates the function of DCs through NF-κB signalling pathway. Our findings support that CYLD serves as a potential target for immunotherapy in PA.
Introduction
Lung cancer is one of the most common cause of tumour-related deaths with about 2 million deaths worldwide in 2018 [Citation1]. PA is the predominant of lung cancer with high incidence rate every year [Citation2]. Although surgical resection, radiotherapy, immunotherapy and chemotherapy are effective therapeutic strategies for PA, the 5-year survival rate of these patients was only ∼10% [Citation3]. Thus, it is imperative to reveal the molecular mechanisms of PA.
Dendritic cells (DCs) is a class of antigen-presenting cell and involved in the innate and adaptive immunity. Several studies have focussed on the important role of DCs in the progression of tumour and in the immunotherapy on tumour. For example, Li et al. showed that DCs dysfunction was observed in lung cancer, and the repression of NF-κB/STAT3 signalling involved in the DC dysfunction, which led to cancer immune escape [Citation4]. Lee et al. and zhang et al. showed that DCs were involved in the immune response against tumours via producing the inflammatory cytokines to recruit and activate immune cells including CD4+ and CD8+ T cells [Citation5–7]. The influenza mimetic protein–polymer nanoparticles and an extract of Pinellia pedatisecta Schott was used for cancer immunotherapy via regulating dendritic cell activation and maturation [Citation7,Citation8]. Our previous study also showed that IL33 repressed the progression of PA via regulating the maturation and the function of DCs [Citation9].
The cylindromatosis (CYLD), a 107 kDa polypeptide, was reported as a deubiquitination enzyme, involved in the progression of various cancer, including skin cancer [Citation10], acute lymphoblastic leukemia [Citation11], chronic lymphocytic leukemia [Citation12], colon carcinoma [Citation13] and hepatocellular carcinoma [Citation14]. CYLD was also reported as a regulator in the inflammation of acute lung injury [Citation15], rheumatoid arthritis [Citation16] and colitis [Citation17]. Recently, accumulating studies demonstrated the high expression of CYLD in immune cell and is involved in the regulation of the activation and function of these cells, including Treg cells and Th17 cells [Citation17], macrophages [Citation18,Citation19] and DCs [Citation20]. Our previous study showed that CYLD involved in DCs-mediated regulation of T cell function, however, the contributions and potential role and regulatory mechanism of CYLD on DCs in the tumour progression of PA remains unclear.
In this study, we used a CYLD–/– mice to reveal the role of CYLD in the progression of PA, and demonstrated that CYLD regulated the function of DCs via NF-κB signalling pathway.
Methods
Mice
CYLD–/– mice were obtained from Southern model organisms. All experiments were carried out and approved by the Institutional Animal Care and Use Committee of Provincial Clinical College of Fujian Medical University, Fujian Provincial Hospital.
Cells isolation and culture
DCs and CD4+ T cells were generated from lung tissues and splenocytes as described previously [Citation21]. DCs were pre-treated with 20 μM PDTC (Sigma-Aldrich, St.Louis, MO, USA) for 12 h and then co-cultured with CD4+ T cells. 24 h latter, the expression and secretion of cytokines were detected using qPCR analysis and ELISA analysis. For the effects of DCs on T cell proliferation and differentiation, we co-cultured T cells with DCs for 72 h.
qPCR analysis
The NF-κB inhibitor PDTC (Sigma,USA) was used to treat DCs, and then qPCR analysis was used for IL-17A expression in DCs. In brief, total RNA from DCs was obtained Trizol (Thermo Fisher Scientific), cDNA was obtained using reverse transcription kit (Applied Biosystems). The mRNA expression of IL-17A was detected using a SYBR Green PCR Master Mix (Applied Biosystems, USA). The primers were IL17A forward: 5′-TTTAACTCCCTTGGCGCAAAA-3′; reverse 5′-CTTTCCCTCCGCATTGACAC-3′; GAPDH forward: 5′-AGGTCGGTGTGAACGGATTTG-3′; reverse 5′-TGTAGACCATGTAGTTGAGGTCA-3′.
Enzyme-linked immunosorbent assay (ELISA)
Cytokine produced by DCs and CD4 cells were detected by ELISA using IL-6, IL-1β, IL-23, IL-17 (ExCell Bio, China) according to the manufacturer’s protocol.
Flow cytometry staining and analysis
The cells were stained with FITC- anti-CD45, A647- anti-CD11c, PE- anti-CD11b and PE- anti-CD4, for 30 min on ice for CD4+ T cell and DCs, BV711-conjugated anti-IL17 was added for IL17 labelled CD4+ T cells. FACSCalibur cytometer (BD Biosciences, Mountain View, CA) was used for detecting cells and FlowJo10 software (BD Biosciences) was used for data analysis.
Western blotting
The DCs from WT mice and CYLD–/– mice were lysed in RIPA (Sigma Aldrich, USA), and the protein were separated using 10% polyacrylamide gels. The PVDF membrane was treated with primary antibodies p-p65 (Ser536) (Sigma, USA), p65 (Sigma, USA) and GAPDH (Sigma, USA) at 4 °C overnight, and then incubated with secondary antibodies at room temperature for 1 h.
Statistics
The data were analysed using SPSS 20.0, using Student’s t-test (for two groups), or one-way ANOVA (for more than groups). * Represents p value of less than .05 was considered significant.
Results
CYLD−/− mice have shorter survival rate of PA
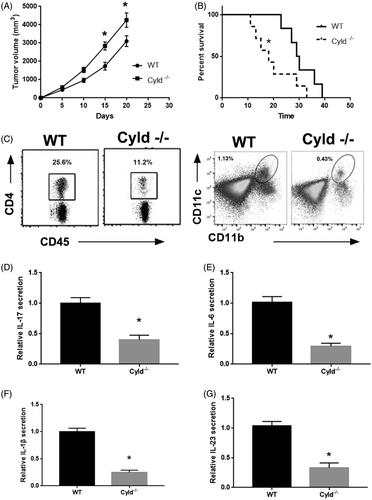
To explore the role of CYLD in the progression of PA, we analysed the growth of PA in CYLD-deficient mice (CYLD–/–) and wild type (WT) mice. Each group had 7 mice. showed that the tumour growth was evidently increased and the survival rate was significantly reduced in CYLD–/– mice compared to WT mice. The median survival time were 29.5 day in WT group and 18 day in CYLD–/– group. Moreover, the infiltration of CD4+ T cells and DCs remarkably reduced in PA tissue from CYLD–/– mice compared to that from WT mice using flow cytometry. The secretion of IL-17 was also reduced in CYLD–/– mice compared to WT mice using ELISA. Moreover, CYLD deficiency evidently reduced IL-6, IL-1β and IL-23 expression in DCs from WT and CYLD–/– mice. In conclusion, these results indicated that CYLD involves the progression of PA.
Figure 1. CYLD knockout suppressed the progression of lung adenocarcinoma. (A) The tumour growth in WT mice and CYLD−/− mice. (B) The survival rate of WT mice and CYLD−/− mice with tumour volume reached ∼3 cm3. (C) The CD4+ T cell and DCs infiltration in lung adenocarcinoma. The secretion of IL-17, (D) IL-6, (E) IL-1β, (F) IL-23 and (G) in lung adenocarcinoma. *p < .01 vs WT mice.
CYLD in DCs involved the progression of PA
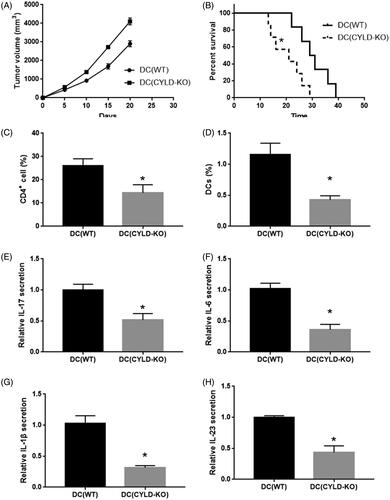
CYLD is wildly expressed in various immune cells including DCs, CD4+ T cells and macrophages. To define the role of CYLD in DCs during the progression of PA, we built a DC(CYLD-KO) mice which reconstituted their DC compartment with DC precursors from mice were defined as DC(CYLD-KO) mice. Each group had 7 mice. We found that the tumour growth was evidently increased and the survival rate was significantly reduced in CYLD–/– mice compared to WT mice. The median survival time was 29.5 days in WT group and 21 days in CYLD-KO group. Moreover, the infiltration of CD4+ T cells and DCs remarkably reduced in PA tissue from CYLD–/– mice using flow cytometry. The secretion of IL-17 was also reduced in CYLD–/– mice using ELISA. Moreover, CYLD deficiency evidently reduced IL-6, IL-1β and IL-23 expression in DCs sorted from WT and CYLD–/– mice ().
Figure 2. Knockout CYLD in DCs suppressed the progression of lung adenocarcinoma. (A) The tumour growth in WT mice and DC(CYLD-KO) mice. (B) The survival with tumour volume reached ∼3 cm3 in WT mice and DC(CYLD-KO) mice. (C) The CD4+ T cell and DCs infiltration in lung adenocarcinoma in WT mice and DC (CYLD-KO) mice. The secretion of IL-17, (D) IL-6, (E) IL-1β, (F) IL-23 and (G) in lung adenocarcinoma in WT mice and DC(CYLD-KO) mice. *p < .01 vs WT mice.
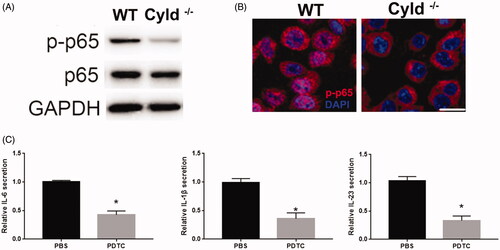
Reduced NF-κB signalling in CYLD knockout DCs
Previous studies revealed the regulation of CYLD on NF-κB pathway [Citation16,Citation22,Citation23]. To reveal the mechanism of CYLD in the function of DCs, we detected the activation of the NF-κB pathway. Similar to previous studies, we detected the reduction of p-p65 in DC from CYLD–/– mice compared to WT mice, indicating that silenced CYLD reduced the activation of NF-κB pathway in DCs (). Furthermore, silenced CYLD decreased the nuclear translocation of p-p65 in the DCs (). In addition, NF-κB inhibitor (PDTC) significantly reduced the expression of IL-6, IL-1β and IL-23 in DCs ().
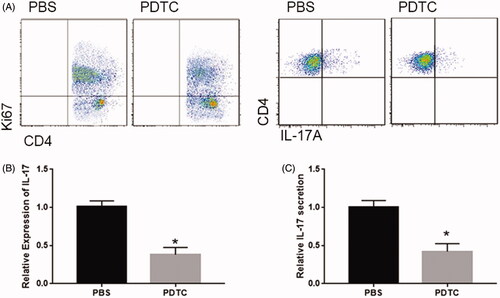
NF-κB pathway involved the DCs-induced proliferation and function of CD4+ T cells
Next, we detected the involvement of NF-κB pathway on the proliferation of T cells by co-cultured T cells with PDTC/PBS treated DCs. As shown in , PDTC significantly repressed DCs-induced T cell proliferation at 72 h (). In addition, the number of IL17A+CD4+ T cells was significantly reduced by PDTC treatment (). Finally, the expression and secretion of IL-17 were evidently reduced by PDTC in T cell co-cultured with DCs (). Together, these data indicated that NF-κB pathway involved DCs-induced proliferation and function of CD4+ T cells.
Discussion
CYLD functioned as tumour suppressor in various cancer including lung cancer. Our previous study showed that CYLD was involved IL-33-mediated tumour suppression in PA; however, the potential role and mechanism of CYLD in the progression of PA are yet largely unexplored. The current study showed that CYLD knockout significantly repressed the growth of PA, and CYLD regulated the function of DCs via NF-κB signalling pathway.
Immune response characterised by various immune cell infiltration, playing an important role in the tumorigenesis [Citation24]. Studies showed that the B cell and CD8+ T cell, CD3+ and FOXP3+ T cells infiltration were significantly relative with the prognostic value in lung adenocarcinoma [Citation25,Citation26]. Moreover, DCs was also reported to be contributed to the immunotherapy of patient with lung adenocarcinoma [Citation27]. Our previous study also showed that IL33 repressed the progression of PA via the regulation on DCs. In this study, CYLD−/− mice have shorter survival rate of PA, and knockout CYLD in DCs repression the progression of PA, indicating that CYLD in DCs was involved in the immunoregulation in PA.
CYLD is a deubiquitination enzyme, it was involved in the progression of physiological processes via regulating various signalling pathways. Zhang et al. showed that CYLD could activate TGF-β-activated kinase 1 (TAK1), which regulates the inflammatory signalling pathway via repressing Toll-like receptor signaling [Citation28]. CYLD was also reported to regulate cGAS-STING signalling pathway in HSV-1 stimulation via by stabilising the STING protein [Citation29]. Moreover, the relationship between CYLD and NF-κB pathway is puzzling [Citation30]. CYLD negatively regulates NF-κB signalling in high glucose-induced mesangial cells [Citation31] and in cerebral Ischemia/Reperfusion rats [Citation32]. However, T cells from CYLD overexpressed mice showed an increase of inflammatory cytokines and evident activation of the NF-κB pathway [Citation33]. In this study, decreased expression and reduced the nuclear translocation of NF-κB signalling was observed in CYLD knockout DCs. Inhibiting NF-κB pathway repressed DCs-induced proliferation and function of CD4+ T Cells.
In summary, this study indicates that CYLD function as a tumour suppresser in PA via regulates the function of DCs through NF-κB signalling pathway.
Disclosure statement
The authors declare that they have no conflicts of interest to disclose.
References
- Khosravi N, Caetano MS, Cumpian AM, et al. IL22 promotes kras-mutant lung cancer by induction of a protumor immune response and protection of stemness properties. Cancer Immunol Res. 2018;6(7):788–797.
- Reppert S, Boross I, Koslowski M, et al. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun. 2011;2(1):600.
- Caronni N, Simoncello F, Stafetta F, et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in Lung Cancer. Cancer Res. 2018;78(7):1685–1699.
- Li R, Fang F, Jiang M, et al. STAT3 and NF-kappaB are simultaneously suppressed in dendritic cells in Lung Cancer. Sci Rep. 2017;7(1):45395.
- Lee JM, Lee M-H, Garon E, et al. Phase I trial of intratumoral injection of ccl21 gene-modified dendritic cells in Lung Cancer elicits tumor-specific immune responses and CD8(+) T-cell infiltration. Clin Cancer Res. 2017;23(16):4556–4568.
- Zhang Y, Hu X, Hu Y, et al. Anti-CD40-induced inflammatory E-cadherin + dendritic cells enhance T cell responses and antitumour immunity in murine Lewis lung carcinoma. J Exp Clin Cancer Res. 2015;34:11.
- Wang Y, Huang H, Yao S, et al. A lipid-soluble extract of Pinellia pedatisecta Schott enhances antitumor T cell responses by restoring tumor-associated dendritic cell activation and maturation. J Ethnopharmacol. 2019;241:111980.
- Lee C, Jose L, Shim K, et al. Influenza mimetic protein-polymer nanoparticles as antigen delivery vehicles to dendritic cells for cancer immunotherapy. Nanoscale. 2019;11(29):13878–13884.
- Zhang J, Chen Y, Chen K, et al. IL-33 drives the antitumour effects of dendritic cells via upregulating CYLD expression in pulmonary adenocarcinoma. Artif Cells Nanomed Biotechnol. 2019;47(1):1335–1341.
- Alameda JP, et al. CYLD regulates keratinocyte differentiation and skin cancer progression in humans. Cell Death Dis. 2011;2:e208.
- Yang Y, Ran J, Sun L, et al. CYLD regulates noscapine activity in acute lymphoblastic leukemia via a microtubule-dependent mechanism. Theranostics. 2015;5(7):656–666.
- Liu P, Xu B, Shen W, et al. Dysregulation of TNFalpha-induced necroptotic signaling in chronic lymphocytic leukemia: suppression of CYLD gene by LEF1. Leukemia. 2012;26(6):1293–1300.
- Hellerbrand C, Bumes E, Bataille F, et al. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis. 2007;28(1):21–27.
- Pannem RR, Dorn C, Ahlqvist K, et al. CYLD controls c-MYC expression through the JNK-dependent signaling pathway in hepatocellular carcinoma. Carcinogenesis. 2014;35(2):461–468.
- Lim JH, Stirling B, Derry J, et al. Tumor suppressor CYLD regulates acute lung injury in lethal Streptococcus pneumoniae infections. Immunity. 2007;27(2):349–360.
- Zhang LM, Zhou JJ, Luo CL. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-kappaB activation. Arthritis Res Ther. 2018;20(1):219.
- Tang Y, Reissig S, Glasmacher E, et al. Alternative splice forms of CYLD mediate ubiquitination of SMAD7 to prevent TGFB signaling and promote colitis. Gastroenterology. 2019;156(3):692–707.
- Wex K, Schmid U, Just S, et al. Receptor-interacting protein kinase-2 inhibition by CYLD impairs antibacterial immune responses in macrophages. Front Immunol. 2016;6:650.
- Legarda D, Justus SJ, Ang RL, et al. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by lps and licensed by type I IFN. Cell Rep. 2016;15(11):2449–2461.
- Srokowski CC, Masri J, Hövelmeyer N, et al. Naturally occurring short splice variant of CYLD positively regulates dendritic cell function. Blood. 2009;113(23):5891–5895.
- Jang JS, Lee JH, Jung NC, et al. Rsad2 is necessary for mouse dendritic cell maturation via the IRF7-mediated signaling pathway. Cell Death Dis. 2018;9(8):823.
- Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17(1):25–34.
- Hahn M, Bürckert J-P, Luttenberger CA, et al. Aberrant splicing of the tumor suppressor CYLD promotes the development of chronic lymphocytic leukemia via sustained NF-kappaB signaling. Leukemia. 2018;32(1):72–82.
- Gurusamy D, Clever D, Eil R, et al. Novel “elements” of immune suppression within the tumor microenvironment. Cancer Immunol Res . 2017;5(6):426–433.
- Varn FS, Tafe LJ, Amos CI, et al. Computational immune profiling in lung adenocarcinoma reveals reproducible prognostic associations with implications for immunotherapy. Oncoimmunology. 2018;7(6):e1431084.
- Dong Z-Y, Zhang C, Li Y-F, et al. Genetic and immune profiles of solid predominant lung adenocarcinoma reveal potential immunotherapeutic strategies. J Thoracic Oncol. 2018;13(1):85–96.
- Wojas-Krawczyk K, Krawczyk P, Buczkowski J, et al. Immunotherapy of lung adenocarcinoma patient with Peptide-pulsed dendritic cells: a case report. Arch Immunol Ther Exp. 2012;60(1):69–77.
- Zhang J, Ou J, Wan X. LRRC62 attenuates Toll-like receptor signaling by deubiquitinating TAK1 via CYLD. Exp Cell Res. 2019;383(1):111497.
- Zhang L, Wei N, Cui Y, et al. The deubiquitinase CYLD is a specific checkpoint of the STING antiviral signaling pathway. PLoS Pathog. 2018;14(11):e1007435.
- Massoumi R, Chmielarska K, Hennecke K, et al. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125(4):665–677.
- Li Y, Huang W, Xu Y, et al. CYLD deubiquitinase negatively regulates high glucose-induced NF-kappaB inflammatory signaling in mesangial cells. BioMed Res Int. 2017;2017:1.
- Jiang J, Luo Y, Qin W, et al. Electroacupuncture suppresses the NF-kappaB signaling pathway by upregulating cylindromatosis to alleviate inflammatory injury in Cerebral Ischemia/Reperfusion Rats. Front Mol Neurosci. 2017;10:363.
- Reissig S, Hövelmeyer N, Weigmann B, et al. The tumor suppressor CYLD controls the function of murine regulatory T cells. J Immunol. 2012;189(10):4770–4776.