Abstract
Background
Involvements of microRNA-22 (miR-22) in cancer have attracted much attention, but its role in diagnosis of hepatocellular carcinoma (HCC) is still largely unknown. Therefore, the aim of this study was to investigate the expression level and the prognostic value of miR-22 in HCC patients.
Methods
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to evaluate serum level of miR-22 in 108 HCC patients and 67 healthy controls. The relationship between miR-22 expression level and clinicopathologic characteristics was analysed via chi-square test. Receiver operating characteristic (ROC) curve was built to estimate the diagnostic value of serum miR-22 in HCC.
Results
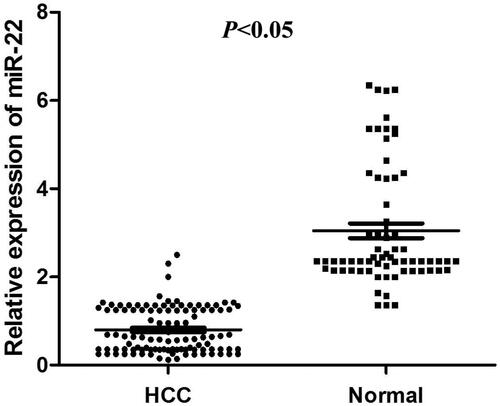
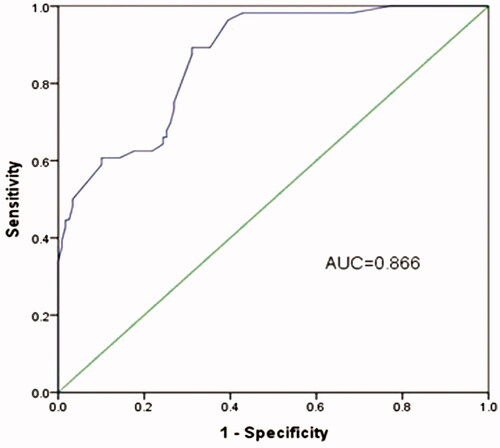
miR-22 expression was significantly down-regulated in HCC compared to that in healthy controls (p < .05). And the low miR-22 expression was significantly associated with vein invasion (p = .002), TNM stage (p = .013) and high serum levels of AFP (α-fetoprotein), ALT (alanine aminotransferase), AST (aspartate aminotransferase) and ALP (alkaline phosphatase. miR-22 had a high diagnostic value with area under the curve of 0.866 corresponding with a sensitivity of 89.3% and a specificity of 68.9%, respectively.
Conclusion
miR-22 expression was down-regulated in HCC patients. Serum miR-22 might be a novel diagnostic marker in HCC.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumour and the third leading cause of cancer-related deaths worldwide [Citation1]. Nearly 700,000 people die of it annually [Citation2,Citation3]. With the improvement of early diagnosis and surgical treatment for the disease in recent years, mortality and incidence are decreasing. But the recurrence of HCC still staying at a high level which lead to a poor prognosis which is strongly correlated with diagnostic delay [Citation4]. Many immunohistochemical markers are available for the diagnosis of HCC, but they have their own strength and limitations [Citation5]. Therefore, it is of great importance to find some accurate bio-markers in the diagnosis or prognosis of HCC.
MicroRNAs (miRNAs) are a recently discovered category of small (∼22 nucleotides), non-coding and single-stranded endogenous RNA molecules [Citation6]. They have been considered to be new approaches of tumour biomarkers for early diagnosis of cancers and have been suggested to play important roles in HCC development [Citation7]. And some of them have been identified to correlate with diagnosis or accepted to be potential therapeutic targets in liver cancer [Citation8–10]. miR-22 is located at a fragile cancer-relevant genomic region in chromosome 17 (17p13.3) and is mapped to an exon of the C17orf91 gene [Citation11]. It has been shown to be abnormally expressed in various types of cancers, such as prostate cancer, breast cancer, cholangiocarcinoma, multiple myeloma, lung cancer, ovarian cancer and colon cancer [Citation12–18]. miR-22 was reported to be down-regulated in HCC and could be a prognostic marker according to previous studies [Citation19,Citation20]. However, the roles of miR-22 in the diagnosis of HCC remain unknown.
In this study, we detected the expression of miR-22 in HCC patients and healthy controls as well as analysed the relationship between miR-22 expression and clinicopathologic characteristics. Then we evaluated the value of miR-22 in the early detection of HCC.
Materials and methods
Patients and samples
Serum was collected from 108 patients suffering from primary HCC 67 healthy volunteers matched for age and gender. None of the patients recruited in this study had undergone preoperative chemotherapy or radiotherapy. This study was authorized by the Ethics Committee of the Fifth Medical Center, Chinese PLA General Hospital. All specimens and clinical materials were obtained and used after getting written informed consent from the patients in the Fifth Medical Center, Chinese PLA General Hospital. Tumours were classified according to the TNM cancer staging system (2009) set by the Union of International Cancer Control. The clinicopathological features of the HCC patients including age, gender, tumour size, vein invasion, liver cirrhosis, TNM stage, histologic grade, hepatitis B virus infection and serum AFP level were shown in .
Table 1. Relationship between serum miR-22 expression and clinicopathological features of HCC patients.
RNA extraction and qRT-PCR analysis
Total RNA was extracted from the serum with miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Then the cDNA was synthesized using One Step PrimeScript miRNA cDNA Synthesis Kit (Takara, Dalian, China) according to the manufacturer’s instructions. RT-PCR reaction was conducted using the PrimeScript RT reagent Kit (Takara, Dalian, China) in the Applied Biosystems 7900 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). Small nucleolar RNA U6 was used as an internal control. The comparative cycle threshold (CT) was used to calculate the expression of miR-22. Each sample was in triplicate.
Statistical analysis
The software of SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Data were presented as means ± SD. The differences between two groups were analysed using t-test. χ2 test was used to analyse the relationship between miR-22 expression and clinicopathologic characteristics. A receiver operating characteristic (ROC) curve was plotted and the area under the curve (AUC) was calculated to evaluate the diagnostic performance of miR-22. Differences were considered to be statistically significant when p was less than .05.
Results
The expression level of miR-22 in serum of HCC patient and healthy controls
The expression level of serum miR-22 was detected in 108 patients with HCC and 67 normal people by qRT-PCR analysis. Compared with the healthy controls, the expression level of serum miR-22 was significantly lower in HCC patients (p < .05, ).
Relationship between miR-22 expression and clinicopathological features of HCC patients
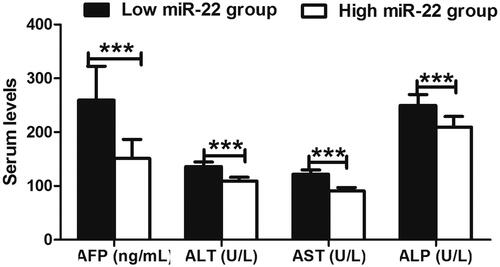
To further investigate whether the expression level of serum miR-22 was correlated with the clinicopathological variables, we further associated their relationship. As shown in , the expression of miR-22 was tightly associated with vein invasion (p = .002) and TNM stage (p = .013). However, there was no association between miR-22 expression and age, gender, tumour size, liver cirrhosis, hepatitis B virus infection, histologic grade (p > .05 for all). Biochemical data analyses found that serum AFP (α-fetoprotein), ALT (alanine aminotransferase), AST (aspartate aminotransferase) and ALP (alkaline phosphatase) levels were significantly higher in HCC patients with low miR-22 expression than in high miR-22 expression (p < .001 for all) ().
Figure 2. The comparison of biochemical parameters in HCC patients based on their expression levels of miR-22, including AFP (α-fetoprotein), ALT (alanine aminotransferase), AST (aspartate aminotransferase) and ALP (alkaline phosphatase). All the mentioned factors exhibited significantly higher level in the serum of HCC patients with low miR-22 expression than in high expression. ***p < .001 represented the significant difference between the compared two groups.
Diagnostic value of miR-22 for HCC
ROC curve was established to explore the diagnostic value of miR-22 in HCC. AUC of 0.866 corresponding with a sensitivity of 89.3% and a specificity of 68.9% was obtained which demonstrated a high diagnostic value of miR-22 (). Besides, the ideal cut-off value for miR-22 expression level was 1.352.
Discussion
Studies report that most of HCC result from the chronic infections of hepatitis B virus and/or hepatitis C virus, but the roles of other non-viral factors are relatively small [Citation21–23]. Patients with HCC have no overt symptoms until the tumour is in its advanced stages, making the treatment of this disease difficult and ineffective [Citation24,Citation25]. The only curative treatment methods for HCC patients are surgical resection and liver transplantation [Citation26,Citation27]. However, the frequent tumour metastasis and recurrence after surgical therapy still led to the dismal outcome of patients with HCC and the five-year survival rate was only approximately 35% [Citation28,Citation29]. Although a growing number of novel treatment strategies have been developed for HCC, such as molecular targeted therapy and gene therapy, satisfactory therapeutic outcomes have not been achieved. Under this circumstance, a novel diagnostic biomarker for HCC is of great importance.
Numbers of studies have proved that miRNAs are involved in multiple physiological and pathological processes, and strongly correlated with tumorigenesis [Citation30]. Recently, aberrant expression of miR-22 was found in many types of cancers, which involved in various cellular processes related to carcinogenesis. Xiong et al. found that miR-22 was frequently down-regulated in ERα-positive human breast cancer cell lines and clinical samples [Citation13]. Yamakuchi et al. found that miR-22 expression in human colon cancer was lower than in normal colon tissue, and it might have an anti-angiogenic effect in this cancer [Citation18]. Ling et al. observed the down-regulation of miR-22 in lung cancer tissues and lung cancer cell lines, and suggested that miR-22 might exhibit excellent anti-lung cancer activity in vitro and in vivo [Citation16]. In contrast, Poliseno et al. showed that miR-22 was aberrantly overexpressed in human prostate cancer [Citation12]. Liu et al. had reported that miR-22 might act as a micro-oncogene in transformed human bronchial epithelial cells induced by anti-BPDE [Citation31]. These controversial findings of miR-22 expression in cancer development suggest the diverse roles of miR-22 in different types of cancer. It could act as a tumour suppressor or oncogene. In our study, we found that the level of serum miR-22 was lower in HCC patients than that in healthy controls. This result indicated that miR-22 acted as a tumour suppressor in HCC. Then we further explored the role of miR-22 in the development of HCC via analysing the potential relationship between the miR-22 expression and various clinicopathological characteristics of HCC patients. The result indicated that low expression levels of miR-22 were significantly correlated with vein invasion and tumour stage. However, there was no relationship with other clinical features, such as gender, age, tumour size, liver cirrhosis, histologic grade and serum AFP level. This result might manifest that miR-22 was involved in the progression of HCC.
Based on the function of miR-22 mentioned above, we inferred the aberrant expression of miR-22 must have some meaningful clinical significance. As its prognostic value was confirmed in the previous study [Citation19,Citation20]. In the present study, we estimated its diagnostic value. After establishing the ROC curve with miR-22 expression, we obtained a curve with AUC of 0.866 corresponding with a sensitivity of 89.3% and a specificity of 68.9%. This was the first report to show that serum miR-22 level could distinguish HCC from the healthy individuals with well performance. However, due to the relatively small sample size, the diagnostic value of serum miR-22 for HCC requires further verification in a further investigation with large sample size. Moreover, in our study, all the individuals in the control group were healthy. Whether serum miR-2 could discriminate between HCC and benignant liver disease cases remained unclear. In addition, the exact molecular mechanisms of miR-22 acting on HCC were poorly known. Therefore, well-designed studies are in urgent need to improve our results.
In conclusion, these findings provide convincing evidence for the first time that the miR-22 can serve as a novel molecular marker for the diagnosis of HCC.
Disclosure statement
We declare that we do not have any conflict of interest.
References
- Lai EC, Lau WY. The continuing challenge of hepatic cancer in Asia. Surgeon. 2005;3(3):210–215.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–1127.
- Attwa MH, El-Etreby SA. Guide for diagnosis and treatment of hepatocellular carcinoma. World J Hepatol. 2015;7(12):1632–1651.
- Nguyen T, Phillips D, Jain D, et al. Comparison of 5 immunohistochemical markers of hepatocellular differentiation for the diagnosis of hepatocellular carcinoma. Arch Pathol Lab Med. 2015;139:1028–1034.
- Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–511.
- Gramantieri L, Fornari F, Callegari E, et al. MicroRNA involvement in hepatocellular carcinoma. J Cell Mol Med. 2008;12(6a):2189–2204.
- Li W, Xie L, He X, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123(7):1616–1622.
- Ji J, Shi J, Budhu A, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361(15):1437–1447.
- Xiong Y, Fang JH, Yun JP, et al. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology. 2010;51:836–845.
- Wan WN, Zhang YQ, Wang XM, et al. Down-regulated miR-22 as predictive biomarkers for prognosis of epithelial ovarian cancer. Diagn Pathol. 2014;9(1):178.
- Poliseno L, Salmena L, Riccardi L, et al. Identification of the miR-106b∼25 microRNA cluster as a proto-oncogenic PTEN-targeting intron that cooperates with its host gene MCM7 in transformation. Sci Signal. 2010;3(117):ra29.
- Xiong J, Yu D, Wei N, et al. An estrogen receptor alpha suppressor, microRNA-22, is downregulated in estrogen receptor alpha-positive human breast cancer cell lines and clinical samples. FEBS J. 2010;277(7):1684–1694.
- Kawahigashi Y, Mishima T, Mizuguchi Y, et al. MicroRNA profiling of human intrahepatic cholangiocarcinoma cell lines reveals biliary epithelial cell-specific microRNAs. J Nippon Med Sch. 2009;76(4):188–197.
- Lionetti M, Agnelli L, Mosca L, et al. Integrative high-resolution microarray analysis of human myeloma cell lines reveals deregulated miRNA expression associated with allelic imbalances and gene expression profiles. Genes Chromosom Cancer. 2009;48:521–531.
- Ling B, Wang GX, Long G, et al. Tumor suppressor miR-22 suppresses lung cancer cell progression through post-transcriptional regulation of ErbB3. J Cancer Res Clin Oncol. 2012;138(8):1355–1361.
- Li J, Liang S, Yu H, et al. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119(3):543–548.
- Yamakuchi M, Yagi S, Ito T, et al. MicroRNA-22 regulates hypoxia signaling in colon cancer cells. PLoS One. 2011;6(5):e2029.
- Zhang J, Yang Y, Yang T, et al. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer. 2010;103(8):1215–1220.
- Zhou L, He J, Zhang Y. MicroRNA-22 expression in hepatocellular carcinoma and its correlation with ezrin protein. J Int Med Res. 2013;41(4):1009–1016.
- Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat. 2014;21(3):153–162.
- Chan DL, Alzahrani NA, Morris DL, et al. Systematic review of efficacy and outcomes of salvage liver transplantation after primary hepatic resection for hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):31–41.
- Zhang ZY, Zheng W, Hai J. MicroRNA-148b expression is decreased in hepatocellular carcinoma and associated with prognosis. Med Oncol. 2014;31(6):984.
- Zhuang L, Zeng X, Yang Z, et al. Effect and safety of interferon for hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2013;8(9):e61361.
- Ren W, Qi X, Jia J, et al. Prevalence of hepatocellular carcinoma in Chinese Budd-Chiari syndrome patients: an extended systematic review using Chinese-language databases. Eur J Gastroenterol Hepatol. 2013;25:1241–1243.
- Shen A, Zhang H, Tang C, et al. Systematic review of radiofrequency ablation versus percutaneous ethanol injection for small hepatocellular carcinoma up to 3 cm. J Gastroenterol Hepatol. 2013;28(5):793–800.
- Yamanaka K, Petrulionis M, Lin S, et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma – systematic review and meta-analysis. Cancer Med. 2013;2(6):862–871.
- Yin Z, Fan X, Ye H, et al. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20(4):1203–1215.
- Zavaglia C, Airoldi A, Mancuso A, et al. Adverse events affect sorafenib efficacy in patients with recurrent hepatocellular carcinoma after liver transplantation: experience at a single center and review of the literature. Eur J Gastroenterol Hepatol. 2013;25:180–186.
- Wang WB, Li FJ, Zhang Y, et al. Reduced expression of miR-22 in gastric cancer is related to clinicopathologic characteristics or patient prognosis. Diagn Pathol. 2013;8(1):102.
- Liu L, Jiang Y, Zhang H, et al. miR-22 functions as a micro-oncogene in transformed human bronchial epithelial cells induced by anti-benzo[a]pyrene-7,8-diol-9,10-epoxide. Toxicol In Vitro. 2010;24(4):1168–1175.