Abstract
Combining DNA damage repair inhibitors and chemotherapeutic agents is an emerging strategy in cancer treatment. In this study, we engineered the polycation nanoparticle (NP), which co-encapsulated DNA damage repair inhibitor Dbait and chemotherapeutic drug Docetaxel (Dtxl), using H1 nanopolymer (folate–-polyethylenimine600–cyclodextrin), and the size of H1/Dbait/Dtxl was about 117 nm. We demonstrated that H1/Dbait/Dtxl enhanced the efficiency of radio-chemotherapy in prostate cancer cells by CCK-8 assay and colony-forming assay. Importantly, the improvement of radio-chemotherapy of H1/Dbait/Dtxl in prostate cancer was also validated in vivo, and the NP did not have a high toxicity profile. The results of immunohistochemistry and western blot supported that the improved therapeutic efficacy was through inhibiting DNA damage repair signalling pathway. Our study supports further investigations using NP to co-deliver DNA damage repair inhibitors and chemotherapeutics to improve the therapeutic efficacy of cancer.
Introduction
Prostate cancer (PCa) is the most common malignancy in men and the second leading cause of cancer-related male mortality [Citation1]. Although potentially curable when confined to the prostate, about 20% of PCa patients will present with metastatic disease, and the current initial treatment of metastatic PCa remains androgen deprivation therapy (ADT). However, ADT leads to remissions lasting about 2 to 3 years, the disease inevitably progresses to castration-resistant PCa (CRPC), which is associated with a poor prognosis and poses considerable therapeutic challenges [Citation2]. Among the anti-cancer methods, cytotoxic chemotherapy, such as Docetaxel (Dtxl), and radiotherapy are the mainstay of CRPC therapy [Citation3]; however, the occurrence of radio-chemotherapy resistance will further make the treatment of PCa, especially CRPC, to be a thorny question.
It is well known that the radio-chemotherapy resistance of CRPC is associated with DNA damage repair pathways [Citation4]. Dbait, a short double-stranded DNA molecule with a free double-strand blunt end, is a new class of DNA damage repair inhibitors [Citation5]. Dbait could target key DNA damage signal transducers, trigger their activation and amplify false damage signalling; consequently, the recruitment of down-stream DNA repair enzymes was impaired, inhibiting several DNA repair pathways [Citation5,Citation6]. Previously, studies have shown that Dbait was effective in combination with radiotherapy and chemotherapy on several radio-resistant and chemo-resistant tumours [Citation5,Citation7–9]. However, as a kind of nucleic acid-based therapeutics, Dbait requires an efficient delivery vehicle for administration to increase the efficiency of cellular uptake.
Herein, in order to improve the efficacy and reduce the toxicity of radio-chemotherapy for CRPC, we engineered a polycation nanoparticle (NP), H1/Dbait/Dtxl, which co-encapsulated Dbait and Dtxl using H1 nanopolymer (folate–-polyethylenimine600–cyclodextrin). H1 is biodegradable and has the capacity to target tumour cells because of the folate-targeting moiety [Citation10], Of note, PCa has been shown to overexpress folate receptor [Citation11]. In this study, H1/Dbait/Dtxl formulation was characterised firstly. We then found that NP form of H1/Dbait/Dtxl could significantly improve the efficiency of radio-chemotherapy in CRPC in vitro using CRPC cell lines, including androgen receptor (AR) positive-expressed cell lines (PC-3 and DU145) and AR negative-expressed cell line (LNCaP). The results were further validated with subcutaneous transplanted model of PC-3 tumour in nude mice.
Materials and methods
Cell lines, dbait molecule and dtxl drug
Three CRPC cell lines, PC-3, DU145 and LNCaP, were obtained from Cell Bank of Chinese Academy of Sciences (No.TCHu158, No.TCHu222 and No.TCHu173, respectively). PC-3 and LNCaP cells were cultured in RPMI-1640 medium (HyClone, USA) supplemented with 10% foetal bovine serum (FBS) (Gibco, USA), and DU145 cells were cultured in DMEM medium (HyClone, USA) supplemented with 10% FBS, both RPMI-1640 and DMEM medium contained 100 μg/mL streptomycin and 100 μg/mL penicillin. Cells were grown at 37 °C in monolayer cultures under conditions of 100% humidity, 95% air and 5% CO2. Dbait was synthesised by Genscript Biotechnology (Jiangsu, China), the sequence is: 5′-GCTGTGCCCACAACCCAGCAAACAAGCCTAGA-(H)-TCTAGGCTTGTTTGCTGGGTTGTGGGCACAGC-3′, where H is a hexaethylene glycol linker and the letters underlined and in bold are phosphorodiamidate nucleosides. Dtxl injection drug was bought from Hengrui Pharma, Jiangsu, China.
Synthesis of H1 and preparation of H1/dbait/dtxl
H1 was synthesized by a method reported previously [Citation10]. H1, Dbait and Dtxl were dissolved in sterile double-distilled water. H1/Dbait was constructed as described in our previous study, and its N/P ratio (where “N” is the amount of nitrogen in polycation and “P” is the amount of phosphate in 1 µg Dbait) was 12, which had the best bioactivity for nucleic acid delivery [Citation8]. In order to form H1/Dbait/Dtxl and select the best synthetic method, different amounts of Dtxl were added into H1/Dbait with the mass ratio of H1 and Dtxl at 1, 6, 12, 18 and 24, respectively. The H1/Dbait/Dtxl was required to be incubated for 20 min at room temperature prior to performing the following experiments.
Characterization of H1/dbait/dtxl
The size and zeta potential of H1/Dbait/Dtxl were determined using dynamic light scattering (DLS). Measurements were carried out at 25 °C using a Zetasizer Nano-ZS from Malvern Instruments (Worcestershire, United Kingdom). In order to detect the stability of H1/Dbait/Dtxl nanoparticles in biological environment, Size of nanoparticles was observed after incubation with RPMI-1640 medium supplemented with 10% FBS for 5 minutes, 24 h and 48 h, respectively, and measurements were carried out at 37 °C using a Zetasizer Nano-ZS. Z average mean (nm) and zeta potential (mV) were used for data analysis. The transmission electron microscopy (TEM) image of H1/Dbait/Dtxl was obtained using a Tecnai G220 TEM (FEI, USA), the samples were prepared by dropping 10 μL H1/Dbait/Dtxl on the copper grid, and the average diameter was determined based on 100 particles, which were randomly selected from the representative TEM images.
CCK-8 assay
Cell proliferation was detected by using a CCK-8 kit (Beyotime, China) according to the manufacturer’s instruction. Briefly, cells were plated at 5000cells/well in 96-well plates and each group had eight replicated wells. 12 h later, Dtxl (0.256 µg), H1/Dtxl(containing 0.256 µg Dtxl), H1/Dbait (containing 0.2 µg Dbait) or H1/Dbait/Dtxl (containing 0.2 µg Dbait and 0.256 µg Dtxl) was added into the 200 µL culture medium of each well, respectively, and incubated for another 24 h. Then, 20 µL CCK-8 reagent was added to each well and cells were incubated for 1 h. The absorbance at 450 nm of each well was measured on an ELX-800 Spectrometer Reader (Bio-Tek, USA). Cell survival fraction was calculated by OD450test/OD450control.
Colony formation assay
The clone formation assay was done as described in the published paper [Citation8]. Briefly, cells were seeded in 6-well plates at appropriate cell densities and incubated routinely until cells were adhered to the wall. The original culture medium of each well was replaced with 2 ml culture medium containing 2% FBS, then cells were treated with phosphate buffer (PBS), Dtxl (2.56 µg/well), H1/Dtxl (containing 2.56 µg/well Dtxl), H1/Dbait (containing 2 µg/well Dbait) or H1/Dbait/Dtxl l(containing 2.56 µg/well Dtxl and 2 µg/well Dbait), respectively. 5 h later, cells were treated with irradiation (IR) at the dose of 0 Gy, 2 Gy, 4 Gy or 6 Gy (0.3 Gy/minutes). About 14 days later, cell clones were fixed with 4% paraformaldehyde (Beyotime, China) and stained with crystal violet (Beyotime, China). All colonies with over 50 cells were counted, and cell survival curves were obtained by GraphPad Prism software 5.0.
Immunofluorescence assay
Cells were grown on coverslips placed in 6-well plates and treated with PBS, IR, IR plus Dtxl, IR plus H1/Dtxl, IR plus H1/Dbait or IR plus H1/Dbait/Dtxl, respectively. PBS, Dtxl, H1/Dtxl, H1/Dbait and H1/Dbait/Dtxl were given 5 h before IR treatment (4 Gy, 0.3 Gy/minutes) and the doses of them were in accordance with “Colony formation assay” part. 24 h later, cells were fixed with 4% paraformaldehyde for 20 min and permeabilized with 0.3% TritonX-100 (Sigma, USA) for 10 min. Then cells were blocked with 5% bovine serum albumin (BSA) at room temperature for 1 h and incubated with rabbit polyclonal antibody against γ-H2AX (Ser139) (Cell Signalling Technology, USA) overnight at 4 °C. After being incubated with secondary antibody conjugated with tetramethylrhodamine (Vicmed, China) for 1 h at room temperature, cell nuclei were stained with Hochest33342 (Invitrogen, USA) for 20 min at 25 °C, and coverslips were mounted on microslides with glycerol mounting medium (Beyotime, China). Images were acquired using a fluorescence microscope (Nikon, Japan).
Western blot
Cells were seeded and treated as described above in “Immunofluorescence assay” part. Then, cells were harvested and suspended in cell lysis buffer (Beyotime, China) with protease inhibitor and phosphatase inhibitor (Selleck, USA) and incubated for 20 min on ice. Total proteins were isolated by centrifugation at 12,000 rpm for 10 min at 4 °C. After protein concentration detection and denaturation, the protein samples were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis and electro-transferred to polyvinylidene fluoride membranes (Millipore, USA). Subsequently, the membranes were subjected to blocking with 5% BSA, and incubated with the corresponding primary antibodies at 4°C overnight: γ-H2AX (Ser139), DNA-PKcs (Cell Signalling Technology, USA), PARP (Cell Signalling Technology, USA) and actin (Bioworld, China). Membranes were then incubated with secondary antibody conjugated with horseradish peroxidase (HRP) (Vicmed, China) for 1 h at room temperature. Finally, the immunoreactive bands were developed with a chemiluminescent HRP substrate (Millipore, USA) and pictured by a Chemiluminescence Image System (Tanon, China). The band intensity was analysed by ImageJ software.
Establishment and treatment of subcutaneous PCa-xenografted mice model
The experiments in vivo were carried out according to the guidelines of Jiangsu Council on Animal Care. Male nude mice (HuaFuKang biological technology, China), four to six weeks old, were housed in SPF laboratory animal room at least 1 week before commencing experiments. Xenograft tumours were inoculated in the right lower limbs of mice by subcutaneous injection of 4 × 106 PC-3 cells in 100 μL PBS. When the volume of subcutaneous tumours reached 100 ∼ 150 mm3, the tumour-bearing mice were randomly divided into six groups (10 mice per group) for different treatments. The mice of the control group were intratumoral injected with 100 μL PBS per day for three consecutive days. The mice of IR group, IR plus Dtxl group, IR plus H1/Dtxl group, IR plus H1/Dbait group and IR plus H1/Dbait/Dtxl group were first respectively intratumoral injected with 100 μL PBS, 100 μL Dtxl (containing 76.8 µg Dtxl), 100 μL H1/Dtxl (containing 76.8 µg Dtxl), 100 μL H1/Dbait (containing 60 µg Dbait) or 100 μL H1/Dbait/Dtxl (containing 60 µg Dbait and 76.8 µg Dtxl). 5 h later, the mice were radiated with 3 Gy (0.3 Gy/minutes), and only the tumour regions were irradiated, as the remaining parts of the body were lead-shielded, the above treatments were given for three consecutive days. Tumour volumes were calculated by measuring two perpendicular diameters with a calliper every three days and by using the formula V = 0.5 × a × b2, where a and b were the larger and smaller diameters, respectively. When tumour volumes reached 3000 mm3, mice were euthanized by an overdose of carbon dioxide. Tumour growth curves and overall survival curves of tumour-bearing mice were obtained by GraphPad Prism 5.0 software.
Immunohistochemistry
Twenty-four hour after treatment, four mice of each group were sacrificed randomly and tumours were excised for western blot and immunohistochemistry. After dewaxing at 65 °C for 4 h followed by two 15-min washes with xylene, paraffin sections of tumour tissues were rehydrated with graded ethanol and distilled water. Antigen retrieval was carried out by heating the sections in 10 mM citrate buffer (pH 6.0) (Beyotime, China) at 95 °C for 30 min, and endogenous peroxidases were interdicted by 3% hydrogen peroxide for 10 min. The sections were then blocked with 5% BSA for 30 min and incubated overnight at 4 °C with γ-H2AX (Ser139) antibody. Subsequently, the sections were incubated with HRP-conjugated secondary antibody for 30 min at room temperature. After dyeing target proteins with 3,3-diaminobenzidine and staining nuclei with haematoxylin, sections were dehydrated and sealed with coverslips. Images were recorded by a light microscope (Nikon, Japan).
Statistical analysis
All experiments were carried out at least three times. Quantitative data were presented as mean ± standard deviation (SD), and statistical analysis was conducted by SPSS16.0. For comparisons between two groups, a Student’s t-test or chi-squared test was used. p < .05 was considered to be statistically significant.
Results
Characterization of H1/dbait/dtxl
As shown in Supplementary Figure S1, at the mass ratio of 6, H1/Dbait/Dtxl had the best effect on inducing the improvement of γ-H2AX, which indicated the presence of DNA damages produced by radiotherapy, in PC-3 cells. Hence, the mass ratio of 6 was used to synthesise H1/Dbait/Dtxl in the following experiments.
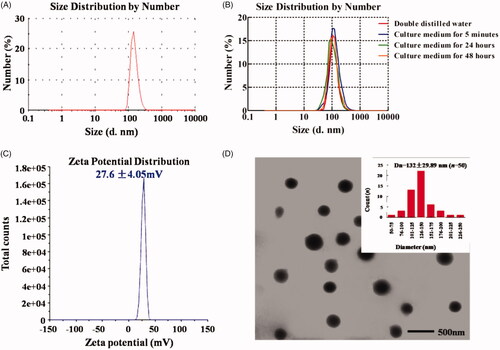
H1/Dbait/Dtxl was found to have the size of 117 ± 12.9 nm (), and its surface charge was 27.6 ± 4.06 mV (). TEM image of H1/Dbait/Dtxl showed well-defined spherical NPs with a median diameter at 132 ± 29.89 nm (). In comparison, the particle size of H1/Dbait/Dtxl remained unchanged in culture medium over the experimental time period of 48 h (), suggesting that H1/Dbait/Dtxl nanoparticles have great stability.
H1/dbait/dtxl inhibited proliferation of CRPC cells and improved their radiosensitization in vitro
The influence of H1/Dbait/Dtxl on CRPC cells survival and proliferation was analysed by CCK-8 assay and clone formation assay. As shown in , Dtxl, H1/Dtxl, H1/Dbait and H1/Dbait/Dtxl could significantly inhibit the cell proliferation of CRPC, and a significant difference existed between Dtxl group and H1/Dtxl group, the addition of Dbait further improved the inhibitory effect of H1/Dtxl.
Figure 2. NP H1/Dbait/Dtxl inhibited the proliferation and colony-forming ability of CRPC cells in vitro. (A) The effect of H1/Dbait/Dtxl on proliferation of PC-3 cell (Ai), DU145 cell (Aii) and LNCaP cell (Aiii) detected by CCK-8 assay. (B) Clonogenic survival curves of PC-3 cell (Bi), DU145 cell (Bii) and LNCaP cell (Biii) treated with H1/Dbait/Dtxl combined with radiotherapy. **p < .01, n = 3.
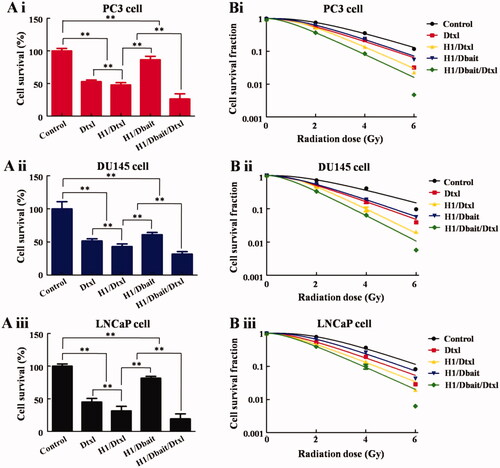
The results of clone formation assay () also indicated that the growth rates and colony formation abilities of CRPC cells were significantly inhibited by Dtxl, H1/Dtxl, H1/Dbait and H1/Dbait/Dtxl, the inhibitory effect of H1/Dbait/Dtxl was strongest. In addition, the parameter, radiation sensitivity ratio (SERD0), was calculated to quantify the degree of radiosensitization, the SERD0s of H1/Dbait/Dtxl in PC-3, DU145 and LNCaP cells were 1.60, 1.85 and 1.28, respectively, which indicated that H1/Dbait/Dtxl could be used as a radiosensitizer without increasing toxicity of chemotherapy or radiotherapy.
H1/dbait/dtxl inhibited the repair of CRPC cell DNA damage in vitro
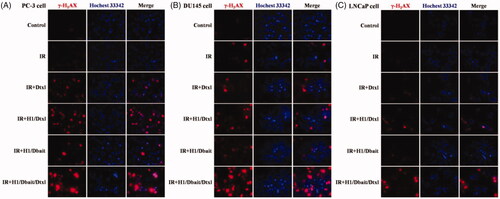
In this study, we investigated whether H1/Dbait/Dtxl would lead to increased H2AX phosphorylation (γ-H2AX) in CRPC cells, which was formed at DSB damage sites and resolved when the damage was repaired. We found that compared with a control group and IR group, the red fluorescence signal of γ-H2AX of the other four groups were higher, and the number of γ-H2AX foci induced by H1/Dbait/Dtxl was more than H1/Dtxl, which induced higher expression of γ-H2AX than Dtxl ().
Figure 3. NP H1/Dbait/Dtxl enhanced H2AX phosphorylation (γ-H2AX) in CRPC cells, including PC-3 cell (A), DU145 cell (B) and LNCaP cell (C) detected by immunofluorescence assay, n = 3.
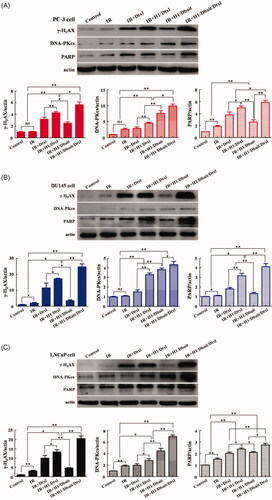
We also examined the effect of H1/Dbait/Dtxl on other DNA damage associated proteins, such as DNA-PKcs and PARP. As the results of western bolt showed in , compared with control and IR treatments, the levels of γ-H2AX, DNA-PKcs and PARP were obviously increased in CRPC cells treated with IR plus Dtxl, IR plus H1/Dtxl, IR plus H1/Dbait and IR plus H1/Dbait/Dtxl. Furthermore, after analysing the band intensities, significant differences were existed between IR plus H1/Dtxl group and IR plus Dtxl group, IR plus H1/Dbait/Dtxl group and IR plus H1/Dtxl group (), indicating that H1/Dbait/Dtxl improved the radiosensitization of CRPC by mimicking DNA DSB and inhibiting DNA damage repair signalling pathways.
Figure 4. NP H1/Dbait/Dtxl increased the expression levels of DNA damage response effectors in vitro. Effect of H1/Dbait/Dtxl on γ-H2AX, DNA-PKcs and PARP expression in PC-3 cell (A), DU145 cell (B) and LNCaP cell (C) was evaluated by Western blot the band intensity were quantified after normalised to actin, respectively. *p < .05, **p < .01, n = 3.
H1/dbait/dtxl improved efficiency and reduced side effects of radio-chemotherapy of CRPC in vivo
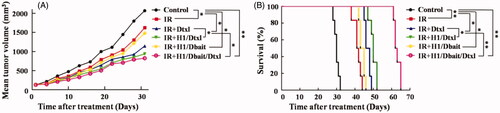
To validate our in vitro results and evaluate the efficacy of H1/Dbait/Dtxl in vivo, mice bearing PC-3 xenograft tumours were established, tumour growth and overall survival were observed. The results () showed that the combination therapy of IR and H1/Dbait/Dtxl led to significantly longer tumour growth delay and longer survival time when compared with the other five treatments. As for tumour growth analysis, significant differences also existed between the following groups, IR plus Dtxl versus IR plus H1/Dtxl, p = .03; IR plus H1/Dtxl versus IR plus H1/Dbait/Dtxl, p = .04. In addition, the survival time of IR plus H1/Dbait/Dtxl group was longer than IR plus H1/Dtxl group, and IR plus H1/Dtxl treatment could significantly prolonged survival time when compared with IR plus Dtxl treatment.
Figure 5. NP H1/Dbait/Dtxl improved the efficiency of radio-chemotherapy of CRPC in vivo. (A) Tumour growth curves of mice bearing PC-3-xenograft tumours. (B) Survival curves of mice bearing PC-3-xenograft tumours. *p < .05, **p < .01, n = 6.
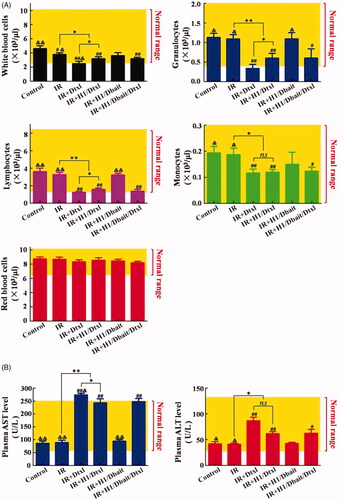
Haematologic toxicity and hepatotoxicity are two major side effects of traditional chemotherapy and radiotherapy that often lead to early termination of treatments. summarised the haematologic toxicity and hepatotoxicity profiles recorded for healthy tumour-free nude mice four days after treatments. As shown in , IR, IR plus Dtxl, IR plus H1/Dtxl and IR plus H1/Dbait/Dtxl treatments had inhibition on medullary haematopoiesis function, indicated by the decreasing counts of white blood cells (WBC, including lymphocytes, granulocytes and monocytes), but had no effect on red blood cell (RBC) counts. The addition of Dtxl or H1/Dtxl to radiotherapy further significantly decreased the counts of WBC, lymphocytes, granulocytes and monocytes, and the numbers of WBC, lymphocytes and granulocytes in IR plus Dtxl group were lower than the lower limit of the normal range. However, H1/Dtxl significantly reduced the inhibition of WBC, lymphocytes and granulocytes induced by free Dtxl drug (PWBC = 0.04, Pgranulocytes = 0.04, Plymphocytes = 0.04). On the other hand, Dbait did not show any significant hematological toxic effects. Compared with control and IR groups, the combination treatment of IR and Dtxl significantly increased the levels of plasma aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and the AST level was higher than the normal range (). Encapsulation of Dtxl into H1 could obviously reduce the AST level and maintain it at a normal range. Besides, compared with IR plus H1/Dtxl treatment, co-encapsulation of Dbait and Dtxl did not increase liver toxicity (PAST = 0.69, PALT = 0.77), and there was no significant difference between IR group and IR plus H1/Dbait group (PAST = 0.44, PALT = 0.29) (). The above results confirmed that H1/Dbait/Dtxl was a potent radiosensitizer.
Figure 6. Haematologic toxicity and hepatotoxicity of NP H1/Dbait/Dtxl. (A) Effect of H1/Dbait/Dtxl on white blood cells, lymphocytes, granulocytes, monocytes and red blood cells. (B) Effect of H1/Dbait/Dtxl on aspartate aminotransferase and alanine aminotransferase. “#”denotes significant difference existed between control group and the labelled groups, #p < .05, ##p < .01; “&” denotes significant difference existed between control group and the labelled groups, &p < .05, &&p < .01; “ns” denotes statistical non-significance; *p < .05, **p < .01, n = 3.
H1/dbait/dtxl inhibited DNA damage repair of CRPC in vivo
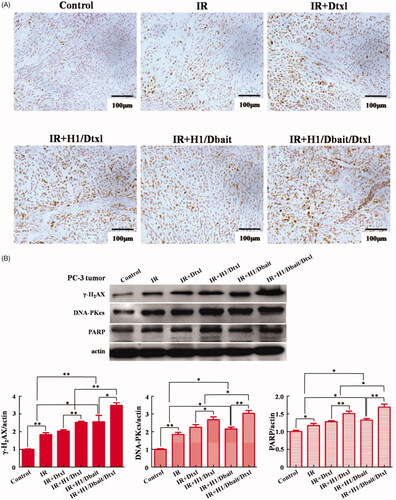
The molecule mechanism of H1/Dbait/Dtxl on improving the radio-chemotherapy effect of CRPC was further investigated in vivo. Consisting with the in vitro studies, the two independent assays of immunohistochemistry and western blot all indicated that, compared with control and IR group, IR plus Dtxl, IR plus H1/Dtxl, IR plus H1/Dbait and IR plus H1/Dbait/Dtxl treatments significantly increased the protein expression levels of γ-H2AX, DNA-PKcs and PARP in tumours tissues and the improvement of IR plus H1/Dbait/Dtxl was most obvious, the expression levels of the three molecules in IR plus H1/Dtxl group were all higher than IR plus Dtxl group (). These results confirmed that H1/Dbait/Dtxl retained the ability of mimicking DSB and inhibiting DNA damage repair in vivo.
Figure 7. NP H1/Dbait/Dtxl increased the expression levels of DNA damage response effectors in vivo. (A) Effect of H1/Dbait/Dtxl on γ-H2AX expression in PC-3 tumours detected by immunohistochemistry. (B) Effect of H1/Dbait/Dtxl on γ-H2AX, DNA-PKcs and PARP expression in PC-3 tumours detected by Western blot, the band intensity were quantified after normalised to actin, respectively. *p < .05, **p < .01, n = 3.
Discussion
Over the past 5 years, paradigm-changing developments have been made in the treatment of CRPC, some new therapeutic options have sprung up, such as novel endocrine therapies [Citation3], vaccines [Citation12], nano-drugs [Citation13]. Among them, nanotechnology-based drugs have attracted more and more concern, they offer many advantages over the conventional drug dosage form which includes increased efficacy, reduced toxicity and improved patient conveniences [Citation13].
In this study, we aimed to improve the radio-chemotherapy of CRPC by directly targeting the treatment resistance mechanism-DNA damage repair pathways. First of all, we synthesised the NPs which encapsulated Dbait, a novel radiosensitizer, and Dtxl, a well-established chemotherapeutic agent, using H1 nanopolymer. Our in vitro evaluation of H1/Dbait/Dtxl demonstrated particle size and surface charge () that were conducive to in vivo drug delivery [Citation14]. We confirmed that, as shown in /Dbait/Dtxl significantly inhibited the proliferation and improved the radiosensitization of CRPC cells with desirable SERD0 values, which manifested H1/Dbait/Dtxl was an excellent radiosensitizer. It is also important to note that the survival curves with H1/Dbait/Dtxl as a radiosensitizer were almost linear in shape (), consistent with NP's mechanism of action as a DNA damage repair inhibitor. Our mechanistic studies () further confirmed that H1/Dbait/Dtxl improved the radio-chemotherapy effect of CRPC through inhibiting the DNA damage repair.
Our previous study had demonstrated that folate-targeted NPs could efficiently accumulated in PCa tumour sites in vivo [Citation15]; hence, the targeting ability of H1 could further improve the anti-cancer effect of Dbait combined with Dtxl. The results of in vivo study showed that H1/Dbait/Dtxl not only increased the therapeutic effects, indicated by the inhibition of tumour progression and extension of survival time of CRPC-bearing mice (), but also decreased the hepatotoxicity and haematotoxicity caused by radio-chemotherapy ().
Our study strongly suggests that nanotechnology-based therapy of combining DNA repair inhibitors with conventional chemotherapy may prove to be a safe and effective therapeutic strategy in the treatment of CRPC, future studies will focus on identifying other novel combination of agents that are synergistic in chemo-radiotherapy.
Figure_S1.tif
Download TIFF Image (7.2 MB)Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29.
- Yap TA, Smith AD, Ferraldeschi R, et al. Drug discovery in advanced prostate cancer: translating biology into therapy. Nat Rev Drug Discov. 2016;15(10):699–718.
- Pal SK, Sartor O. Prostate cancer: the best fit for enzalutamide in metastatic prostate cancer. Nat Rev Clin Oncol. 2014;11(9):504–506.
- Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161(5):1215–1228.
- Quanz M, Berthault N, Roulin C, et al. Small-molecule drugs mimicking DNA damage: a new strategy for sensitizing tumors to radiotherapy. Clin Cancer Res. 2009;15(4):1308–1316.
- Quanz M, Chassoux D, Berthault N, et al. Hyperactivation of DNA-PK by double-strand break mimicking molecules disorganizes DNA damage response. PLoS One. 2009;4(7):e6298.
- Coquery N, Pannetier N, Farion R, et al. Distribution and radiosensitizing effect of cholesterol-coupled Dbait molecule in rat model of glioblastoma. PLoS One. 2012;7(7):e40567.
- Yao H, Qiu H, Shao Z, et al. Nanoparticle formulation of small DNA molecules, Dbait, improves the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomedicine. 2016;12(8):2261–2271.
- Herath NI, Devun F, Lienafa MC, et al. The DNA repair inhibitor DT01 as a novel therapeutic strategy for chemosensitization of colorectal liver metastasis. Mol Cancer Ther. 2016;15(1):15–22.
- Yao H, Ng SS, Tucker WO, et al. The gene transfection efficiency of a folate-PEI600-cyclodextrin nanopolymer. Biomaterials. 2009;30(29):5793–5803.
- Xia W, Low PS. Folate-targeted therapies for cancer. J Med Chem. 2010;53(19):6811–6824.
- Rotow J, Gameiro SR, Madan RA, et al. Vaccines as monotherapy and in combination therapy for prostate cancer. Clin Transl Sci. 2010;3(3):116–122.
- Hema S, Thambiraj S, Shankaran DR. Nanoformulations for targeted drug delivery to prostate cancer: an overview. J Nanosci Nanotechnol. 2018;18(8):5171–5191.
- Prabhakar U, Maeda H, Jain RK, et al. Challenges and key considerations of the enhanced permeability and retention effect for nanomedicine drug delivery in oncology. Cancer Res. 2013;73(8):2412–2417.
- Zhang X, Liu N, Shao Z, et al. Folate-targeted nanoparticle delivery of androgen receptor shRNA enhances the sensitivity of hormone-independent prostate cancer to radiotherapy. Nanomedicine. 2017;13(4):1309–1321.