Abstract
Oxidative stress can induce apoptosis and decrease activities of osteoblasts. Hyperoside (HYP) is a potent antioxidant derived from Chinese herb. This study aims to evaluate the protective effects provided by HYP to osteoblastic MC3T3-E1 cells. MC3T3-E1 cells were pre-treated with HYP for 24 h before being treated with 0.3 mM hydrogen peroxide (H2O2) for 24 h. Cell viability, flow cytometric analysis and mRNA expression of alkaline phosphatase (ALP), collagen I (COL-I) and osteocalcin (OCN) in MC3T3-E1 cells were examined. We next examined apoptosis-related and mitogen-activated protein kinase (MAPK) related proteins in HYP and H2O2 groups. HYP over the dose of 40 μmol/L could obviously increase the MC3T3-E1 cell viability at 24 h and 48 h (p < .05). HYP significantly (p < .05) increased mRNA expression of ALP, COL-I and OCN than H2O2 group. Moreover, HYP decreased the apoptosis rate and apoptosis-related proteins that induced by H2O2. In addition, HYP decreased the production of phosphorylated Jun N-terminal kinase (JNK) and p38 levels of osteoblastic MC3T3-E1 cells induced by H2O2. These results demonstrated that the protective effect provided by HYP to osteoblastic MC3T3-E1 cells was mediated, at least in part, via inhibition of MAPK signalling pathway and oxidative damage of the cells.
Introduction
Osteoporosis (OP) is a bone disease that affected almost 200 million older people in the world [Citation1,Citation2]. Patients with OP are prone to complicated with fractures. The pathogenesis of OP is not clear. Oxidative stress is a crucial initiating risk factor for OP [Citation3]. Reactive oxygen species (ROS) accumulation and osteoblast apoptosis involved in the pathogenesis of various types of OP [Citation4,Citation5]. Therefore, substances with antioxidant activity may be new targets for preventing OP [Citation6,Citation7].
Hyperoside (HYP), chemical name is quercetin 3-O-β-D-galactoside, which belongs to the flavonol glycosides [Citation8]. Since HP possess the presence of alcoholic hydroxyl groups in the molecule, and is widely present in plants such as Campanulaceae and Rosaceae. HYP exerts a variety of biological activities, including myocardial protection, against redox and anti-inflammatory activities. Zhang et al. [Citation9] reported that HYP could stimulates osteoblastic differentiation in osteosarcoma cells. Chen et al. [Citation10] reported that HYP has potential applications as a drug for osteoporosis treatment.
Some studies showed that JNK and p38 MAPK pathways were involved in bone formation [Citation11]. However, whether HYP has the effect on bone formation via MAPK pathways is still unknown. What’s more, no studies have investigated the application of HYP in treating osteoblast MC3T3-E1 cells. In this study, we hypothesized that HYP may be effective in protecting against oxidative stress injury and prevent osteoblast apoptosis through inhibited JNK and p38 MAPK pathways.
Material and methods
Reagents and antibodies
HYP (purity >98%) was purchased from Yuanye Company (Shanghai, China), MC3T3-E1 cells was purchased from the Cell Resource Centre of Shanghai Institutes for Biological Sciences. JNK, P-JNK, P-38, P-P38 and β-actin were obtained from Cell Signalling Technology (Beverly, MA, USA). P38 inhibitor SB203580 and PI3K inhibitor LY294002 were purchased from Selleckchem (Houston, TX, USA). Concentration of SB203580 and LY294002 were reference from previous study and identified as 10 μM [Citation12]. Foetal bovine serum (FBS) and α-Modified minimal essential medium (a-MEM) were purchased from Gibco (Gaithersburg, MD, USA).
Cell culture
Osteoblast-like MC3T3-E1 was obtained from Fenghui Bio and cultured in high-Dulbecco’s modified eagle medium (DMEM) supplemented with 10% FBS, 100 U/ml double-antibody, and cultured at 37 °C and 5% CO2 saturated humidity in a cell culture incubator. Observe and record the changes in the colour and cell morphology of the medium. In order to keep the culture fresh, it is necessary to change the solution every 48 h. When the cells reach 80–90% fusion state, cell subculture was conducted to keep the cells activity.
Cell viability assay
MC3T3-E1 were seeded in a 96-well plate and treated with DMEM, H2O2 (100 µM, control), H2O2 + HP (10 μmol/L), H2O2 + HP (20 μmol/L), and H2O2 + HP (40 μmol/L). After treatment for 12 h, 24 h, 48 and 72 h, cells were washed by Hank’s for three times. Then, MC3T3-E1 were incubated with 200 μl fresh medium containing 0.5 mg/ml MTT. Cells were incubated in cell culture incubator for 4 h in a light-shielded environment. The enzyme detection wavelength was set to 490 nm, and the data was measured and recorded.
Flow cytometric analysis for apoptosis
AnnexinV-FITC cell apoptosis detection kit was used to detect the apoptotic rate of each group of cells. At the end of the experiment, cells were centrifuged at 1000 rpm for 5 min, and stained with Annexin V-FITC and PI (BD Biosciences, San Diego, CA, USA) for 15 min in darkness. After the incubation for 30 min in the dark, the flow cytometry (BD, the U.S.) The osteoblast apoptosis was assessed using a FACSCalibur flow cytometer (BD Biosciences, San Diego, CA, USA). The apoptotic rate = Q2 cell number/total cell number × 100%.
PCR
Total RNA in these five groups was extracted by Trizol reagent (Invitrogen, USA) as recommended by the manufacturer. cDNA was synthesized by ReverTra Ace qPCR RT Kit (Toyobo Co. Ltd., Osaka, Japan). Primer sequences of target genes are listed in . The QRT-PCR analysis was performed using SYBR® Green Realtime PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan). Reaction conditions were referred to the instruction of the manufacture. Relative expression levels of the target genes were normalized with the control gene β-actin.
Table 1. Real-time PCR primers for amplification of specific MC3T3-E1 mRNA.
Western blot
Western blotting was conducted as reported previously. In brief, total proteins were extracted by RIPA (Beyotime, Shanghai, China) and 0.01% protein inhibitor cocktail (Sigma, Shanghai, China). The isolated protein concentration was measured by BCA Quantitation Kit (Boster, Wuhan, China). Equivalent amounts of proteins (100 μg) in each groups were seperated on 8–15% SDS-PAGE gels and electrotransferred to nitrocellulose membranes (Millipore, Bedford, MA). After blocking within 5% skim milk, membrane was incubated with primary antibodies overnight at 4 °C. Membranes were then incubated with its corresponding HRP-conjuncted secondary antibody (Boster, Wuhan, China) and were visualized using enhanced chemiluminescence (EMD Millipore, Billerica, MA, USA).
Statistical analysis
Data were represented as mean ± SD. of multiple repeats of the same experiment (n = 5). The data for these measurements were analyzed with one-way analysis of variance (ANOVA) with subsequent post hoc multiple comparison by Dunnett’s test. All analyses were calculated by SPSS 19.0(SPSS Corp., Chicago, USA). Statistically significant values defined as p < .05.
Results
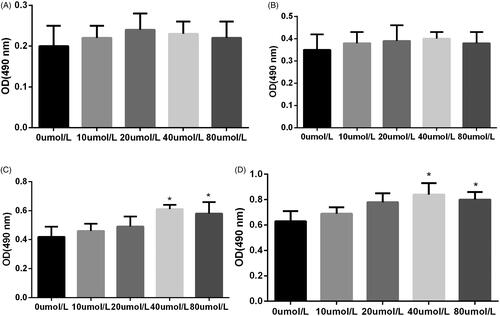
HYP exhibits no cytotoxicity on MC3T3-E1 cells
As shown in , after treatment of HYP (10 μmol/L, 20 μmol/L, 40 μmol/L and 80 μmol/L) for 6 h, 12 h, 24 h and 48 h. HYP over the dose of 40 μmol/L could obviously increase the MC3T3-E1 cell viability at 24 h and 48 h (p < .05). However, there was no significant difference between the OD values for the HP (40 μmol/L) and HP (80 μmol/L).
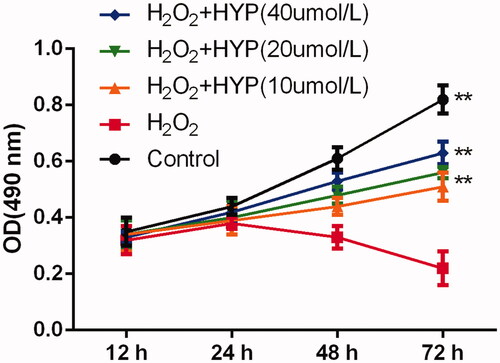
HYP could increase cell viability insulted by H2O2
As shown in , compared with control group, H2O2 significantly decrease the cell viability with statistically significant at 72 h. There were no statistically significant for cell viability at 24 h and 48 between these five groups (p > .05). However, 20 μmol/L and 40 μmol/L of HYP could increase cell viability than H2O2 treatment group at 72 h (p < .05).
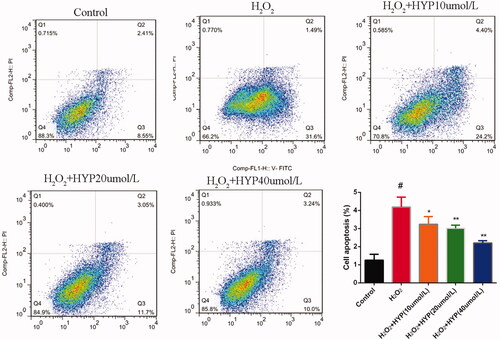
Effects of HP on apoptosis of H2O2-insulted MC3T3-E1 cells
showed that cell apoptosis rate of control, H2O2, and HYP treated groups. Compared with control group, H2O2 significantly increased the apoptosis rate with statistically significant. However, HYP significantly decreased the apoptosis rate in a dose-dependent manner than control group (p < .05).
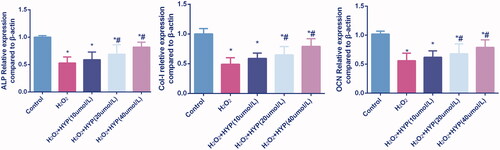
Effects of HYP on the expressions of osteogenesis-related mRNA
ALP, collagen-I and OCN mRNA expressions were determined by PCR. Results are presented in . When treated with H2O2, ALP, collagen-I and OCN was decreased than control group. When co-cultured with HYP and H2O2, the ALP, collagen-I and OCN was increased than H2O2 group.
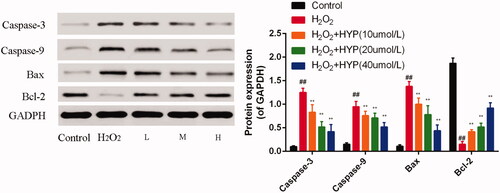
Effects of HYP on the expressions of apoptosis-related proteins and MAPK pathways
When treated with H2O2, caspase-3, caspase-9 and Bax was increased and Bcl-2 was decreased than control group (p < .05). However, caspase-3, caspase-9 and Bax were significantly decreased when treated with H2O2 and HYP (10, 20 and 40 μM) (). Bcl-2 in HYP (10, 20 and 40 μM) groups were significantly increased than H2O2 group.
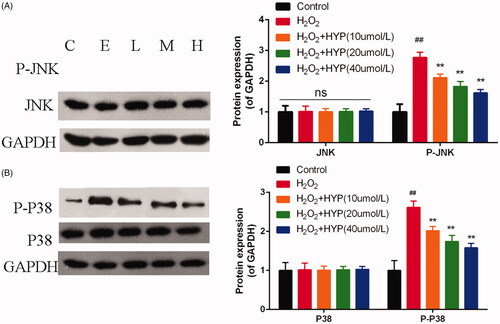
We next examine the protein expression of MAPK-related pathways (). When treated with H2O2, p-JNK and p-P38 were increased than control group (p < .05). However, compared with control group, HYP groups have lower expression of p-JNK and p-P38 with statistically significant (p < .05).
HYP supports and protects activation of P38 MAPK and PI3K-Akt signalling Cascades
As demonstrated in , treatment with SB203580, a p38 inhibitor, or LY294002, a PI3K inhibitor, in MC3T3-E1 cells decreased expression of p-p38 or p-Akt respectively with or without HYP. However, level of p-p38 or p-Akt in groups co-treated with HYP and inhibitors was lower than that of incubation within inhibitors alone. And the expression of total p38 and Akt remained unchangeable across the different treating groups respectively.
Figure 7. Immunoblotting showing the level of phosphorylated p38 and Akt in MC3T3-E1 cells incubated within HYP with or without SB203580 and LY294002.
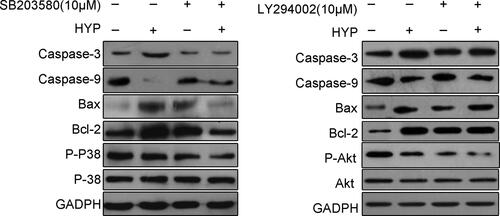
Moreover, we found that apoptosis-associated (Bax, Caspase-3 and Caspase-9) and anti-apoptotic protein (Bcl-2) was increased and decreased respectively in SB203580 or LY294002 with or without HYP. Thus, we concluded that HYP exhibited anti-apoptotic effects through P38 MAPK and PI3K-Akt signalling cascades.
Discussion
In this study, we found that: (1) HYP attenuated H2O2-induced MC3T3-E1 cells apoptosis; (2) H2O2 treatment in MC3T3-E1 cells significantly increased the expression of p-JNK and p-P38, which can be revised by HYP. Current study shown that HYP may be effective in providing protection against osteoporosis associated with oxidative stress.
Oxidative stress mainly through increase in osteocytes number and apoptosis, and thus leading to form the OP [Citation13]. Several studies have been identified that oxidative stress was the risk factor for OP in human beings [Citation14]. Thus, we used H2O2 as the model of oxidative stress. Recently, Chen et al. reported that HYP protects human kidney-2 cells against oxidative damage induced by oxalic acid [Citation9]. In our experiment, we used flow cytometry analysis to determine the apoptosis rates between these groups. When added H2O2, the apoptosis rate significantly increased than control group. When co-cultured with HYP, apoptosis rates were significantly decreased than H2O2 group. Park et al. [Citation15] found that HYP is an effective compound to protect cells against oxidative stress via haem oxygenase-1 (HO-1) induction. Yang et al. [Citation16] revealed that HYP protected the human primary melanocytes against oxidative damage through phosphoinositide 3-kinase/AKT signally pathways .
Bcl-2 is a family of evolutionarily related proteins. The Bcl-2 protein family is essential in the mitochondrial apoptosis pathway [Citation17]. Bcl-2 family included anti-apoptotic members and pro-apoptotic members. Several studies have demonstrated that the ratio of Bcl-2/Bax determines the fate of cells to apoptosis or survival. The decrease of Bcl-2/Bax induced the increase of mitochondrial permeability and the release of cytochrome c, which resulted in the activation of caspase-3 and apoptosis.
In this study, after treatment with H2O2, Bax, caspase-3 and caspase-9 were significantly increased, which indicated that H2O2 could increase the apoptosis rates. And Bcl-2 significantly decreased after H2O2 treatment compared with the negative control. When co-cultured with HYP, the Bcl-2 expression was significantly increased, which indicated that HYP could protect the MC3T3-E1 cells against the apoptosis induced by H2O2. Caspase-3 is cleaved and activated in the process of apoptosis. HYP significantly inhibited the caspas-3 expression induced by H2O2 in MC3T3-E1 cells, which demonstrated the anti-apoptotic effect of HYP on oxidative stress-induced MC3T3-E1 cells apoptosis.
H2O2, as an oxidative stress inducer, may activate ERK and p38 proteins in cells, which may be closely related to MAPK signalling pathway [Citation18,Citation19]. Xu et al. [Citation20] found that after treatment with H2O2, ERK and p38 proteins was activated. In our experiments, when treatment with H2O2, protein related to MAPK significantly increased than control group. Treatment with HYP notably inhibited the H2O2-induced increase in phosphorylated p38 and phosphorylated JNK levels. Moreover, we used p38 and PI3K inhibitors to further identify whether p38 MAPK and PI3K-Akt signalling cascades participate into the anti-apoptosis effects of HYP. When added SB203580 or LY294002, the p-p38 and p-Akt was decreased. When co-cultured with HYP with or without SB203580 or LY294002, the p-p38 and p-Akt was decreased than HYP alone. Thus, we speculated that HYP has anti-oxidative effects through regulating MAPK signalling.
Conclusion
HYP has anti-oxidative effects and mainly through the regulate of MAPK-mediated responses in MC3T3-E1 cells. These findings suggested that HYP protected MC3T3-E1 cells from oxidative stress-induced injury, which was likely associated with the prevention of OP. However, more studies should be focussed on the HYP receptor for regulating oxidative stress.
Abbreviation
ALP: alkaline phosphatase; a-MEM: α-modified minimal essential medium; COL-I: collagen I; FBS: foetal bovine serum; HYP: hyperoside; JNK: Jun N-terminal kinase; OCN: osteocalcin; OP: osteoporosis; ROS: reactive oxygen species.
Authors’ contributions
GX conceived the idea of this study. GX and ZD cultured cells and performed Western blot and qRT-PCR. HFS performed the staining assays. HFS and GX analyzed the data. GX wrote the manuscript, which was revised by all the other authors. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The ethics committee of Xuzhou Medical University affiliated Hospital of Lianyungang approved this study and consent to participate.
Availability of data and materials
Please contact author for data requests.
Disclosure statement
The authors declare that they have no competing interest.
References
- Cheng J, Wang H, Zhang Z, et al. Stilbene glycoside protects osteoblasts against oxidative damage via Nrf2/HO-1 and NF-kappaB signaling pathways. Arch Med Sci. 2019;15(1):196–203.
- Yao H, Yao Z, Zhang S, et al. Upregulation of SIRT1 inhibits H2O2-induced osteoblast apoptosis via FoxO1/β-catenin pathway. Mol Med Rep. 2018;17(5):6681–6690.
- Zhu XW, Ding K, Dai XY, et al. Beta-aminoisobutyric acid accelerates the proliferation and differentiation of MC3T3-E1 cells via moderate activation of ROS signaling. J Chin Med Assoc. 2018;81(7):611–618.
- Guo CC, Zheng LH, Fu JY, et al. Antiosteoporotic effects of Huangqi Sanxian decoction in cultured rat osteoblasts by proteomic characterization of the target and mechanism. Evid Based Complement Alternat Med. 2015;2015:1–10.
- Callaway DA, Jiang JX. Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab. 2015;33(4):359–370.
- Jian J, Sun L, Cheng X, et al. Calycosin-7-O-beta-d-glucopyranoside stimulates osteoblast differentiation through regulating the BMP/WNT signaling pathways. Acta Pharm Sin B. 2015;5(5):454–460.
- Schroder K. NADPH oxidases in bone homeostasis and osteoporosis. Free Radic Biol Med. 2019;132:67–72.
- Gong Y, Yang Y, Chen X, et al. Hyperoside protects against chronic mild stress-induced learning and memory deficits. Biomed Pharmacother. 2017;91:831–840.
- Zhang N, Ying MD, Wu YP, et al. Hyperoside, a flavonoid compound, inhibits proliferation and stimulates osteogenic differentiation of human osteosarcoma cells. PLoS One. 2014;9(7):e98973.
- Chen Y, Dai F, He Y, et al. Beneficial effects of hyperoside on bone metabolism in ovariectomized mice. Biomed Pharmacother. 2018;107:1175–1182.
- Zha X, Xu Z, Liu Y, et al. Amentoflavone enhances osteogenesis of human mesenchymal stem cells through JNK and p38 MAPK pathways. J Nat Med. 2016;70(3):634–644.
- Lv J, Sun X, Ma J, et al. Netrin-1 induces the migration of Schwann cells via p38 MAPK and PI3K-Akt signaling pathway mediated by the UNC5B receptor. Biochem Biophys Res Commun. 2015;464(1):263–268.
- Pereira CS, Stringhetta-Garcia CT, da Silva Xavier L, et al. Llex paraguariensis decreases oxidative stress in bone and mitigates the damage in rats during perimenopause. Exp Gerontol. 2017;98:148–152.
- Han D, Gu X, Gao J, et al. Chlorogenic acid promotes the Nrf2/HO-1 anti-oxidative pathway by activating p21(Waf1/Cip1) to resist dexamethasone-induced apoptosis in osteoblastic cells. Free Radic Biol Med. 2019;137:1–12.
- Park JY, Han X, Piao MJ, et al. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J Cancer Prev. 2016;21(1):41–47.
- Yang B, Yang Q, Yang X, et al. Hyperoside protects human primary melanocytes against H2O2-induced oxidative damage. Mol Med Rep. 2016;13(6):4613–4619.
- Hao XL, Kang Y, Li JK, et al. Protective effects of hyperoside against H2O2-induced apoptosis in human umbilical vein endothelial cells. Mol Med Rep. 2016;14(1):399–405.
- Ruffels J, Griffin M, Dickenson JM. Activation of ERK1/2, JNK and PKB by hydrogen peroxide in human SH-SY5Y neuroblastoma cells: role of ERK1/2 in H2O2-induced cell death. Eur J Pharmacol. 2004;483(2–3):163–173.
- Kwon SH, Kim JA, Hong SI, et al. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem Int. 2011;58(4):533–541.
- Xu Y, Yao H, Wang Q, et al. Aquaporin-3 attenuates oxidative stress-induced nucleus pulposus cell apoptosis through regulating the P38 MAPK pathway. Cell Physiol Biochem. 2018;50(5):1687–1697.