Abstract
Superparamagnetic iron oxide nanoparticles (SPIONs) have been employed in several biomedical applications where they facilitate both diagnostic and therapeutic aims. Although the potential benefits of SPIONs with different surface chemistry and conjugated targeting ligands/proteins are considerable, complicated interactions between these nanoparticles (NPs) and cells leading to toxic impacts could limit their clinical applications. Hence, elevation of our knowledge regarding the SPION-related toxicity is necessary. Here, the present review article will consider current studies and compare the potential toxic effect of SPIONs with or without identical surface chemistries on different cell lines. It centers on cellular and molecular mechanisms underlying toxicity of SPIONs. Likewise, emphasis is being dedicated for toxicity of SPIONs in various cell lines, in vitro and animal models, in vivo.
Introduction
Past decades have witnessed growing progress towards the application of nanomaterials in research, industry, and medicine Nanomaterials are engineered structures with 1–3 external dimensions ranging from 1 to 100 nm, while the term “nanoparticle (NP)” is used to descibe material having three external dimensions at a nanoscale level. These materials are increasingly being used for cosmetics, microelectronics, and drug carriers [Citation1].
All these engineered NPs show a common feature: their physiochemical and biological characteristics are considerably different as compares to those bulk materials of the similar constituents, allowing them to pose suitable characteristics, like conductivity, optical sensitivity and reactivity. However, undesirable effects like harmful interactions with biological systems-surroundings, with the possibility to generate toxicity can be exerted [Citation2].
Among various NPs, Superparamagnetic Iron Oxide Nanoparticles (SPIONs) are one of the most vital and fascinating metallic nanomaterials which expose valuable biomedical applications, such as targeted delivery of molecules or genes, stem cell tracking, magnetic resonance imaging (MRI), hyperthermia, tissue repair, transfection, magnetic separation technologies (e.g. rapid DNA sequencing), and in vivo cell tracking. SPIONs comprise magnetite (Fe3O4) or maghemite (g-Fe2O3) cores of about 5–20 nm, most often prepared by precipitation [Citation3]
SPIONs are primarily interesting due to their superparamagnetism, which means the particles do not display magnetic property without an external magnetic field. This behavior helps in preventing coagulation, which in-turn lowers the chance of agglomeration in vivo compared to other MNPs. They also have some unique characteristics such as high saturation field, high field irreversibility and extra anisotropy contributions or shifted loops following field cooling. Currently, there are several FDA-approved magnetite-based NPs including GastroMARK™; Umirem®, Feridex®/Endorem®, Feraheme®, Feridex I.V.R, and Gastromark®, as well as numbers of applicable SPIONs are in ongoing clinical trials [Citation4]. Thus, establishing test procedures to ensure safe fabrication and use of NPs, especially SPIONs, in the market place is urgently needed. This review presents an overview of recent in vitro and growing body of toxicity data to take an important step in this direction. The reader will find out that the toxicity data obtained change considerably depending on size distribution, surface modifications (including coating), and targeted-cell type.
Biomedical applications of SPIONs
SPIONs have been greatly attained due to their desirable characteristics, such as biocompatibility, biodegradability, and ease of fabrication. The superparamagnetic nature of SPIONs is also an important criterion influencing applications in biomedicine. SPIONs offer many uses in biomedicine; the most known are considered in different diagnostic and therapeutic applications, for example, as a contrast substance in magnetic resonance imaging (MRI), cell labeling, hyperthermic destruction of tumor tissue when subjected to alternating magnetic fields, drug delivery, and many more (). These particles have large magnetic moments and are widely used as T2 contrast agents in MRI. T2 agents alter the transverse relaxation times (T2) of water protons and provide dark negative signal intensity in images [Citation5].
Table 1. Some of the commercialized SPIONs which are used for different diagnostic and therapeutic applications.
In the field of targeted imaging and drug delivery, SPIONs can be guided to the demanded target area by means of an external magnetic field while concurrently tracking NPs biodistribution. This method makes them theragnositc (therapeutic and diagnostic) [Citation13]. Moreover, as magnetic cell labeling agents, commercially available SPIONs, in specific Endorem and Resovist, are most universally used. To date, NPs of both types are easily phagocyted by reticuloendothelial system (RES) Kupffer cells upon intravenous administration and are clinically developed for spleen and liver imaging by MRI [Citation14].
Hyperthermia has been also considered as one of the most promising non-invasive technique for cancer therapy because it can effectively kill cancer cells and simultaneously keeping normal cells intact. This is because tumor cells are more sensitive to temperature enhancement compared to normal cells [Citation15]. The mechanism of hyperthermia treatment involves the application of high temperature stress (usually around 41–46 °C) intracellularly, resulting in the activation and/or initiation of several extracellular and intracellular degradation mechanisms.
This degradation effects of hyperthermia include denaturation, folding, and aggregation of proteins, signal transduction alteration, apoptosis induction, changes in hydrogen potential, and reduction in the perfusion and oxygenation of the cancer [Citation16]. The National Cancer Institute (www.nci.nih.gov) has verified three kind of hyperthermia treatments including but not limited to local hyperthermia, regional hyperthermia, and whole body hyperthermia. In local hyperthermia small area of tumor tissue is heated using various techniques that heat-up the tumor. Large areas of tissue and whole body are heated in regional hyperthermia and whole body hyperthermia, respectively. Thus, MNPs are dispersed throughout the target tissue via direct injection or specific antibody targeting, after which the tissue is exposed to an alternating magnetic field. This field causes the particles to generate heat by magnetic relaxation mechanisms [Citation4,Citation17].
Potential mechanisms of SPIONs toxicity
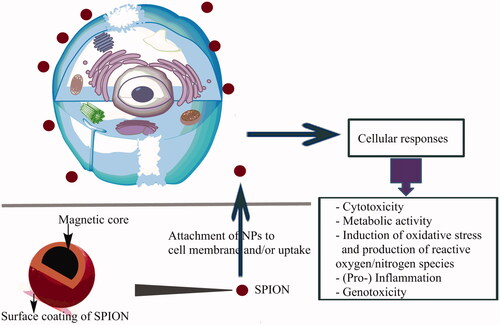
Characterization of physical properties, such as size and surface charge, together with in vitro assays for reactive oxygen species (ROS) production and induction of oxidative stress-mediated responses, such as inflammation and mitochondria-mediated apoptosis, plus in vivo markers of oxidative stress, such as lipid peroxidation and signature cytokines, are famous examples of a predictive paradigm for toxicity screening [Citation18]. Additionally, some of the nanomaterial interactions may also lead to other forms of injury, such as membrane damage, protein denaturation, genotoxicity, immune reactivity, and foreign body granulomas formation () [Citation19]. They have also been reported to cause cellular perturbations like actin cytoskeleton modulation, dysregulation of gene expression profiles, disturbance in iron homeostasis, as well as alteration of cellular responses like signaling pathways activation and cell cycle regulation impairment [Citation20]. It is also possible that new nanomaterial properties may emerge unknown mechanisms of toxicity. However, there is a strong likelihood that biological activities will depend on physicochemical properties that are not commonly measured in toxicity screening studies. Thus, any test instance must aim to characterize the test material as regards chemical composition (purity, crystallinity, electronic properties, etc.), size (distribution and surface area), surface structure (functional groups, reactivity, and inorganic/organic coatings, etc.), shape, solubility, and aggregation.
ROS-mediated toxicity
Due to unique physicochemical characteristics, SPIONs display a larger surface area for the generation of free radicals. The most intracellular and in vivo toxicities of SPIONs arise from extreme production of ROS, such as free radicals like hydroxyl radicals, superoxide anion, and the nonradical hydrogen peroxide, which have the potential to affect the protein and lipid materials of the cell, and cellular organelles (mitochondria, nucleus, and endoplasmic reticulum) [Citation21]. Enzymatic activity in the body can degrade the surface of NP and thereby the leached iron ions causing ROS generation. Subsequently, the ROS internalize in cells where they can cause oxidative stress by activating pro-inflammatory mediators. Furthermore, ROS due to reaction with macromolecules can damage cells by peroxidizing lipids, altering proteins, damage to the genetic materials, interfering with signaling functions, and modulating gene transcription and finally leading to cell death either by necrosis or apoptosis [Citation22].
Toxicity mediated by accumulation in the tissues
Another mechanism of SPIONs toxicity arises from enhanced accumulation of the SPIONs in different storage sites of the body. Since SPIONs should magnetically target a specific tissue/organ in biomedical application, high iron concentration could be localized in the same tissue/organ [Citation23]. Of note, a large number of studies have been demonstrated that at concentrations of 100 g/mL or higher, SPIONs with altering physicochemical properties may cause toxicity or cytotoxicity. Subsequently, high concentration of free Iron ions in that tissue could lead to homeostasis imbalance and aberrant cellular responses such as oxidative stress, cytotoxicity, epigenetic events, DNA damages, and inflammatory processes, which would consequently result in carcinogenesis or have a significant effect on subsequent generations. One of the key mechanisms is that iron, as a reactant and as a catalyst, may trigger free radical production, which can promote the proteins oxidation, membrane lipids peroxidation, and modification of nucleic acids [Citation24].
Effect of surface coating on SPIONs toxicity
It is well recognized that optimizing the physicochemical parameters is highly effective to minimize the immune response and toxicity of SPIONs. Characteristic parameters of SPIONs, including charge, surface coating, and morphology, influence the efficacy of SPIONs and also their biomedical fate within cells [Citation9,Citation25]. Proper surface coatings can stabilize iron oxide NPs and avoid agglomeration. It is also an efficient way of preventing the dissolution and release of toxic ions [Citation9]. In this regards, Malvindi et al. compared the cytotoxicity of bare SPIONs and thin silica shell-coated SPIONs (Fe3O4/SiO2 NPs) on A549 and HeLa cells. They showed that surface engineering of Fe3O4 NPs can reduce the oxidative stress and iron homeostasis alteration and, thus, the overall toxicity, in spite of bare and passivated NPs showing similar cell internalization efficiency. It was observed from their study that the toxicity of bare NPs was as a result of their stronger in situ degradation, with larger intracellular release of Fe3+ compares to surface passivated Fe3O4/SiO2 NPs. They confirmed that Fe3O4/SiO2 NPs surface engineering is essential in improving the stability of particles in biological environments reducing both genotoxic and cytotoxic effects [Citation26].
Pongrac et al. analyzed the levels of ROS, mitochondrial membrane potential, intracellular glutathione, DNA damage, cell-membrane potential, and activities of glutathione peroxidase (GPx) and Superoxide dismutase (SOD), to show the potential mechanism of toxicity of uncoated SPIONs, coated with d-mannose, or coated with polyl-lysine on murine neural stem cells (NSCs). Their results indicated all tested SPION types affected the NSCs similarly, signifying that mitochondrial homeostasis is their main cellular target [Citation18]. Mahmoudi et al. also considered the effects of bare and polyvinyl alcohol (PVA) coated SPIONs on mouse primary fibroblast (L929) cell line, and reported that the coated SPIONs at highest doses (400 mM) present acceptable cell viability levels. A medium dose (200 mM) of the PVA-coated SPIONs fails to show cell cycle arrest or apoptosis but at doses of 400 mM apoptosis and cell cycle alterations were observed because of protein corona formation and DNA damages [Citation5].
Effect of cell “vision” on SPIONs toxicity
An important issue regarding SPIONs is various toxic effects induced by same SPIO NPs with identical physicochemical properties among different organs and cell types. A possible justification could be attributed to concept of “cell vision” which is to the first contact point of cells with the surface of nanomaterial. This point of contact is the cell’s membrane sugar, proteins, and the phospholipid components, which together define how the cell “sees” NPs [Citation27,Citation28]. Laurent et al. evaluated the effect of SPIONs with various surface chemistries on different cell lines and showed that the uptake of SPIONs and their corresponding side effects are strongly dependent on the cell type. For instance, the identical concentration of SPIONs which caused significant toxicity on the brain-derived neuronal glial and lung cells caused a minute toxicity on the other cell types. There are also similar in vitro studies that reported effect of cell vision on SPIONs toxicity [Citation25,Citation29].
In vitro toxicity response of SPIONs
Growing evidence has shown that uptake and biomedical fate—toxic or non-toxic effects—of SPIONs are primarily dependent on their size, surface modifications and dose, as well as exposed-cell type. Laurent et al. investigated cytotoxicity effects of carboxyethylsilanetriol (CES)-coated SPIONs on a panel of various cell lines from different origins including the brain, lung, heart, liver, kidney, skin, colon, and cervix. Their results showed that the same concentration of CES-coated SPIONs which resulted in significant toxicity against the brain-derived neuronal and glial cells and lung cells caused very low toxicity on the other cell types. CES-coated SPIONs exerted toxic effects on neuronal and lung cells at a concentration of 2 mM while reduced the cell viability of glial cells by less than 80% at a particle concentration of 4 mM. It was also found that CES-coated SPIONs at the highest assayed concentration of 32 mM showed significant toxicity for the majority of the cell lines. These results can confirm cell-type dependency of cellular uptake and toxicity of SPIONs [Citation28].
Charges of cellular membrane is one of the most vital factors that determines uptake of NPs, hence, various cell types with distinct membrane charge can exhibit different cellular response. Hereupon, surface charge of NPs and treated cells can significantly affect uptake and subsequent cellular fate of these particles. Investigation of the cellular toxicities of the bare and surface-modified SPIONs (COOH and/or NH2) in three human cell lines including BE-2-C (brain), HCM (heart), and 293 T (kidney) which showed that positively charged SPIONs-NH2 exerted higher cell toxicity, in comparison to negatively charged SPIONs-COOH [Citation25]. It is underpinned by another in vitro study that showed positively charged amino polyvinyl alcohols (PVAs)-SPIONs were adhered to microglial cells and isolated brain-derived endothelial cells at a much higher level than other SPIONs with negative or neutral surface charge [Citation30]. Higher cytotoxicity of positive SPIONs was suggested to be due to their significant and considerable cellular uptake rate in comparison with negative SPIONs, which can be referred to Coulombic interaction of negatively charged cellular membranes with positively charged SPIONs [Citation25].
The effect of particle surface charge and exposed cell type on toxicity profile of SPIONs is further supported by an in vitro study on human alveolar epithelial cells that showed SPIONs coated with either negative (COO−) or positive (NH3+) functional groups can be used as nontoxic agents at the high concentrations (1 and 2 mg/mL), while non-toxic levels of bare SPIONs, whose surfaces are highly rich in hydroxyl groups, ranged at concentration below 100 μg/mL. Therefore, it was suggested that the presence of hydroxyl groups impacts cellular toxicity of SPIONs in alveolar epithelial cells [Citation31]. In the view-point of toxic dose, these results are in accordance with findings of other cytotoxicity studies on SPIONs, that demonstrated SPIONs with several physicochemical properties, which exhibited no cytotoxicity when applied in a concentration range less than 100 μg/mL [Citation32].
It is well-documented that it is not only the efficacy of SPIONs that are highly reliant on their surface charges, but also their fate within cells. Actually, the positive SPIONs, after cellular uptake, dwell in lysosomes or endosomes where they disrupt proton pump balance which leads to lysosomal swelling and rupture resulting in iron deposition in the cytoplasm. Lysosomal-deposited SPIONs may also be degraded into free iron ions which are released to the cytoplasm and finally contributes to the cellular iron pool. The successive fate of the iron and its participation in cellular viability and physiology is very complicated, and extends from the induction of cell proliferation to alterations in the ferritin expression and production of ROS as a frequent consequence of intracellular SPION processing [Citation28]. It is important to note that SPIONs modify genes related to cell proliferative responses as a result of their ROS characteristics [Citation28,Citation33]. ROS are generated inside lysosomes acidic environment via the reaction of free iron in the form of Fe2+ with lead and hydrogen peroxide to the production of hydroxyl radicals (Fenton reaction) [Citation34]. Following intracellular release, the free iron can cross the mitochondrial or nuclear membrane. The hydrogen peroxide and oxygen produced in the mitochondria also undergoes the Fenton reaction, producing ferric ions (Fe3+) and hydroxyl radicals. The hydroxyl radicals might then damage the DNA indirectly, proteins and lipids. Overall, it was reported that different types of cells show different ROS levels subject to the pathways use to defend themselves against SPIONs [Citation28,Citation33].
represents the researches where in vitro toxicity of SPIONs is observed by different cytotoxicity assays. This table considers effects of SPIONs with various physicochemical characteristics on both various human and nonhuman cell lines. The viability was measured after different time point. This table is segmented according to the cells or tissue type used and corresponding research publications cited against them. Results of these studies show (1) the surface engineering can exert vigorous effects on NP stability, aggregate size, and cellular interaction, and considerably affecting the fate and SPIONs uptake in intercellular medium. (2) Toxicity in response to uptake of SPIONs was strongly cell type dependent. Also, the responses of various tested cell lines to SPIONs could be considerably different. (3) The number of NPs per cell (independent of concentration) as the number of cells tested might also affect the toxicological results.
Table 2. Cytotoxicity of Fe3O4 nanoparticles in different cell lines.
SPIONs toxicity in different tissues; in vivo studies
Cell-culture studies are the first step towards understanding an agent reaction in the body. Though the toxicity studies of SPIONs by in vitro assays are more cost-effective, faster, and easier without ethical problems, in vivo experiments in animal models are indispensable for the investigation of SPION biological effects. Interestingly, there is a low correlation between in vivo and in vitro toxicity of SPIONs, and it has been found that in vitro toxicity of SPIONs is not exactly translated in vivo [Citation43]. These contradictory results can be due to regulation of changes in pH, ionic strength and chemical composition of the circulating blood by the liver and kidney hemostasis in the body. However, the cell cultures are extremely sensitive to changes in their environment such as fluctuations in temperature, pH, and nutrient and waste concentrations. Also, insufficient pretreatment of the particle dispersion is an important factor that can lead to erroneous results or artefacts. Moreover, NPs can adsorb dyes and be redox active; therefore, controlling the experimental conditions and choice of an appropriate cytotoxicity assay are important factors to ensure that the cell death measured relates to the added NPs toxicity versus the unstable culturing conditions [Citation44].
In vivo experiments present several advantage for toxicity studies, including determination of the toxico-kinetics in the body (i.e. absorption, distribution, metabolism, and excision) and investigating the neurological, reproductive, immunological, cardiovascular and developmental toxicities to determine the chronic systemic toxicity of SPIONs [Citation45]. As concluded from in vivo assays, SPIONs can be categorized into biocompatible and nontoxic particles, however, these particles have been found to exert the slight toxic effects in a dose-dependent manner [Citation47]. It was reported that intravenous administration of dextran-coated SPIONs (Ferumoxtran-10) at a dose of 2.6 mg/kg in rat promoted no hemodynamic alterations in animals, whereas dose elevation up to 13 mg Fe/kg considerably led to intensified aortic blood flow but importantly exerted no cardiovascular or respiratory toxicity. Hematological parameters were moderately altered when a repeat-dose of 17.9 mg/kg (3–5 times) were intravenously injected in rats. Some treatment-related clinical toxicities were recognized when administrated a very high single dose up to 126 mg/kg which is 45 folds greater than that used as MRI contrast agents in human. As revealed by neurotoxicity study, ferumoxtran-10 exerted some minor adverse effects on the central nervous system, including a diminished or elevated activity of spontaneous locomotor, mydriasis or exophthalmos, and rearing [Citation48].
A mouse study showed that subcutaneous injection of SPIONs at a dose of 100 mg/kg could exert a low toxicity profile, without treatment-related deaths and light transient clinical signs. Administrated SPIONs were found to be distributed in brain, heart, liver, lungs, kidneys, and spleen diminishingly within 24 h after injection. After repeated injections for 10 days, the deposition of iron in the studied organs was very low, showing that the iron was quickly cleared at 100 mg/kg subcutaneously administrated in mice. It was suggested that demonstrated low hazard effects in mice following acute injection of SPIONs are unlikely to be translated in clinical practice because of the single low dosing in human [Citation49].
In vivo toxic response of SPIONs has also been found to be tissue dependent. For example, a rat study exhibited that a single intravenous injection of SPIONs (0.8 mg/kg) in rat could promote toxic effects in the liver, lung, and kidney organs whereas heart and brain remained unaffected [Citation46]. It is well supported by another in vivo study that showed intravenous administration of SPIONs at a dose of 10 mg/kg in rat increased oxidative stress in liver, spleen, and kidney, peaking at about 3 days and then slowly decreasing afterward, while no significant changes were observed for lung, brain, and heart tissues. The results showed a tissue-dependent toxic response, with liver showing a marginal elevation of oxidative stress and returning to normal condition within 1 week, while the kidney and spleen took 3 weeks to return to normal condition. Interestingly, there were no apparent histopathological changes in the cellular structures of the liver, spleen, and kidney at 1 day or 7 days following SPIONs injection. Furthermore, despite the injected SPIONs predominantly accumulate in the liver, the persistent iron overload has been demonstrated not to damage the liver function nor cause noticeable immunotoxicity responses in the health models [Citation47].
Clinical evidence
An additional aspect about SPIONs needing to be mentioned is detailed understanding of the behavior of nanoparticles in living systems that is partially known or predictable. In this regard, Richards et al. labeled, for the first time, human peripheral blood mononuclear cells (PBMC) under Good Manufacturing Practice (GMP)-compliant conditions with Feridex® (Endorem®) without affecting their viability or function. Afterward, SPION-labeled cells were intravenously administrated. The results showed that MRI cell tracking using iron oxide is clinically safe and 1–5 × 109 SPION-labeled autologous PBMCs can be given safely: 100-fold greater than that needed for detection on MRI scanning [Citation54]. Furthermore, there are other trials that probed Feridex® in humans and recognized it as a safe and efficient agent. The most frequent side effect was focused on back pain, which was detected in 4% of patients. Although lumbar pain has been associated with administration of a variety of colloids and emulsions, the physiological causes are unknown, because no significant changes in chemistry values, vital signs, and electrocardiographic findings were found [Citation4]. Vadhan-Raj et al. assessed the safety and efficacy of ferumoxytol® for the episodic treatment of iron deficiency anemia (IDA) over a 6-month period (NCT01114217). Their study demonstrated Ferumoxytol could be well tolerated with no new safety signals identified among patients who received repeat dosing. The most frequent side effect was focused on headache, which was detected in 7% of patients and urinary tract infection 5.6% of these. Moreover, nausea, vomiting, dizziness, diarrhea, nasopharyngitis, fatigue, back pain, constipation, hypokalemia, cough, were found in 2–5% of patients, respectively [Citation55].
To sum up, Aforementioned in vivo findings and clinical evidence suggest that SPIONs can be considered as biocompatible and nontoxic particles, although administration of high doses of iron may accompany with the chronic iron toxicity in human ().
Table 3. Toxicity results of SPIONs on various tissues; in vivo studies.
Conclusion
Despite the use of multifunctional SPIONs with the potential for biomedicine is going to dramatically enhance the coincident diagnosis and local therapeutic applications, two important issues need to be considered when selecting SPIONs for in vivo applications: First, the relevant side effects and the fate of the SPIONs, i.e. elimination route or retention time in the body system if they are biodegradable. Because, once the surface-derivatized NPs are inside the cells, the coating is likely digested, leaving the bare particles exposed to other cellular components and organelles, thereby potentially influencing the overall integrity of the cells. The second one is their destabilization due to the adsorption of plasma proteins and their non-specific uptake by the RES, which may cause agglomeration and recognition by innate immune system, respectively. Some studies had hypothesized that rigid coatings are suitable candidates to overcome these shortcomings. Moreover, for NPs translation to medical application, including superparamagnetic particles, with unique physicochemical properties, there are fundamental but undoubtedly acceptable barriers given by the regulatory bodies has to be overcome. In this regard, this study tried to compare cytotoxicity of NPs with iron oxide as core and a biocompatible polymer as first layer. Although, there are not adequate examples of a case studies about SPIONs that were performed in the past, where the same particles were tested with the various cell lines using the same method/protocol, which would allow real comparison and estimation of the experimental blunder between different research team but since SPIONs show a cell-specific response, this article has connived this issue to consider side effects of SPIONs toxicity depend on dose, concentration, and tissue susceptibility for safe medical applications. Authors propose to future researches that study various cells/tissues exposed to SPIONs presenting identical physicochemical properties to estimate susceptibility of cells/tissues to used dose of NPs to allow NPs engineering is cleverly performed directive and effective.
Disclosure statement
The authors declare that there are no conflicts of interest and financial support for the present review article.
References
- Vakili‐Ghartavol R, Mombeiny R, Salmaninejad A, et al. Tumor‐associated macrophages and epithelial–mesenchymal transition in cancer: nanotechnology comes into view. J Cell Physiol. 2018;233(12):9223–9236.
- Canu IG, Schulte PA, Riediker M, et al. Methodological, political and legal issues in the assessment of the effects of nanotechnology on human health. J Epidemiol Commun Health. 2018;72(2):148–153.
- Wang F, Lu C-H, Willner I. From cascaded catalytic nucleic acids to enzyme–DNA nanostructures: controlling reactivity, sensing, logic operations, and assembly of complex structures. Chem Rev. 2014;114(5):2881–2941.
- Fubini B, Ghiazza M, Fenoglio I. Physico-chemical features of engineered nanoparticles relevant to their toxicity. Nanotoxicology. 2010;4(4):347–363.
- Mosayebi J, Kiyasatfar M, Laurent S. Synthesis, functionalization, and design of magnetic nanoparticles for theranostic applications. Adv Healthcare Mater. 2017;6(23):1700306.
- Ramirez-Nuñez A, Jimenez-Garcia L, Goya G, et al. In vitro magnetic hyperthermia using polyphenol-coated Fe3O4@ γFe2O3 nanoparticles from Cinnamomun verum and Vanilla planifolia: the concert of green synthesis and therapeutic possibilities. Nanotechnology. 2018;29(7):074001.
- Stark WJ, Stoessel PR, Wohlleben W, et al. Industrial applications of nanoparticles. Chem Soc Rev. 2015;44(16):5793–5805.
- Mahmoudi M, Milani AS, Stroeve P. Synthesis, surface architecture and biological response of superparamagnetic iron oxide nanoparticles for application in drug delivery: a review. IJBNN. 2010;1(2/3/4):164–201.
- Shagholani H, Ghoreishi SM, Sharifi SH. Conversion of amine groups on chitosan-coated SPIONs into carbocyclic acid and investigation of its interaction with BSA in drug delivery systems. J Drug Deliv Sci Technol. 2018;45:373–377.
- Mahmoudi M, Sant S, Wang B, et al. Superparamagnetic iron oxide nanoparticles (SPIONs): development, surface modification and applications in chemotherapy. Adv Drug Deliv Rev. 2011;63(1-2):24–46.
- Mohammed L, Gomaa HG, Ragab D, et al. Magnetic nanoparticles for environmental and biomedical applications: a review. Particuology. 2017;30:1–14.
- Mahmoudi M, Hofmann H, Rothen-Rutishauser B, et al. Assessing the in vitro and in vivo toxicity of superparamagnetic iron oxide nanoparticles. Chem Rev. 2012;112(4):2323–2338.
- Nune SK, Gunda P, Thallapally PK, et al. Nanoparticles for biomedical imaging. Expert Opin Drug Deliv. 2009;6(11):1175–1194.
- Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62(3):284–304.
- Andreas K, Georgieva R, Ladwig M, et al. Highly efficient magnetic stem cell labeling with citrate-coated superparamagnetic iron oxide nanoparticles for MRI tracking. Biomaterials. 2012;33(18):4515–4525.
- Li L, Jiang W, Luo K, et al. Superparamagnetic iron oxide nanoparticles as MRI contrast agents for non-invasive stem cell labeling and tracking. Theranostics. 2013;3(8):595–615.
- Liao S-H, Liu C-H, Bastakoti BP, et al. Functionalized magnetic iron oxide/alginate core-shell nanoparticles for targeting hyperthermia. Int J Nanomed. 2015;10:3315–3327.
- Verma J, Lal S, Van Noorden CJ. Nanoparticles for hyperthermic therapy: synthesis strategies and applications in glioblastoma. Int J Nanomed. 2014;9:2863–2877.
- Laurent S, Dutz S, Häfeli UO, et al. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166(1–2):8–23.
- Pongrac IM, Pavičić I, Milić M, et al. Oxidative stress response in neural stem cells exposed to different superparamagnetic iron oxide nanoparticles. Int J Nanomed. 2016;11:1701–1715.
- Clift MJ, Gehr P, Rothen-Rutishauser B. Nanotoxicology: a perspective and discussion of whether or not in vitro testing is a valid alternative. Arch Toxicol. 2011;85(7):723–731.
- Liu Y, Li J, Xu K, et al. Characterization of superparamagnetic iron oxide nanoparticle-induced apoptosis in PC12 cells and mouse hippocampus and striatum. Toxicol Lett. 2018;292:151–161.
- Singh N, Jenkins GJ, Asadi R, et al. Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1(1):5358.
- Møller P, Jacobsen NR, Folkmann JK, et al. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44(1):1–46.
- Lei L, Ling-Ling J, Yun Z, et al. Toxicity of superparamagnetic iron oxide nanoparticles: research strategies and implications for nanomedicine. Chin Phys B. 2013;22(12):127503.
- Laffon B, Fernández-Bertólez N, Costa C, et al. Cellular and molecular toxicity of iron oxide nanoparticles. In: Cellular and molecular toxicology of nanoparticles. Berlin (Germany): Springer; 2018. p. 199–213.
- Elias A, Tsourkas A. Imaging circulating cells and lymphoid tissues with iron oxide nanoparticles. ASH Educ Program Book 2009;2009(1):720–726.
- Kawanishi M, Ogo S, Ikemoto M, et al. Genotoxicity and reactive oxygen species production induced by magnetite nanoparticles in mammalian cells. J Toxicol Sci. 2013;38(3):503–511.
- Walsh TJ, Finberg RW, Arndt C, et al. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N Engl J Med. 1999;340(10):764–771.
- Mahmoudi M, Laurent S, Shokrgozar MA, et al. Toxicity evaluations of superparamagnetic iron oxide nanoparticles: cell “vision” versus physicochemical properties of nanoparticles. ACS Nano. 2011;5(9):7263–7276.
- Liu WH, Liu SM, Lin SF, et al. Role of berberine in fibronectin expression via S1P2-MAPK signaling pathway in diabetic nephropathy. Chin Pharmacol Bull. 2013;29(5):723–728.
- Malvindi MA, De Matteis V, Galeone A, et al. Toxicity assessment of silica coated iron oxide nanoparticles and biocompatibility improvement by surface engineering. PLoS One 2014;9(1):e85835.
- Mahmoudi M, Simchi A, Imani M. Cytotoxicity of uncoated and polyvinyl alcohol coated superparamagnetic iron oxide nanoparticles. J Phys Chem C. 2009;113(22):9573–9580.
- Laurent S, Burtea C, Thirifays C, et al. Crucial ignored parameters on nanotoxicology: the importance of toxicity assay modifications and “cell vision”. PLoS One 2012;7(1):e29997.
- Mahmoudi M, Saeedi-Eslami SN, Shokrgozar MA, et al. Cell “vision”: complementary factor of protein corona in nanotoxicology. Nanoscale 2012;4(17):5461–5468.
- Cengelli F, Maysinger D, Tschudi-Monnet F, et al. Interaction of functionalized superparamagnetic iron oxide nanoparticles with brain structures. J Pharmacol Exp Ther. 2006;318(1):108–116.
- Mbeh DA, Mireles LK, Stanicki D, et al. Human alveolar epithelial cell responses to core–shell superparamagnetic iron oxide nanoparticles (SPIONs). Langmuir 2015;31(13):3829–3839.
- Ankamwar B, Lai T-C, Huang J-H, et al. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 2010;21(7):075102.
- Karlsson HL, Cronholm P, Gustafsson J, et al. Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol. 2008;21(9):1726–1732.
- KöNczöL M, Ebeling S, Goldenberg E, et al. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: role of ROS, JNK, and NF-κB. Chem Res Toxicol. 2011;24((9):1460–1475.
- Soenen SJ, De Cuyper M. Assessing cytotoxicity of (iron oxide‐based) nanoparticles: an overview of different methods exemplified with cationic magnetoliposomes. Contrast Media Mol Imaging. 2009;4(5):207–219.
- Raynal I, Prigent P, Peyramaure S, et al. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39(1):56–63.
- Moore A, Marecos E, Bogdanov A, Jr., et al. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214(2):568–574.
- van Landeghem FKH, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009; 30(1):52–57.
- Lewinski NA. Biodistribution of cadmium selenide/zinc sulfide quantum dots in aquatic organisms. Houston (TX): Rice University; 2011.
- Lewinski N, Colvin V, Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4(1):26–49.
- Sharifi S, Behzadi S, Laurent S, et al. Toxicity of nanomaterials. Chem Soc Rev. 2012;41(6):2323–2343.
- Hanini A, Schmitt A, Kacem K, et al. Evaluation of iron oxide nanoparticle biocompatibility. Int J Nanomed. 2011;6:787–794.
- Jain TK, Reddy MK, Morales MA, et al. Biodistribution, clearance, and biocompatibility of iron oxide magnetic nanoparticles in rats. Mol Pharma. 2008;5(2):316–327.
- Reddy LH, Arias JL, Nicolas J, et al. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem Rev. 2012;112(11):5818–5878.
- Muldoon LL, Sàndor M, Pinkston KE, et al. Imaging, distribution, and toxicity of superparamagnetic iron oxide magnetic resonance nanoparticles in the rat brain and intracerebral tumor. Neurosurgery. 2005;57(4):785–796.
- Liu SY, Han Y, Yin LP, et al. Toxicology studies of a superparamagnetic iron oxide nanoparticle in vivo. Adv Mater Res. 2008; 47–50, 1097–1100.
- Richards JM, Shaw CA, Lang NN, et al. In vivo mononuclear cell tracking using superparamagnetic particles of iron oxide: feasibility and safety in humans. Circ Cardiovasc Imaging. 2012;5(4):509–517.
- Mahmoudi M, Simchi A, Imani M, et al. Superparamagnetic iron oxide nanoparticles with rigid cross-linked polyethylene glycol fumarate coating for application in imaging and drug delivery. J Phys Chem C. 2009;113(19):8124–8131.
- Vadhan‐Raj S, Ford DC, Dahl NV, et al. Safety and efficacy of ferumoxytol for the episodic treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy: results of a phase III, open‐label, 6‐month extension study. Am J Hematol. 2016;91(2):E3–E5.