?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Abdominal wall defects are associated with abdominal wall surgery, infection and tumour resection. Polypropylene (PP) mesh, which has excellent mechanical strength, is currently the primary clinical repair material. In repairing the abdominal wall, the mesh can erode the bowel and cause other problems. Constructing a barrier that induces a weak inflammatory response and promotes rapid recovery of the peritoneum is important. We used electrospinning technology to construct a silk fibroin coating on the abdominal surface of a PP patch. A rat model was used to compare the inflammatory responses, regeneration of peritoneal tissue, and antiadhesion effects of electrospun regenerated silk fibroin (RSF) coatings, polycaprolactone (PCL) coatings, and noncoated PP meshes. The inflammatory responses, antiadhesion fractions, and areas of RSF and PCL were better than those of PP at 6 weeks. RSF was associated with complete peritoneal regeneration, in contrast to PCL. At 12 weeks, the structure of the PCL peritoneum was unstable, and the adhesion fraction and area were significantly higher than those of RSF. The intact peritoneum could not be effectively regenerated. The RSF group exhibited lower IL-6 levels than the PCL and PP groups but higher VEGF, IL-10 and TGF-β levels, making RSF more conducive to the regeneration of peritoneal and abdominal wall tissues.
Introduction
Abdominal wall defects caused by ventral hernia, trauma, infection, and tumour resection are frequent problems in abdominal surgery [Citation1,Citation2]. Reconstruction of these defects can be challenging if tissue loss is significant and can result in surgery failure. Tissue repair is typically achieved using synthetic, nonabsorbable meshes, which leads to a considerable reduction in the rate of recurrence [Citation3]. However, artificial prostheses are permanent foreign materials in the body and can cause chronic inflammation, fibrosis, and other complications [Citation4].
After general abdominal surgery, abdominal peritoneal adhesions are reported in 67–93% of patients who receive an artificial synthetic mesh [Citation5]. These adhesions cause severe morbidity due to complications, such as intestinal obstruction, enterocutaneous fistulas, and pain [Citation6].
Large abdominal wall defects are often accompanied by the absence of a peritoneum, and polypropylene (PP) and other materials in an artificial mesh are directly in contact with the internal organs of the abdominal cavity [Citation7]. However, the surface of the large structural mesh is not smooth, causing damage to the intestinal mucosa and severe inflammatory reactions [Citation8,Citation9]. Severe intra-abdominal adhesions are prone to complications, such as intestinal obstruction and bowel spasms, and increase the risk of secondary or abdominal surgery [Citation10]. Current knowledge of the pathophysiology of adhesion formation has led to two major preventative approaches: the use of pharmacological agents to arrest the cascade of events leading to adhesion formation and the implementation of a physical barrier to separate the peritoneum from the underlying organs during the critical period of peritoneal healing [Citation9]. Several biodegradable materials assembled in layers are currently used as barriers. Although such layers can reduce the inflammatory response, they lack the sites and microstructures that favour the adhesion and migration of surrounding myoblasts, fibroblasts, and mesothelial cells [Citation11,Citation12]. Seprafilm, genzyme, and polylactide (PLA) are biodegradable solid barrier devices that have been commercialized [Citation13]. These films are difficult to apply, cover the tissue surface with contours, and adhere instantly to any moist surface, including the surgeon’s gloves, which makes their handling difficult during laparoscopic surgery [Citation14,Citation15].
Electrostatic fields are used to prepare nanosized fibres, which have a high porosity and a large specific surface area, similar to extracellular matrix collagen fibres [Citation16]. They are widely used in skin and bone tissue regeneration, can imitate the natural extracellular matrix to the greatest extent and can facilitate cell adhesion. At the same time, the processing parameters can be altered to optimize the three-dimensional structure of the material [Citation17,Citation18]. Various studies have shown that nanoscale microstructures constructed by electrospinning can promote the adhesion and migration of osteoblasts, fibroblasts, skeletal muscle stem cells, adipose stem cells, etc. as well as facilitate the exchange of substances between cells and promote abdominal tissue continuity [Citation19,Citation20]. In addition, by adjusting the electrospinning parameters, dense fibre membranes with pore diameters of 3–5 μm can be obtained, and this structure can effectively form barriers and isolate abdominal organs and rough PP patches [Citation21].
Regenerated silk fibroin (RSF) is a nonphysiological natural biological macromolecule derived from silk that has obvious advantages over other natural macromolecules [Citation22]. Furthermore, RSF has good biocompatibility and is nontoxic, nonpolluting, nonirritating, and biodegradable [Citation23]. Studies have shown that RSF facilitates cell adhesion while reducing inflammation [Citation24]. All of these characteristics have important implications for reducing postoperative complications, such as those associated with meshes. An experimental study on urethral repair by Wei et al. showed that polyelectrolyte/silk fibroin/collagen hybrid electrospun materials have better biocompatibility than nonhybrid materials and are conducive to the growth of mucosal epithelial cells [Citation25].
In this study, we fabricated electrospun-coated membranes of composite RSF with a PP material to promote tissue regeneration at the early peritoneal surface and reduce mesh and intra-abdominal adhesions. We hypothesize that silk fibre can potentially be translated into clinical practice as an alternative for abdominal wall repair.
Materials and methods
Mesh preparation
We first chose a type of PP mesh (produced by the Institute of Textiles, Donghua University), and its mechanical strength and parameters are shown in . The mesh was cut into 10 × 10 cm samples and fixed on the receiving platform of the electrospinning machine (self-developed by the Institute of Textiles, Donghua University, schematic). Next, 8%, 10%, 12%, 14%, 15% and 17% RSF in formic acid solutions were developed. Spinning was performed using different concentrations of RSF with a spinning time of 3 h, a voltage of 20 kV, and a receiving distance of 15 cm. After completion, the samples were treated with 75% ethanol for 1 h and then removed, washed twice with phosphate-buffered saline (PBS) and sealed in a sterile Petri dish (). PCL with a spinning time of 2 h, a voltage of 18 kV, and a receiving distance of 17 cm was prepared for the control group.
Table 1. The mechanical strength and parameters of the PP mesh.
Scanning electron microscopy
The RSF fibre was histologically examined to assess its stability. Briefly, the scaffold was prefixed with 2% glutaraldehyde for 2 h at 4 °C, washed twice with PBS, and post fixed in 1% osmic acid for 2 h at 4 °C. After washing twice with PBS, the samples were dehydrated with an ethanol gradient and dried to a critical point (HCP-2, Hitachi, Tokyo, Japan). The samples were then mounted, sputter-coated with gold (BAL-TEC, Philips, Amsterdam, Netherlands), and examined with a scanning electron microscope (Philips-XL-30, Philips, Amsterdam, Netherlands).
Tensile fracture strength test of the fibre membrane
Five pieces of 0.5 × 3 cm rectangular samples were uniformly cut from the prepared fibre membranes. The tensile strength of the rectangular specimen was tested using a medical fabric multifunctional strength tester with a gauge of 10 mm and a tensile speed of 5 mm/min. The measurement was repeated 5 times.
Pore size distribution and porosity test
Each fibre membrane specimen was 3.5 × 3.5 cm in size. The pore size and the distribution of the fibrous membranes were measured using a pore size analyzer (CFP-1100AI, PMI, Cape Coral, FL). The principle of the test requires that the sample is fully infiltrated by the wetting agent with a known surface tension and then placed into the sample chamber. The gas that passes through the capillary pores of the sample in dry and wet states under pressure is calculated. Changes in pressure and flow are analyzed, and the pore size and pore distribution of the sample are calculated.
The porosity of a fibrous membrane is the percentage of the volume of the pores in the fibre within the total volume of the fibrous membrane. EquationEquation (1)(1)
(1) was used to calculate porosity:
(1)
(1)
Cytological detection
The effects of different compound meshes on the proliferation of rat skeletal muscle cells (L6, donated by the Chinese Academy of Sciences) and human umbilical vein endothelial cells (HUVECs, donated by the Chinese Academy of Sciences) were tested. The CCK-8 assay (KeyGEN, Beijing, China) was used to measure the cytotoxicity of composite scaffolds of PP, RSF + PP and PCL + PP at different time points (1, 3, 5, and 7 days). An identical procedure was used for each time point. The composite scaffolds (2 cm × 2 cm) were immersed in 20 ml of Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% foetal bovine serum (FBS) and shaken in an incubator for 24 h, after which the released medium was collected. Fibroblasts were plated in quadruplicate at a density of 2000 cells/well in 100 μl of DMEM containing 10% FBS. After 24 h of culture in a 5% CO2 incubator at 37 °C, the cell culture medium was replaced with the released medium. After an additional 24 h of culture, 10 μl of CCK-8 solution was added to each well, and the 96-well plate was incubated for an additional 1.5 h. We used a hybrid multimode microplate reader (Synergy H1, BioTek, Winooski, VT) to measure the 96-well plate at 450 nm.
SEM cell profiling
The RSF fibre was histologically examined to assess its suitability for cell profiling. The fibre was fixed in the 6-pore blank, and L6 cells were added to each pore at a density of 5 × 106/L in 1 ml. After 3 and 5 days, the fibre was fixed in 4% paraformaldehyde for 24 h. To assess the surface morphology of the composite scaffold, the samples were analyzed using a SEM.
Tissue repair in a full-thickness abdominal wall defect model
For the in vivo study, 24 Sprague–Dawley rats (180–200 g) were used (all experiments were approved by the institutional review committee of Shanghai Jiao Tong University School of Medicine). The animals were divided into three groups. The experimental group (n = 8) received a PP mesh with RSF fibre (PP + RSF), while the two scaffold control groups (each n = 8) received a PP mesh composed of PCL (PP + PCL) fibre or PP mesh only (PP). To create abdominal wall defects, rats were anaesthetized by intraperitoneal injections of 10% chloral hydrate (4 ml/kg). The abdominal wall was shaved, disinfected, and covered with sterile draping. A full-thickness abdominal wall defect (2 cm × 2 cm) was made under the peritoneum (IPOM), and a mesh was placed. The edge of the defect was sutured directly to the implanted scaffold with 4–0 silk sutures, and the skin was closed in layers with 3–0 silk sutures.
General observation
After 6 weeks and 12 weeks, each group of rats was euthanized by inhalation of 7% isoflurane. We measured and photographed the gross appearance of abdominal wall defect regeneration. The abdomen was subjected to necropsy to analyze and score adhesion formation according to the adhesion scoring system [Citation26] (Table S1, Supporting Information).
Histological examination
To harvest the composite scaffold and surrounding tissues, rats were sacrificed by administration of high-dose chloral hydrate at 6 or 12 weeks after surgery. Tissue samples were fixed with 4% paraformaldehyde, embedded in paraffin, and sectioned at a 6-mm thickness for haematoxylin and eosin (HE) staining to examine the tissue structure.
RNA expression assay
The mRNA expression of TGF-β, vascular endothelial growth factor B (VEGF-B), interleukin (IL)-6, and IL-10 in the retrieved specimens was determined by quantitative real-time PCR as described previously [Citation25]. The retrieved samples (4 from each group per time point) were immediately immersed in liquid nitrogen and stored at 80 °C until analysis. The samples were cut into small pieces, and approximately 100 mg of the sample was processed for RNA extraction as previously described. Briefly, total RNA was extracted from muscle tissues using a Nucleospin RNA kit (Clontech, Shiga, Japan). cDNA was synthesized with SuperScript II reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. cDNAs and primers were added to SYBR Green PCR master mix (TaKaRa, Tokyo, Japan) according to the manufacturer's instructions. Then, quantitative analysis was performed using QuantStudio™ 6 Flex (Applied Biosystems, Foster City, CA). All data were normalized to rat β-actin, which was used as an internal control, and then further normalized by presenting the values in terms of the relative expression compared with the 6w PP mesh group.
Statistical analysis
All results are presented as the mean ± standard deviation. Differences in the elastic modulus and maximal load were analyzed by one-way ANOVA. p Values less than .05 were considered statistically significant. SPSS 22.0 software (SPSS, Chicago, IL) was used for all statistical analyses.
Results
The RSF concentration is a novel method for determining the form of electrostatic spinning
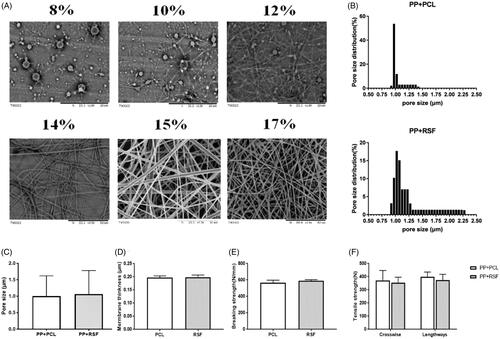
The concentration of the RSF spinning solution is the determining factor that affects spinning, not only affecting spinnability but also greatly influencing the fibre morphology. When the concentration of the spinning solution was low, the fibre morphology was not uniform, and many beads were present. As the concentration of the spinning solution increased, the number of beads in the fibre membrane significantly decreased, and the fineness of the fibres tended to be more uniform. At a spinning solution concentration of 15%, the bead numbers were significantly reduced. However, as the concentration increased, the fibre diameter also increased significantly because the viscosity of the solution increased as the concentration of the spinning solution increased. If the solution was too thin, sufficient entanglement could not occur between molecules, and polymer beads were produced ().
Figure 2. Mechanical properties and construction of the mesh. (A) Effects of different concentrations of RSF solution on electrospinning. (B,C) The pore size was controlled in a limited area. (D–F) The mechanical properties of the two types of mesh. No significant differences were observed between RSF and PCL fibres.
The pore size was controlled in a limited area
The tensile fracture load–displacement curves of PCL and RSF nanofiber membranes were collected after 2 and 3 h of spinning, respectively. To control the time of spinning, the tight fibre was in the same area () (p < .05).
The tensile strengths of the two types of composite mesh were not significantly different (p > .05). Thus, the RSF fibre composed of the PP mesh could provide the required strength.
Analysis of the pore size and the distribution of the PCL nanofiber membrane prepared at a spinning time of 2 h () showed an average pore size of 1.06 μm and a maximum pore size of 2.27 μm. The pore size and the distribution of the RSF nanofiber membrane prepared at a spinning time of 3 h exhibited an average pore size that was decreased by 1.2 μm.
Electrostatic spinning of RSF did not influence skeletal muscle cell or mesothelial cell proliferation
The CCK-8 assay revealed no significant differences (p>.05) among PP, PP + RSF, and PP + PCL in coculture with L6 cells and mesothelial cells on days 1, 3, 5 and 7 ().
Figure 3. (A,B) PP mesh with electrostatic spinning did not increase cytotoxicity. (C) SEM shows that the L6 cells adhered better on the RSF fibre across the area of the cell than on the other surfaces.
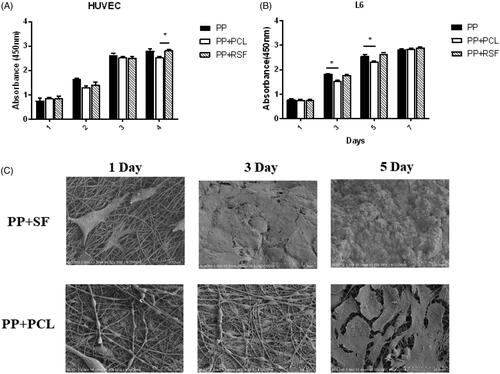
SEM showed that the L6 cells adhered better to the RSF fibre across the area of the cell than to the other surfaces. On day 3, the surface of the RSF-adhered area was nearly equal to that of the fibre. On day 5, the cells completely covered the RSF fibre, and the PCL was not covered ().
RSF electrospinning membrane coating can effectively reduce intra-abdominal adhesion
Animals were sacrificed at 6 and 12 weeks, and gross adhesion scores were observed. The 24 Sprague–Dawley (SD) rats did not show any recurrence, infection or incision dehiscence.
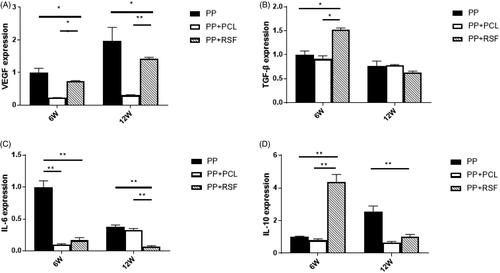
At 6 weeks, almost no adhesion was observed between RSF and PCL, and the peritoneum completely protected the PP part. The PP had many bowel adhesions and could not be easily separated. The PP adhesion score was 8.3 ± 1.0 at 6 weeks, with an average adhesion area of 5.23 ± 2.10 cm2; the RSF adhesion score was 2.8 ± 1.0, with an average adhesion area of 1.10 ± 0.60 cm2; and the PCL adhesion score was 2.3 ± 1.0, with an average adhesion area of 1.12 ± 1.70 cm2. Compared with the PP mesh, the PCL and RSF composite meshes had lower antiadhesion fractions and fewer adhesion areas (p < .05), but less new connective tissue coverage was observed on the peritoneal surface of the PCL compared with that of RSF ().
Figure 4. (A) The result of tissue repair in a full-thickness abdominal wall defect model. (B,C) The area and score of mesh adhesion. The RSF fibre could significantly reduce adhesion.
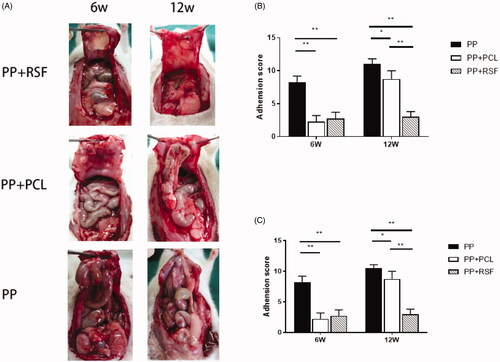
At 12 weeks, the PP adhesion score was 10.5 ± 0.6, with an average adhesion area of 7.81 ± 1.34 cm2. The RSF adhesion score was 3.0 ± 0.8, with an average adhesion area of 1.51 ± 1.32 cm2, and the PCL adhesion score was 8.8 ± 1.3, with an average adhesion area of 6.31 ± 2.90 cm2. The 12 weeks PCL adhesion fraction and area ratio increased over 6 weeks, and the RSF adhesion score and adhesion area were lower than those of the PCL mesh (p < .05). RSF showed peritoneal tissue regeneration and no obvious inflammatory reaction or adhesion ().
RSF electrospun membrane can efficiently regenerate the abdominal wall
As shown in the figure, at 6 weeks, a large amount of adipose tissue was observed between the monofilaments of the PP mesh, adhesion was obvious, and inflammatory cell infiltration was evident. At 12 weeks, intestinal epithelioid tissue appeared around the monofilament. There were obvious physical barriers in the PCL, but fewer new vessels were observed on both sides. At 12 weeks, the PCL was broken due to degradation, and some of the fat and fibrous tissues were as long as the inside. At 12 weeks, the RSF was thicker than the PCL, and at 12 weeks, the PCL layer was broken and damaged, no longer maintaining an ordered structure. The RSF was absorbed in an ordered manner, the thickness of the material was reduced, the material layer was complete, and the tissues on both sides exhibited complete healing ().
Figure 5. The RSF electrospun membrane can efficiently regenerate the abdominal wall. The black arrow shows the mesh covering a complete tissue overlay. The red arrow shows adhesion of the bowel around the mesh.
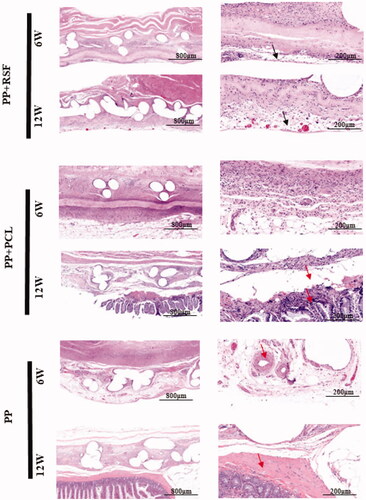
After 6 weeks, Masson staining revealed orderly tissue regeneration around and near the RSF membrane, while the PCL had more adhesions on the abdominal wall. At 12 weeks, both sides of the RSF membrane exhibited stable structures, and the membrane area was further reduced. The regeneration of the peritoneal surface remained intact. However, the PCL showed significant structural fragmentation. The surrounding regenerated fibrous tissue was not continuous ().
RSF exhibits low expression of the peritoneal inflammatory factor IL-6, and VEGF and TGF-β promote the expression of tissue regeneration factor
RT-PCR relative quantification was performed using 2−ΔΔCT values. At 6 w, the expression of VEGF and TGF-Β in the tissues surrounding the RSF membrane was higher than that in the tissues surrounding PCL (p < .05). The expression of VEGF and TGF-Β in the surrounding tissues of the PP mesh was higher than that in the surrounding tissues of RSF or PCL (p < .05) due to the large amount of adherent omental tissue in the PP mesh. At 12 weeks, the expression levels of VEGF and TGF-Β in the surrounding tissues of the PP mesh, RSF and PCL membrane were lower than those at 6 w (p < .05), and there was no significant differences among the groups. At 6 weeks, the expression of IL-6 in the PP group was higher than that in the PCL and RSF groups (p < .05). No significant difference in IL-6 expression was observed between the PCL and RSF groups. At 12 weeks, IL-6 expression was still higher in the PP group than in the PCL and RSF groups (p < .05). At 6 weeks, the expression of IL-10 in the RSF group was higher than that in the PP and PCL groups (p < 0.05). At 12 w, there was no significant difference in IL-10 expression among the PP, PCL, and RSF groups ().
Discussion
Trauma, infection, postoperative complications of abdominal surgery, and abdominal wall tumour resection may cause abdominal hernia or complex abdominal wall defects [Citation2]. Large defect areas are correlated with high direct suture tension, and meshes are required to repair this type of defect. The composite gingiva is an enamel mesh formed by combining two or more materials in different ways, such as PP and ePTFE composite meshes, PP and polyvinylidene fluoride composite meshes, and PP or polylactic acid/polyglycolic acid copolymer meshes [Citation27,Citation28]. The front and back surfaces of composite meshes are usually composed of different materials and exhibit different properties. The portion near the abdominal wall is generally a nonabsorbable woven material with a rough surface, which can stimulate tissue ingrowth and has strong mechanical properties, providing a stable abdominal wall and tensile ability. The portion of the mesh near the gut and other internal organs is generally a material with good biocompatibility and a smooth surface. The mesh is located between the woven material and the viscera and sequesters the viscera and woven material, thereby preventing adhesion.
Electrospinning is an excellent material processing technique for regeneration of the peritoneum. Bolgen et al. applied electrospun PCL membranes to rat abdominal wall repair experiments to investigate the tissue test effects of pure PCL membranes and antibiotic-loaded PCL membranes in abdominal wall repair tests. The results showed that the adhesion of the PCL membrane to the viscera after repair of the abdominal wall was significantly lower than that of the antibiotic-loaded PCL membranes and was very weak for some adhesions [Citation29]. The Proceed mesh uses a method in which PP is combined with absorbable oxidized regenerated fibres to prevent direct contact between the PP and the viscera, thereby reducing adhesion [Citation21,Citation29].
In previous studies, formic acid served as the main RSF solvent, and the residue of formic acid affected regeneration of the surrounding cells [Citation30]. In this study, we used formic acid as a solvent for PCL and RSF and performed cell proliferation experiments. The experimental results show few residues in the RSF electrospun membrane and the PCL, which did not affect skeletal muscle cells or mesothelial cells and could be used for abdominal wall regeneration.
After 2 or more hours of spinning time, the average value of the pore diameter was maintained at approximately 1 μm. Therefore, the pore diameter cannot be further reduced beyond approximately 1 μm. Adhesions result from exposure of the peritoneum mesothelial layer basal membrane to neighbouring tissues following injury or damage to the visceral and parietal peritoneum. The cascade of events leading to adhesion formation has been reported as compromised tissue circulation, inflammation, fibrin deposition, fibrin organization, and collagen formation, finally culminating in adhesion [Citation31]. Antiadhesive agents prevent adhesion by disruption of either the inflammatory process or the fibrin-forming process that leads to adhesion or by provision of a mechanical barrier between affected tissues, preventing their apposition [Citation32,Citation33].
In this experiment, PP meshes exhibited greater adhesion than the other materials tested at 6 and 12 weeks. PCL and RSF films of the same thickness had good antiblocking effects at 6 weeks, and RSF and the surface of the mesh were covered with a new layer of tissue, while the PCL surface adhered. With more adipose tissue, the boundaries of the new peritoneal tissue became unclear. At 12 weeks, the peritoneal tissue regenerated by PCL was not strong. In particular, the material in the central region of the repair material did not exhibit new tissue with a measurable thickness. As the PP material was partially exposed to PCL, adhesion was aggravated, and the adhesion fraction increased. However, the overall adhesion area was still better than that of the PP mesh. PCL showed no complete tissue coverage in the abdominal cavity. With the degradation of the PCL membrane, some PP meshes were exposed to the abdominal organs to form adhesions. The adhesion fraction and the PCL area increased, and some meshes showed more severe adhesions.
Through tissue sectioning and HE staining, many fat cells were observed between the PP mesh nonofilaments, which were directly exposed to the abdominal organs. In addition, the early inflammatory reaction further stimulated intestinal adhesion, and almost no regenerated muscle tissue was observed around the mesh. At 6 weeks, the RSF showed a clear barrier separating the PP mesh from the abdominal organs. The peritoneal side was covered with tissue. At 12 weeks, the thickness of the RSF film was reduced, but its integrity was maintained, and obvious regeneration tissue was visible on both sides of the film.
Because the tissue on the peritoneal side lacks a covered blood supply during repair of the abdominal wall, formation of stable neovascularization on both sides of the early lamella is critical [Citation34]. Moreover, the relative expression levels of factors that promote angiogenesis and tissue regeneration, such as VEGF and TGF-Β, were higher in tissues surrounding RSF than in those surrounding PCL. However, because the PP mesh was covered with a large amount of adipose tissue, the VEGF and TGF-β levels around the PP mesh were relatively high and could not be compared.
The inflammatory response also reduces abdominal adhesions and provides an important basis for peritoneal regeneration [Citation35]. RT-PCR also revealed an inflammatory response, as indicated by IL-6 expression at 6 weeks. The expression of IL-6 was higher in both the PP and PCL groups than in the RSF group, while the expression of the anti-inflammatory factor IL-10 was higher in the RSF group. The reduced stimulation of organs makes RSF a more ideal material.
Conclusion
RSF membranes were constructed by electrospinning technology, and a composite mesh was constructed as a PP-braided mesh. The RSF membranes induced a smaller inflammatory reaction and better promoted the peritoneum and the surrounding abdominal wall than pure PP or PCL electrospun membranes. Tissue migration occurred over the mesh, leading to more stable tissue, promotion of peritoneal regeneration and reduction in the occurrence of a series of complications caused by mesh adhesion.
Supporting_Information.docx
Download MS Word (23.6 KB)Acknowledgements
The authors thank Yan Gu and Peihua Zhang for their very helpful guidance for this study. In addition, the authors thank Xiaoying for her affection in my life.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ventral Hernia Working Group, Breuing K, Butler CE, et al. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148(3):544–558.
- Song Z, Yang D, Yang J, et al. Abdominal wall reconstruction following resection of large abdominal aggressive neoplasms using tensor fascia lata flap with or without mesh reinforcement. Hernia. 2018;22(2):333–341.
- Leber GE, Garb JL, Alexander AI, et al. Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg. 1998;133(4):378–382.
- Tuveri M, Tuveri A, Nicolo E. Repair of large abdominal incisional hernia by reconstructing the midline and use of an onlay of biological material. Am J Surg. 2011;202(1):e7–e11.
- Ellis H, Moran BJ, Thompson JN, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353(9163):1476–1480.
- Kaufman Z, Engelberg M, Zager M. Fecal fistula: a late complication of Marlex mesh repair. Dis Colon Rectum. 1981;24(7):543–544.
- Matthews BD, Pratt BL, Pollinger HS, et al. Assessment of adhesion formation to intra-abdominal polypropylene mesh and polytetrafluoroethylene mesh. J Surg Res. 2003;114(2):126–132.
- Aysan E, Bektas H, Ersoz F. A new approach to postoperative peritoneal adhesions: prevention of peritoneal trauma by aloe vera gel. Eur J Obstet Gynecol Reprod Biol. 2010;149(2):195–198.
- Kamel RM. Prevention of postoperative peritoneal adhesions. Eur J Obstet Gynecol Reprod Biol. 2010;150(2):111–118.
- LeBlanc KA, Elieson MJ, Corder JM. 3rd Enterotomy and mortality rates of laparoscopic incisional and ventral hernia repair: a review of the literature. JSLS. 2007;11(4):408–414.
- Nukavarapu SP, Kumbar SG, Brown JL, et al. Polyphosphazene/nano-hydroxyapatite composite microsphere scaffolds for bone tissue engineering. Biomacromolecules. 2008;9(7):1818–1825.
- Hympanova L, Mori da Cunha M, Rynkevic R, et al. Experimental reconstruction of an abdominal wall defect with electrospun polycaprolactone-ureidopyrimidinone mesh conserves compliance yet may have insufficient strength. J Mech Behav Biomed Mater. 2018;88:431–441.
- Li J, Zhu J, He T, et al. Prevention of intra-abdominal adhesion using electrospun PEG/PLGA nanofibrous membranes. Mater Sci Eng C Mater Biol Appl. 2017;78:988–997.
- Iliopoulos J, Cornwall GB, Evans RO, et al. Evaluation of a bioabsorable polylactide film in a large animal model for the reduction of retrosternal adhesions. J Surg Res. 2004;118(2):144–153.
- Konar S, Guha R, Kundu B, et al. Silk fibroin hydrogel as physical barrier for prevention of post hernia adhesion. Hernia. 2017;21(1):125–137.
- McClellan P, Landis WJ. Recent applications of coaxial and emulsion electrospinning methods in the field of tissue engineering. Biores Open Access. 2016;5(1):212–227.
- Hashizume R, Fujimoto KL, Hong Y, et al. Morphological and mechanical characteristics of the reconstructed rat abdominal wall following use of a wet electrospun biodegradable polyurethane elastomer scaffold. Biomaterials. 2010;31(12):3253–3265.
- Jango H, Gras S, Christensen L, et al. Examinations of a new long-term degradable electrospun polycaprolactone scaffold in three rat abdominal wall models. J Biomater Appl. 2017;31(7):1077–1086.
- Veleirinho B, Coelho DS, Dias PF, et al. Foreign body reaction associated with PET and PET/chitosan electrospun nanofibrous abdominal meshes. PLoS One. 2014;9(4):e95293.
- Liu Z, Li S, Su L, et al. Novel superhydrophilic poly(l-lactic acid-co-epsilon-caprolactone)/fibrinogen electrospun patch for rat abdominal wall reconstruction. J Biomater Appl. 2015;30(2):230–238.
- Zong X, Li S, Chen E, et al. Prevention of postsurgery-induced abdominal adhesions by electrospun bioabsorbable nanofibrous poly(lactide-co-glycolide)-based membranes. Ann Surg. 2004;240(5):910–915.
- Hofmann S, Hagenmuller H, Koch AM, et al. Control of in vitro tissue-engineered bone-like structures using human mesenchymal stem cells and porous silk scaffolds. Biomaterials. 2007;28(6):1152–1162.
- Asakura T, Isobe K, Kametani S, et al. Characterization of water in hydrated Bombyx mori silk fibroin fiber and films by (2)H NMR relaxation and (13)C solid state NMR. Acta Biomater. 2017;50:322–333.
- Hodgkinson T, Yuan XF, Bayat A. Electrospun silk fibroin fiber diameter influences in vitro dermal fibroblast behavior and promotes healing of ex vivo wound models. J Tissue Eng. 2014;5:204173141455166.
- Wei G, Li C, Fu Q, et al. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int Urol Nephrol. 2015;47(1):95–99.
- Conze J, Rosch R, Klinge U, et al. Polypropylene in the intra-abdominal position: influence of pore size and surface area. Hernia. 2004;8(4):365–372.
- Ladurner R, Drosse I, Burklein D, et al. Cyanoacrylate glue for intra-abdominal mesh fixation of polypropylene-polyvinylidene fluoride meshes in a rabbit model. J Surg Res. 2011;167(2):e157–e162.
- Xiaolong Y, Xiaoyan H, Bo W, et al. Ventral hernia repair in rat using nanofibrous polylactic acid/polypropylene meshes. Nanomedicine (Lond). 2018;13(17):2187–2199.
- Bolgen N, Vargel I, Korkusuz P, et al. In vivo performance of antibiotic embedded electrospun PCL membranes for prevention of abdominal adhesions. J Biomed Mater Res B Res. 2007;81(2):530–543.
- Zitting A, Savolainen H, Nickels J. Biochemical and toxicological effects of single and repeated exposures to polyacetal thermodegradation products. Environ Res. 1982;29(2):287–296.
- Kim JY, Cho WJ, Kim JH, et al. Efficacy and safety of hyaluronate membrane in the rabbit cecum-abdominal wall adhesion model. J Korean Surg Soc. 2013;85(2):51–57.
- Hu W, Zhang Z, Lu S, et al. Assembled anti-adhesion polypropylene mesh with self-fixable and degradable in situ mussel-inspired hydrogel coating for abdominal wall defect repair. Biomater Sci. 2018;6(11):3030–3041.
- Lott AJS, Wall DR, Skoien R, et al. Perioperative bleed from superior mesenteric vein to abdominal wall portosystemic shunt via small bowel adhesion. ANZ J Surg. 2017;87(11):E222–E223.
- Takanari K, Hashizume R, Hong Y, et al. Skeletal muscle derived stem cells microintegrated into a biodegradable elastomer for reconstruction of the abdominal wall. Biomaterials. 2017;113:31–41.
- McGinty JJ, Hogle NJ, McCarthy H, et al. A comparative study of adhesion formation and abdominal wall ingrowth after laparoscopic ventral hernia repair in a porcine model using multiple types of mesh. Surg Endosc. 2005;19(6):786–790.