Abstract
Background
CircZNF609 (cZNF609) is previously revealed as an essential mediator in oxidative stress. This paper determined the role of cZNF609 in skin oxidative damage to evaluate its importance in pressure ulcer.
Methods
HaCaT cells treated by H2O2 were considered as a cell model of pressure ulcer. The role of cZNF609 in the model was checked by conducting CCK-8 assay, FITC-PI double-staining, ROS detection and Western blot. The downstream gene and signalling of cZNF609 were studied by utilizing qRT-PCR and Western blot.
Results
HaCaT cells were remarkably damaged by H2O2, as evidenced by the viability loss, apoptosis and ROS generation. It was coupled with the elevated expression of p53, p16, Bax and the activated forms of caspase-3 and PARP. Meanwhile, cZNF609 was high-expressed in response to H2O2. The oxidative stress driven by H2O2 was alleviated by transfection with cZNF609 specific siRNA. Further, the anti-antioxidant impacts of cZNF609 silence were impeded by miR-145 silence. The inhibition of JNK and p38MAPK pathways induced by cZNF609 silence was impeded by miR-145 silence.
Conclusion
The protective function of cZNF609 silence in H2O2-injured HaCaT cells was revealed in vitro. Silence of cZNF609 exhibited its impact possibly through regulating miR-145, and JNK and p38MAPK pathways.
Introduction
Pressure ulcer is a worldwide health problem that increases mortality, prolongs hospitalization, entails high cost of nursing and significantly reduces the quality of life of patients [Citation1–3]. In general, it is happened in paralysis and bedridden patients who are suffered from long-term compression acting on the local tissue. During pressure ulcer, various pathological events are occurred, including local continuous ischaemia, hypoxia, blood circulation obstacle, malnutrition, and finally soft tissue damage or necrosis [Citation4]. More than that, skin oxidative damage is always accompanied with pressure ulcer [Citation5]. The drugs with anti-antioxidant function have been considered as potential agents for improving the treatment of pressure ulcer in clinic [Citation6].
Circular RNAs (circRNAs) are a type of non-coding RNAs that form a covalently closed continuous loop, which was initially misinterpreted as by-products of splicing errors. Due to its specific structures, circRNAs always exhibit features like high stability, sequence conservation, and tissue or cell specificity. And with the advent of high-throughput sequencing and bioinformatics analysis, thousands of circRNAs are identified recently in multiple cell lines and across various species [Citation7]. The abnormal expression of circRNAs was closely related to the development of a number of diseases, including pressure ulcer. A study demonstrated hsa_circRNA_10075 expressed a higher level in pressure ulcers injury in vitro and thus augmenting cell injury [Citation8]. However, circRNA has been rarely studied in pressure ulcers. CircBase retrieval shows circZNF609 (cZNF609) is a circRNA located at human genome chr15:64791491-64792365 [Citation9]. The host gene, ZNF609, is a member of Zinc finger protein family. Zinc finger proteins play important roles in DNA recognition, RNA packaging, transcriptional activation, regulation of apoptosis, protein folding and assembly, and lipid binding [Citation10]. The cZNF609 is abundantly expressed in endothelial cells [Citation11], neurons [Citation12] and myoblasts [Citation13]. The cZNF609 is highly expressed not only in above cells but also in a number of cancer cells. Recent paper mainly focussed on the anti-tumor functions of silencing cZNF609 in a wide range of human cancers, like nasopharyngeal carcinoma [Citation14], colorectal cancer [Citation15], rhabdomyosarcoma [Citation16], renal carcinoma [Citation17] and so forth. Besides, a literature illustrated cZNF609 regulated MEF2A and was likely involved in oxidative stress and the protective function of cZNF609 silence on endothelial cells against oxidative stress [Citation18]. Nonetheless, the role of cZNF609 in skin oxidative damage was not investigated before.
MicroRNAs (miRs or miRNAs) are small, evolutionarily conserved, non-coding RNAs of approximately 18–25 nucleotides in length. They are generally involved in post-transcriptional gene regulation and act as the main downstream genes of circRNAs. The expression pattern of miRNAs is changed in various human diseases, including oxidative stress related diseases such as thalassaemia [Citation19], chronic kidney disease [Citation20] and myocardial ischaemia [Citation21]. In response to oxidative stress like H2O2 and high glucose, miR-145 level was found to be down-regulated in follicular granulosa cells [Citation22] and retinal endothelial cells [Citation23]. Additionally, the up-regulation of miR-145 was able to alleviate oxidative stress-induced damage in cell [Citation22,Citation23]. Those findings evidenced the importance of miR-145 expression in the response following oxidative stress.
The present paper attempted to reveal the role of cZNF609 in a cell model of pressure ulcer made by stimulating keratinocytes with H2O2. The parameters regarding oxidative stress were measured for evaluating the function of cZNF609 silence. Besides, the regulatory association between cZNF609 and miR-145 was studied to uncover the deep mechanisms.
Materials and methods
Cell treatment
Human keratinocyte cell line HaCaT was from CLS Cell Lines Service (Eppelheim, Germany) and cultivated in DMEM/F-12 medium (Gibco, Grand Island, NY) with 10% foetal calf serum (Gibco). The cells were subcultured at 37 °C in an atmosphere with 5% CO2. For the oxidative stress damage, HaCaT cells were treated by a series dose of H2O2 for 12 h.
Cell transfection
The siRNA specific against cZNF609 (si-cZNF609), its negative control (siNC) as well as miR-145 inhibitor and the miRNA NC were all synthesized from GenePharma (Shanghai, China). Transfection was conducted in a 6-well plates in which cells were growth to 90% confluent. Lipofectamine 2000 (Invitrogen, Carlsbad, CA) was added to each well containing cells and the antibiotics-free medium. Incubate cells at 37 °C for 48 h and then the expression of transgenes was tested by qRT-PCR.
Nucleotide sequence of siRNA for circZNF609, miR-145 inhibitor and the negative control was as follows:
si-cZNF609: 5′- GUCAAGUCUGAAAAGCAAUGA-3′;
si-NC: 5′- GGACCCCGUGUUCCAACGACC-3′;
miR-145 inhibitor: 5′- AGGGAUUCCUGGGAAAACUGGAC-3′;
miRNA NC: 5′- CUUAAUAACGCCCAAAUGGGCCGG-3′.
qRT-PCR
Total RNAs in HaCaT cells following the transfection were extracted by utilizing the TRIzol™ Plus RNA Purification Kit (Invitrogen). Collect the RNA samples via centrifugation at 12,000 × g, and the RNAs were reversely transcribed into cDNA by using RevertAid RT Reverse Transcription Kit (Thermo Scientific®, Rockford, IL). Quantitation of PCR products was performed by SYBR™ Green PCR Master Mix (Applied Biosystems, Foster City, CA). To the test of miR-145, TaqMan™ MicroRNA Reverse Transcription Kit (Applied Biosystems) as well as mirVana™ qRT-PCR miRNA Detection Kit (Invitrogen) were applied. Relative expression of cZNF609 and miR-145 was calculated by 2−△△Ct method by normalizing the levels of β-actin and U6 snRNA respectively.
The specific primers of cZNF609, β-actin, miR-145 and U6 were as follows:
cZNF609
Forward primer: 5′-CAGCGCTCAATCCTTTGGGA-3′;
Reverse primer: 5′-GACCTGCCACATTGGTCAGTA-3′.
β-actin
Forward primer: 5′-AGCGAGCATCCCCCAAAGT-3′;
Reverse primer: 5′-GGGCACGAAGGCTCATCATT-3′.
miR-145
Forward primer: 5′-ACACTCCAGCTGGGGTCCAGTTTTCCCAGGA-3′;
Reverse primer: 5′-TGGTGTCGTGGAGTCG-3′.
U6
Forward primer: 5′-CTCGCTTCGGCAGCACA-3′;
Reverse primer: 5′-AACGCTTCACGAATTTGCGT-3′.
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay
HaCaT cells in 96-well plates with 5000 cells per well were treated as indicated. Ten microliters of MTT solution (Beyotime, Shanghai, China) with concentration of 5 mg/mL was added to each well. The cultures were incubated at 37 °C for 4 h and 100 μL DMSO (Sigma-Aldrich, St. Louis, MO) was used to dissolve the Formazan. Set cultures under the wavelength of 570 nm at a Microplate Reader (Bio-Rad Laboratories, Hercules, CA), and the optical density of each well was read for the assessment of cell viability.
Apoptosis assay
HaCaT cells in 6-well plates with 5 × 105 cells per well were treated as indicated. Collect the cells into eppendorf tube and after washing with phosphate buffered saline (Beyotime) for twice, cells were stained by Annexin V-FITC Apoptosis Detection Kit (Beyotime). The staining procedure was conducted in the room temperature and avoided the impact of light. The stained cells were analyzed by utilizing a FACS can (Beckman Coulter, Fullerton, CA). Apoptotic cells are positive to FITC while negative to PI.
Reactive oxygen species (ROS) assay
HaCaT cells in 6-well plates with 5 × 105 cells per well were treated as indicated. The cells were harvested and resuspended in serum-free culture medium with 10 μmol/L DCFH-DA probe (Solarbio, Beijing, China). The samples were incubated at 37 °C for 20 min. After washing with serum-free culture medium for three times, the cells were analyzed by Microplate Reader (Bio-Rad Laboratories) at 488 nm excitation wavelength and 525 nm emission wavelength.
Western blot
HaCaT cells in 24-well plates with 5 × 104 cells per well were treated as indicated. Proteins were isolated from cell by utilizing RIPA buffer (Sangon Biotech, Shanghai, China) with the help of Protease/Phosphatase Inhibitor Cocktail (Thermo Scientific®). The modified BCA Protein Assay Kit (Sangon Biotech) was used to assess protein purity. The protein samples were then suffered from SDS-PAGE and transferred onto the PVDF membranes (Millipore, Bedford, MA). The following primary antibodies were utilized to probe the specific target proteins. Anti-p53 (MA1078) was from BosterBio (Pleasanton, CA), and anti-p16 (orb310837), anti-Bax (orb216030), anti-cleaved-caspase-3 (orb126608), anti-cleaved-PARP (orb251852), anti-JNK (orb95569), anti-p-JNK (orb312293), anti-p38MAPK (orb67545), anti-p-p38MAPK (orb6578), and anti-β-actin (orb86987) were from Biorbyt (San Francisco, CA). After incubation with the secondary antibodies (Biorbyt), the target bands were developed by EasyBlot ECL kit (Sangon Biotech). Quantitation of the grey level of the target bands was performed in Image Lab™ Software (Bio-Rad).
Statistics
All results were from three parallel experiments and presented as mean ± SD. Comparison between groups was conducted by t-test or one way ANOVA in SPSS 19.0 software (SPSS Inc., Chicago, IL). The normal distribution was tested by Kolmogorov-Smirnov test before statistical comparison. Duncan post-hoc analysis was utilized for multiple comparisons. Statistical difference was identified when the p-value was less than .05, and shown as asterisks in figures.
Results
H2O2 evoked oxidative stress damage in HaCaT cells
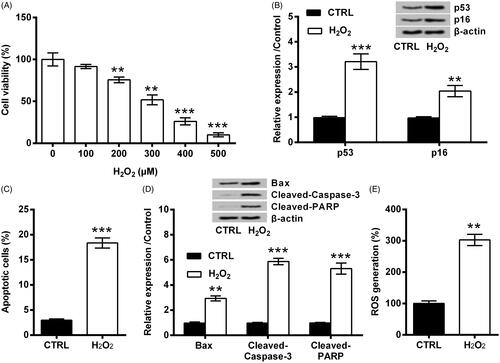
HaCaT cells were stimulated with various doses of H2O2 for 12 h as described by Zhang et al. [Citation6]. As seen in , the viability of HaCaT cells was clearly weakened by H2O2 (p < .01 or p < .001), and the decrease of cell viability was in a dose-dependent fashion. Considering cell viability fell to about a half when 300 μM H2O2 was applied, 300 μM was used in the following. displayed that, protein levels of p53 and p16 were clearly elevated by H2O2 (p < .001 and p < .01). Also, the apoptosis was evoked by H2O2, as evidenced by the increase of apoptosis rate (p < .001, ), the up-regulation of Bax (p < .01) as well as the activation of Caspase-3 and PARP (both p < .001, ). Besides that, ROS level was remarkably elevated by H2O2 (p < .01, ). Those data collectively draw a conclusion that, H2O2 evoked oxidative stress damage in HaCaT cells.
Figure 1. H2O2 evoked oxidative stress damage in HaCaT cells. (A) HaCaT cells were stimulated with various doses of H2O2 for 12 h. The viability of HaCaT cells was examined by CCK-8. The following experiments applied 300 μM H2O2 in HaCaT cells. (B) Expression of p53 and p16, (C) apoptosis rate, (D) expression of Bax, Cleaved-Caspase-3 and Cleaved-PARP, as well as (E) ROS level were examined by Western blot, FITC-PI double staining, and DCFH-DA probe. ** and *** indicate p-values less than .01 and .001.
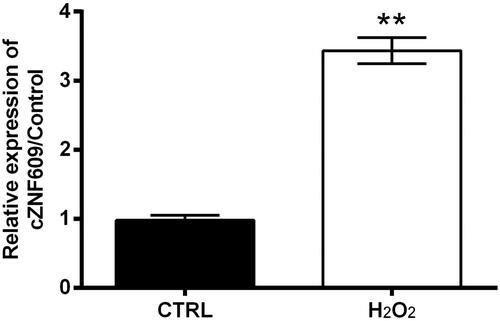
cZNF609 expression induced by H2O2 in HaCaT cells
cZNF609 level in HaCaT cells following the treatment of H2O2 was analyzed by qRT-PCR. Data showed that, cZNF609 level significantly elevated by H2O2 treatment as compared to control (p < .01, ). It seems that cZNF609 expression is sensitive to H2O2 stimulation.
cZNF609 silence alleviated H2O2-evoked oxidative stress damage in HaCaT cells
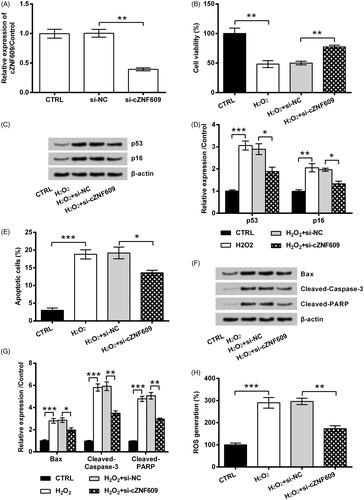
To further reveal if cZNF609 expression was implicated in H2O2-evoked oxidative stress damage in HaCaT cells, its expression in cell was silenced by transfection. showed the successful transfection efficiency, as the level of cZNF609 was clearly repressed by siRNA transfection (p < .01). Subsequently, the oxidative stress damage in HaCaT cells initiated by H2O2 was alleviated by cZNF609 silence. As compared to transfection with si-NC, transfection of cells with si-cZNF609 remarkably promoted cell viability (p < .01, ), and repressed p53 and p16 expression (both p < .05, ) in H2O2-injured HaCaT cells. Also, si-cZNF609 reduced apoptosis rate (p < .05, ), repressed pro-apoptosis proteins expression (p < .05 or p < .01, ), and reduced ROS generation (p < .01, ).
Figure 3. cZNF609 silence alleviated H2O2-evoked oxidative stress damage in HaCaT cells. (A) si-NC or si-cZNF609 was transfected into HaCaT cells. cZNF609 level was checked by qRT-PCR. Following transfection, the cells were stimulated with 300 μM H2O2 for 12 h. (B) Cell viability, (C,D) expression of p53 and p16, (E) apoptosis rate, (F,G) expression of Bax, Cleaved-Caspase-3 and Cleaved-PARP, as well as (H) ROS level were examined by CCK-8, Western blot, FITC-PI double staining, and DCFH-DA probe. *, ** and *** indicate p-values less than .05, .01 and .001.
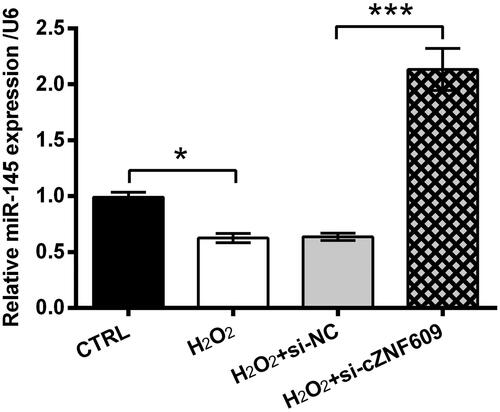
cZNF609 silence induced miR-145 up-regulation
The expression of miR-145 in HaCaT cells following H2O2 treatment and/or si-cZNF609 transfection was then tested. As seen in , miR-145 level was significantly declined by H2O2 (p < .05). Nonetheless, miR-145 level was strongly elevated by si-cZNF609 even under H2O2-treated condition (p < .001). So, the negative regulation network between cZNF609 and miR-145 was revealed.
cZNF609 silence alleviated oxidative stress damage through miR-145
Based on the just announced results, the following experiments focused on investigating the involvement of miR-145 in cZNF609 silence-induced response. To this purpose, miR-145 expression was silenced by transfection with the specific inhibitor (p < .001, ). Following data revealed that, the impact of miR-145 silence towards HaCaT cells was contrary to the impacts of cZNF609 silence. As compared to cZNF609 silence alone, cZNF609 and miR-145 silence concurrently led to a lower cell viability (p < .05, ), and higher levels of p53 and p16 (both p < .05, ). Additionally, as relative to cZNF609 silence alone, cZNF609 and miR-145 silence concurrently result in apoptosis increase (p < .05, ), pro-apoptosis proteins expression (all p < .05, ), and ROS generation (p < .05, ). Moreover, we found that cells co-transfected with si-cZNF609 and miR-145 inhibitor had a higher cZNF609 level rather than only si-cZNF609, while expressed a lower miR-145 level (p < .05, ).
Figure 5. cZNF609 silence alleviated oxidative stress damage through miR-145. (A) NC or miR-145 inhibitor was transfected into HaCaT cells. miR-145 level was checked by qRT-PCR. The cells were transfected with si-cZNF609 alone or together with miR-145 inhibitor. si-NC and NC were transfected as negative controls. The transfected cells were then stimulated with 300 μM H2O2 for 12 h. (B) Cell viability, (C,D) expression of p53 and p16, (E) apoptosis rate, (F,G) expression of Bax, Cleaved-Caspase-3 and Cleaved-PARP, as well as (H) ROS level were examined by CCK-8, Western blot, FITC-PI double staining, and DCFH-DA probe. *, ** and *** indicate p-values less than .05, .01 and .001.
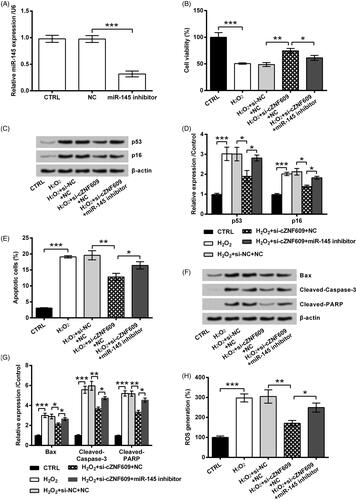
Figure 6. The decrease of miR-145 promoted cZNF609 expression. The cells were transfected with si-cZNF609 alone or together with miR-145 inhibitor. si-NC and NC were transfected as negative controls. The transfected cells were then stimulated with 300 μM H2O2 for 12 h. (A) cZNF609 and (B) miR-145 expression was tested by qRT-PCR. *, ** and *** indicate p-values less than .05, .01 and .001.
cZNF609 silence inhibited JNK and p38MAPK pathways through miR-145
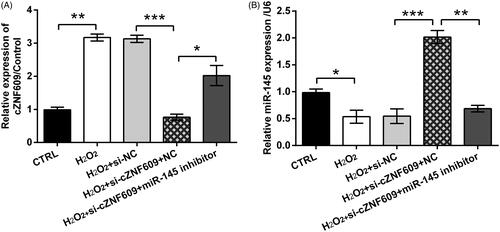
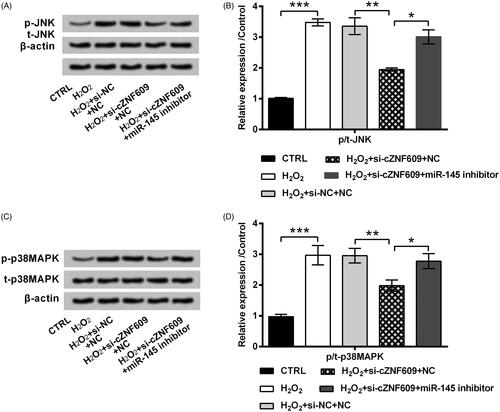
The signalling pathways which are essential in mediating H2O2-evoked oxidative stress damage were finally studied. showed that, the activated form of JNK (p-JNK) was clearly increased by H2O2 in HaCaT cells (p < .001). Transfection of cells with si-cZNF609 inhibited p-JNK expression in H2O2-stimulated cell (p < .01). Besides, the impact of si-cZNF609 on p-JNK expression was partially flatted by miR-145 inhibitor (p < .05). Similar, p-p38MAPK expression was elevated by H2O2 (p < .001) while repressed by si-cZNF609 transfection (p < .01, ). Co-transfection of si-cZNF609 and miR-145 inhibitor led to a higher level of p-p38MAPK as relative to transfection of si-cZNF609 alone (p < .05). Those data uncovered the regulation axis of cZNF609/miR-145 in modulating JNK and p38MAPK pathways.
Figure 7. cZNF609 silence inhibited JNK and p38MAPK pathways through miR-145. HaCaT cells were transfected with si-cZNF609 alone or together with miR-145 inhibitor. si-NC and NC were transfected as negative controls. The transfected cells were then stimulated with 300 μM H2O2 for 12 h. The activation of (A,B) JNK and (C,D) p38MAPK was checked by Western blot. *, ** and *** indicate p-values less than .05, .01 and .001.
Discussion
The pathogenesis of pressure ulcer is quite complex involves in various factors which can interplay with each other [Citation24]. No single factor can explain the risk of this disease [Citation24]. Nonetheless, the processes involved in pathogenesis has been preliminary understood, including duration of ischaemia, hypoxia damage, inflammatory response, and irreversible tissue damage [Citation4]. Thus, in vitro and in vivo investigations have utilized hypoxia-ischaemia as an induction factor to mimic pressure ulcer models [Citation5,Citation25]. More recently, studies have revealed that skin oxidative damage is also closely associated with pressure ulcer [Citation5]. And stimulating keratinocytes with oxidative stress has been considered as a novel cell model of this disease [Citation6]. Herein, the same cell model was established and the role of cZNF609 in the experimental system was studied. cZNF609 was found to be high-expressed in response to H2O2 in HaCaT cells. Silence of cZNF609 alleviated H2O2-evoked oxidative stress damage in HaCaT cells. Besides, the cytoprotective functions of cZNF609 silence may be related with its regulation on miR-145 expression and JNK and p38MAPK pathways.
CircRNAs are new stars in the field of RNA research. They are believed to be critical in the occurrence and development of a wide range of human diseases, for instance acute ischaemic stroke [Citation26], psoriasis [Citation27] and even tumours [Citation28]. Moreover, circRNA may remove the inhibitory effect of a miRNA on its targeted gene, raise the expression of the targeted gene, thereby regulating a series of pathophysiological processes. In the present paper, in vitro data suggested the involvement of cZNF609 in the pathogenesis of pressure ulcer. cZNF609 was highly expressed following H2O2 treatment in HaCaT cells, indicating cZNF609 as a sensitive circRNA in response to oxidative stress. This was in line with a previous finding which was done in high glucose-stimulated endothelial cells [Citation18]. Besides, inhibition of cZNF609 expression by siRNA-mediated transfection protected HaCaT cells against H2O2-evoked oxidative stress damage, as cell viability loss was repressed, apoptosis was reduced and ROS generation was lowered. It seems that intervention of cZNF609 level has promise in impeding oxidative stress damage, which was also confirmed elsewhere [Citation18].
MiRNAs are drawing increasing attention in various human diseases including pressure ulcer as theirs determining roles to stress response, cell proliferation, death, differentiation, etc. [Citation29]. In human skin disorders, miR-145 level was found to be significant changed. For instance, miR-145 was low expressed in psoriatic lesional skin [Citation30]. Of contrast, high expression of miR-145 was observed in a cell model of systemic lupus erythematosus [Citation31] as well as various nonmelanoma skin cancers like basal cell carcinoma and squamous cell carcinoma [Citation32]. The present paper illustrated the low expressed miR-145 in the cell model of pressure ulcer, which was established by stimulating HaCaT cells with H2O2. This finding hint us the importance of miR-145 in mediating oxidative stress during pressure ulcer. Besides, cZNF609 silence was capable of elevating miR-145 expression, indicating miR-145 as one of the downstream genes of cZNF609. This hypothesis was confirmed in the following, as the cytoprotective impacts of cZNF609 silence towards HaCaT cells were alleviated when miR-145 level was suppressed by the specific inhibitor. Previously, cZNF609 has been found to be the molecular sponges of a small fraction of miRNAs, such as miR-615 [Citation33], miR-138-5p [Citation17], miR-150-5p [Citation34] and miR-145 [Citation35]. Our findings agree with the previous observations. But, further investigations are still needed to uncover the complex regulation between cZNF609 and miRNAs.
JNK and p38MAPK belong to the superfamily of MAPK. They are serine/threonine protein kinases and widely expressed in mammalian cells. JNK and p38MAPK signalling pathways both can be activated by various physiological stresses including oxidative stress [Citation36]. The activated signalling is then contributed to mediating the following cellular responses, like inflammatory reaction, the loss of cell proliferation, apoptosis death, and ROS generation [Citation37,Citation38]. In the current paper, JNK and p38MAPK pathways were found to be activated by H2O2 in HaCaT cells. Silence of cZNF609 could suppress the activation of those two signalling. And the inhibition of JNK and p38MAPK pathways induced by cZNF609 silence might be through mediating the expression of miR-145. So, it can be speculated that, cZNF609 silence protected HaCaT cells against H2O2-evoked oxidative stress damage possibly through regulating miR-145, which subsequently mediated its following signalling like JNK and p38MAPK pathways.
In summary, the protective function of cZNF609 silence in H2O2-injured HaCaT cells was revealed in this study. The finding evidenced the anti-antioxidant potential of cZNF609 intervention in pressure ulcer. Mechanically, cZNF609 silence exhibited its impact in the study system possibly through regulating miR-145 expression and thereby mediating JNK and p38MAPK pathways.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Demarre L, Van Lancker A, Van Hecke A, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. Int J Nurs Stud. 2015;52(11):1754–1774.
- Ferris A, Price A, Harding K. Pressure ulcers in patients receiving palliative care: a systematic review. Palliat Med. 2019;33(7):770–782.
- Brown JM, Nelson EA, Gorecki C, on behalf of the European Quality of Life Pressure Ulcer Project group, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. J Am Geriatr Soc. 2009;57(7):1175–1183.
- Kottner J, Balzer K, Dassen T, et al. Pressure ulcers: a critical review of definitions and classifications. Ostomy Wound Manage. 2009;55(9):22–29.
- Liu J, Rybakina EG, Korneva EA, et al. Effects of Derinat on ischemia-reperfusion-induced pressure ulcer mouse model. J Pharmacol Sci. 2018;138(2):123–130.
- Zhang X, Xue H, Zhou P, et al. Angelica polysaccharide alleviates oxidative response damage in HaCaT cells through up-regulation of miR-126. Exp Mol Pathol. 2019;110:104281.
- Ebbesen KK, Hansen TB, Kjems J. Insights into circular RNA biology. RNA Biol. 2017;14(8):1035–1045.
- Li Y, Gao X, Wang Z, et al. Circular RNA 4099 aggravates hydrogen peroxide-induced injury by down-regulating microRNA-706 in L02 cells. Life Sci. 2019;116826.
- Glazar P, Papavasileiou P, Rajewsky N. circBase: a database for circular RNAs. RNA (New York, NY). 2014;20(11):1666–1670.
- Laity JH, Lee BM, Wright PE. Zinc finger proteins: new insights into structural and functional diversity. Curr Opin Struct Biol. 2001;11(1):39–46.
- Boeckel JN, Jae N, Heumuller AW, et al. Identification and characterization of hypoxia-regulated endothelial circular RNA. Circ Res. 2015;117(10):884–890.
- Rybak-Wolf A, Stottmeister C, Glazar P, et al. Circular RNAs in the mammalian brain are highly abundant, conserved, and dynamically expressed. Mol Cell. 2015;58(5):870–885.
- Legnini I, Di Timoteo G, Rossi F, et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol Cell. 2017;66(1):22–37.e9.
- Zhu L, Liu Y, Yang Y, et al. CircRNA ZNF609 promotes growth and metastasis of nasopharyngeal carcinoma by competing with microRNA-150-5p. Eur Rev Med Pharmacol Sci. 2019;23(7):2817–2826.
- Wu L, Xia J, Yang J, et al. Circ-ZNF609 promotes migration of colorectal cancer by inhibiting Gli1 expression via microRNA-150. J BUON. 2018;23(5):1343–1349.
- Rossi F, Legnini I, Megiorni F, et al. Circ-ZNF609 regulates G1-S progression in rhabdomyosarcoma. Oncogene. 2019;38(20):3843–3854.
- Xiong Y, Zhang J, Song C. CircRNA ZNF609 functions as a competitive endogenous RNA to regulate FOXP4 expression by sponging miR-138-5p in renal carcinoma. J Cell Physiol. 2019;234(7):10646–10654.
- Liu C, Yao MD, Li CP, et al. Silencing of circular RNA-ZNF609 ameliorates vascular endothelial dysfunction. Theranostics. 2017;7(11):2863–2877.
- Srinoun K, Sathirapongsasuti N, Paiboonsukwong K, et al. miR-144 regulates oxidative stress tolerance of thalassemic erythroid cell via targeting NRF2. Ann Hematol. 2019;98(9):2045–2052.
- Morii K, Yamasaki S, Doi S. microRNA-200c regulates KLOTHO expression in human kidney cells under oxidative stress. PLos One. 2019;14(6):e0218468.
- Chen Z, Su X, Shen Y, et al. MiR322 mediates cardioprotection against ischemia/reperfusion injury via FBXW7/notch pathway. J Mol Cell Cardiol. 2019;133:67–74.
- Xu L, Sun H, Zhang M, et al. MicroRNA-145 protects follicular granulosa cells against oxidative stress-induced apoptosis by targeting Kruppel-like factor 4. Mol Cell Endocrinol. 2017;452:138–147.
- Hui Y, Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-kappaB signaling. Life Sci. 2018;207:212–218.
- Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974–1003.
- Wei S, Wang H. Ligustrazine promoted hypoxia-treated cell growth by upregulation of miR-135b in human umbilical vein endothelial cells. Exp Mol Pathol. 2019;106:102–108.
- Shen L, Bai Y, Han B, et al. Non-coding RNA and neuroinflammation: implications for the therapy of stroke. Stroke Vasc Neurol. 2019;4(2):96–98.
- Liu R, Wang Q, Chang W, et al. Characterisation of the circular RNA landscape in mesenchymal stem cells from psoriatic skin lesions. Eur J Dermatol. 2019;29(1):29–38.
- Greene J, Baird AM, Casey O, et al. Circular RNAs are differentially expressed in prostate cancer and are potentially associated with resistance to enzalutamide. Sci Rep. 2019;9(1):10739.
- Ji X, Mao J, Zhou S. Rs739837 polymorphism in MiR-885-3p binding site within 3′-untranslated region of Vitamin D receptor is associated with a decreased risk of pressure ulcers. Cell Physiol Biochem. 2017;44(6):2129–2137.
- Yan JJ, Qiao M, Li RH, et al. Downregulation of miR-145-5p contributes to hyperproliferation of keratinocytes and skin inflammation in psoriasis. Br J Dermatol. 2019;180(2):365–372.
- Dong H, Jiang W, Chen H, et al. MicroRNA-145 attenuates IL-6-induced enhancements of sensitivity to UVB irradiation by suppressing MyD88 in HaCaT cells. Int J Immunopathol Pharmacol. 2018;32:205873841879594.
- Balci S, Ayaz L, Gorur A, et al. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346–351.
- Wang JJ, Liu C, Shan K, et al. Circular RNA-ZNF609 regulates retinal neurodegeneration by acting as miR-615 sponge. Theranostics. 2018;8(12):3408–3415.
- Peng L, Chen G, Zhu Z, et al. Circular RNA ZNF609 functions as a competitive endogenous RNA to regulate AKT3 expression by sponging miR-150-5p in Hirschsprung’s disease. Oncotarget. 2017;8(1):808–818.
- Wang S, Xue X, Wang R, et al. CircZNF609 promotes breast cancer cell growth, migration, and invasion by elevating p70S6K1 via sponging miR-145-5p. Cancer Manag Res. 2018;10:3881–3890.
- Ren R, Chen SD, Fan J, et al. miRNA-138 regulates MLK3/JNK/MAPK pathway to protect BV-2 cells from H2O2-induced apoptosis. Bratislava Med J. 2018;119(5):284–288.
- Sinha K, Das J, Pal PB, et al. Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol. 2013;87(7):1157–1180.
- Martinez-Revelles S, Garcia-Redondo AB, Avendano MS, et al. Lysyl oxidase induces vascular oxidative stress and contributes to arterial stiffness and abnormal elastin structure in hypertension: role of p38MAPK. Antioxid Redox Signal. 2017;27(7):379–397.