Abstract
To compare the clinical efficacy of gastric bypass in obese patients with T2DM with different BMI. Serum leptin, adiponectin, triglyceride (TG), cholesterol (CHOL) were measured as the indicators to show clinical efficacy after laparoscopic gastric bypass surgery (LRYGB). For patients with high BMI and patients with low BMI, the therapeutic effect of LRYGB surgical diabetes is more significant. The postoperative remission rate of diabetes in the high BMI group was not correlated with the preoperative lipid metabolism index but was positively correlated with the postoperative lipid metabolism index CHOL, TG, leptin, adiponectin. The postoperative remission rate of diabetes in the low BMI group was positively correlated with the preoperative abnormal lipid metabolism of the patients, and positively correlated with the postoperative remission of leptin and adiponectin, but was not correlated with the postoperative remission of total CHOL and TG. The increase of serum adiponectin level and the decrease of leptin resistance after LRYGB surgery restored the metabolic balance of leptin and adiponectin, improved insulin resistance (IR), and thus improved blood glucose level. Therefore, LRYGB has a definite therapeutic effect on obese patients with T2DM, and elevated adiponectin and improved leptin resistance are some of the mechanisms of surgical treatment of diabetes.
Introduction
Laparoscopic gastric bypass (LRYGB) is one of the common metabolic and bariatric surgical procedures, which can improve glucose metabolism and other metabolic disorders [Citation1–2]. LRYGB is recommended as an effective therapeutic strategy for T2DM and obesity by multiple international diabetes organizations [Citation3]. Insulin resistance is confirmed to be one of the major pathophysiologic mechanisms in T2DM.
Abnormal accumulation of adipose tissue is the pathological essence of obesity-related diseases and an independent risk factor for T2DM [Citation4]. Studies have shown that adipose tissue is a highly active endocrine and important metabolic tissue that secretes adipokines such as leptin and adiponectin, and the leptin and adiponectin pathways are the main metabolic pathways which regulate insulin resistance. Insulin resistance can be significantly reduced after metabolic surgery, a major mechanism of postoperative improvement of glucose homeostasis [Citation5]. In the state of obesity, the metabolic disorder of adiposity leads to the disorder of insulin resistance, glucose metabolism, and energy metabolism, leading to the occurrence and development of T2DM [Citation6].
There was a correlation between fat metabolism and postoperative glucose relief: patients with hyperlipidaemia before the operation had a high remission rate of diabetes after the operation. Preoperative BMI was correlated with postoperative glucose relief: Patients with high preoperative BMI had a higher rate of diabetes remission after surgery. Recently, the role of metabolic surgery has been explored as the treatment of T2DM patients with low BMI [Citation7–10]. Moreover, the mean BMI of T2DM patients is just 24 kg/m2 in China [Citation11]. Accordingly, the recommendations of surgical indications in the Chinese guidelines for the surgical treatment of obesity and T2DM (Patients with diabetes mellitus with BMI ≥ 32.5 kg/m2 can be treated surgically; patients with T2DM with BMI ≥ 27.5 kg/m2 and ineffective medical treatment can also be treated surgically), on the premise of BMI was proposed, according to different BMI of T2DM patients laparoscopic gastric bypass and conduct prospective research, serum leptin, adiponectin, triglyceride, and cholesterol levels were observed before and after the operation. To explore the relationship between the improvement of insulin resistance and the balance of lipid metabolism after surgery, to provide the basis for clinical treatment and the development of reasonable and accurate surgical selection criteria, to maximize the satisfactory clinical efficacy of surgical patients.
Material and methods
Patients
A total of 83 obese patients with T2DM were included in this study from 2015 to 2016. The cases were from Nankai hospital of Tianjin (60 cases) and the general hospital of Tianjin medical university (23 cases). All cases met the above-mentioned inclusion criteria. There were 33 males and 50 females, aged 40.4 ± 4.3 years. Of the 83 cases, 27 cases received oral hypoglycaemic drug therapy, 25 cases received insulin therapy, 18 cases received oral drug + insulin combined therapy, and 13 cases received no systematic treatment. Patients with different BMI were divided into two groups with high and low BMI by taking 32.5 kg/m2 as the boundary, among which 57 cases were in the high BMI group and 26 cases were in the low BMI group.
Inclusion criteria
All patients in the study met the following four criteria [Citation12]: (1) Definite diagnosis of T2DM and poor control of blood glucose (glycosylated haemoglobin >7%); (2) It met the surgical indications in the Chinese guidelines for surgical treatment of obesity and T2DM (2014), and its body mass index (BMI) was ≥27.5; (3) Health and disease conditions are suitable for surgery, without contraindications; (4) Good compliance, can cooperate with treatment and follow-up.
Diagnostic criteria for T2DM, according to the 2010 ADA diagnostic criteria for diabetes(meet one of the following criteria): (1) Haemoglobin A1c (HBA1C) ≥6.5%; (2) Fasting blood glucose (FPG) ≥7.0 mmol/L (126 mg/dL); (3) OGTT test 2 h blood glucose ≥11.1 mmol/L (200 mg/dL); (4) have symptoms of hyperglycaemia or hyperglycaemia crisis, and random blood glucose ≥11.1 mmol/L (200 mg/dL). If there is no hyperglycaemia, item (1) ∼ (3) should be reviewed the next day.
Surgical indications in Chinese guidelines for surgical treatment of obesity and T2DM (2014)[Citation13] are as follows: (1) the course of T2DM is ≤15 years, and the pancreatic islet still has certain insulin secretion function, and fasting serum c-peptide is ≥1/2 of the lower limit of normal value; (2) patients’ BMI is an important clinical standard for determining whether they are suitable for surgery (); (3) When male waist circumference ≥90 cm and female waist circumference ≥85cm, the recommended level of surgery can be improved appropriately; (5) the recommended age range is 16–65 years
Table 1. Inclusion criteria for patients with T2DM treated surgically.
Acidemia, male sexual dysfunction, polycystic ovary syndrome, deformed arthritis, renal dysfunction, etc., especially with cardiovascular risk factors or chronic complications of T2DM; c: It has a certain curative effect, but lack of sufficient evidence of long-term curative effect at home and abroad.
Principles of research design
Prospective studies were used, and laparoscopic gastric bypass (LRYGB) was used for all patients who met the inclusion criteria; patients requiring laparoscopic gastric sleeve resection after admission were excluded from this study. Patients were divided into two groups according to preoperative BMI, the high BMI group (BMI ≥ 32.5), and the low BMI group (32.5 > BMI ≥ 27.5). Subjects in both groups underwent standard gastric bypass surgery and were followed up at 13,691,218 months and 2 years after surgery.
Therapeutic method
Before the operation, patients’ blood glucose was controlled at about 8 mmoL by making a healthy diet plan, exercise plan, and medication. In addition, the levels of glycosylated haemoglobin in patients were decreased to 6.5–7.0%, and urine glucose and urine ketone body were both controlled to negative.
The patient was lying on his back with his head high and feet low. Using the standard 5-hole method, a longitudinal incision about 1 cm long was made on the umbilicus, 10 mm trocar was placed and pneumoperitoneum was established. 13 mm trocar was placed on the outer edge of the left and right rectus abdominis, respectively. 5 mm trocar was placed under the xiphoid process and the lower edge of the costal arch of the left clavicular midline. LRYGB was carried out after no obvious abnormality was found in the whole abdominal cavity. (1) The mesentery was separated from the lesser curvature of the stomach 8–10 cm away from the cardia by the ultrasonic knife until it entered the lesser omental sac 4–5 cm away from the cardia. Under the endoscope, the gastric body was cut and closed by the linear cutting stapler (45 mm blue nail) from the distal end of the lesser curvature of the separated mesentery to the greater curvature (about 45° with the lesser curvature of the stomach). Under endoscopy, the gastric body and the bottom of the stomach were cut and closed to his angle on the left side of the cardia with a straight-line cutting stapler, and the small gastric capsule with a volume of 20–30 mL was made. (2) After lifting the distal jejunum (anterior to colon), a small hole was poked at the lesser curvature of the gastric capsule and the opposite side of the mesentery of the distal jejunum, and the lesser curvature of the stomach was anastomosed with the opposite side of the mesentery. After checking the anastomotic site without tension and active bleeding, cut and close the broken end of gastrojejunal anastomotic site with a straight-line cutting suture device. (3) A small hole was made at a distance of about 100 cm from the distal part of the gastrojejunal anastomosis, and then a small hole was made at the opposite side of the mesenteric edge of the proximal jejunal segment, and then the common opening was closed. (4) After washing the abdominal cavity thoroughly and checking the gastrointestinal and intestinal anastomoses without bleeding, a drainage tube was placed on the left side of the cardia and the operation was completed.
After the operation, antibiotics, which were given to prevent infection, routine nutritional support, fluid supplement, and electrolyte solution were given to patients. 24 h after the operation, the patients were encouraged to get out of bed and drink a little water. Upper gastrointestinal angiography was performed at 48 h to check whether there was a fistula at the gastrointestinal anastomosis in patients. In addition, patients need to take trace elements and multivitamins for a long time, including 1 or 2 pieces of Centrum gamma tablets (1.33 or 2.66 g), 1 piece of Calci D tablet (600 mg), 1 vitamin D capsule (1.2 g) and 2 pieces of complex B vitamin tablets (1100 mg). Of course, it needed to make necessary adjustments or supplement other vitamins or trace elements according to the patient’s situation.
Evaluation indicators
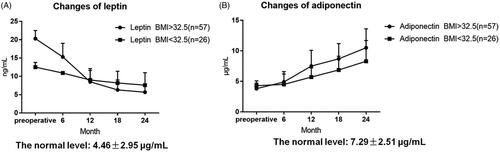
Serum leptin, adiponectin (ELISA), FPG, 2 h postpranonal blood glucose (2hBG), HbA1c, fasting insulin (FIns), TG, CH, and BMI were detected before and after surgery at 6, 12, 18 and 24 months, and insulin resistance (HOMA-IR) and %EWL were calculated. In addition, the levels of fasting serum leptin and adiponectin in normal person were 4.46 ± 2.95 μg/mL and 7.29 ± 2.51 μg/mL, respectively [Citation14,Citation15].
Analysis of Results
Remission criteria for type 2 diabetes [Citation16]:
Complete remission: FBG < 5.6 mmol/L and HbA1c <6% at 1 year after discontinuation of medication;
Partial remission: FBG at 5.6–6.9 mmol/L and HbA1c <6.5% after 1 year of discontinuation of medication;
Improvement: glycosylated haemoglobin decreased more than 1% or FBG decreased more than 25 mg/dL significantly for at least 1 year, or a decrease in glycosylated haemoglobin or FBG accompanied by a decrease in the demand for hypoglycaemic drugs (discontinuation of insulin or an oral hypoglycaemic agent; or cut the dosage in half);
No change: no mitigation or improvement as described earlier;
Relapse: FBG or glycosylated haemoglobin remains at diabetic levels after the first complete or partial remission, or hypoglycaemic agents may be needed.
Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, Illinois). Enumeration data were statistically described by frequency and measurement data were expressed as mean ± SD. Group data t-test (anterograde variance homogeneity test and normality test) was used to compare the same time indexes between the two groups. Chi-square test was used, p < .05 indicated statistical difference.
Results
Basic information of the research objects
There was no statistically significant difference in age, gender, and preoperative diabetes-related indicators between the two groups (p > .05; ). All patients received conventional LRGB surgery with full informed consent, and postoperative medication was adjusted according to the patient’s diabetes status.
Table 2. Preoperative general conditions and diabetes indicators in the low and high BMI groups.
General postoperative conditions
All the 83 patients successfully completed the operation, without conversion to laparotomy, and the intraoperative blood loss was (30 ± 2.6) mL, the operation time was (105 ± 13) min. There were no postoperative complications such as anastomotic bleeding, obstruction and anastomotic leakage. The length of stay was (9 ± 1.4) days. No cases were lost to follow-up after 24 months.
Changes in obesity-related indicators
Two years after surgery, %EWL in the high BMI group was 67.9 ± 9.3, and %EWL in the low BMI group was 72.1 ± 8.7. The weight loss effect was significant in both groups ().
Changes in glucose metabolism and insulin function
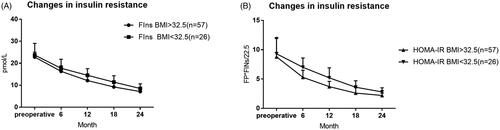
The rate of complete remission and partial remission of T2DM was 48.2% (40/83) and 72.3% (60/83) in all patients 24 months after surgery, respectively. The diabetes remission rate (complete remission + partial remission) in the high BMI group was higher than that in the low BMI group, and the difference was statistically significant (p < .05). Cases of the two groups (high BMI group and low BMI group) gradually decreased after the operation of FPG and 2hPG, the lowest was at 18 months after the operation, compared with the preoperative and postoperative time points were significantly reduced, the difference was statistically significant (p < .05). 24 months after surgery, the HbA1c was significantly different from that before surgery in the two groups (p < .05). After the operation, fasting insulin (FIns) in both groups showed a gradually decreasing trend, reaching the lowest 24 months after the operation. Compared with preoperative FIns, the difference was statistically significant (p < .05). Insulin resistance continued to improve within 2 years in both groups ( and , ).
Figure 1. Changes of %EWL after operation. Percentage of excess weight loss are plotted for the 6, 12, 18 and 24 month time points. Error bars indicate 95% CIs; P values for differences are all <0.05.
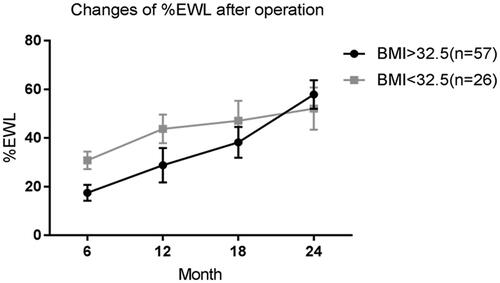
Figure 3. Changes in insulin resistance. Percentage of excess weight loss are plotted for the preoperative, 6, 12, 18, and 24 month time points. p-Values for differences are all <.05 except preoperative. FIns: fasting insulin; HOMA-IR: insulin resistance.
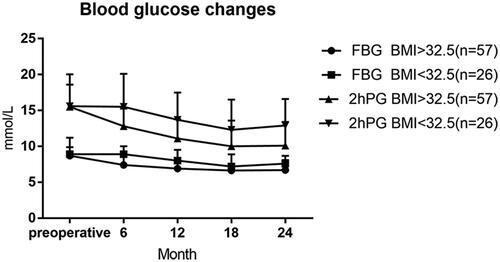
Table 3. Remission and cure of diabetes.
Blood lipid change
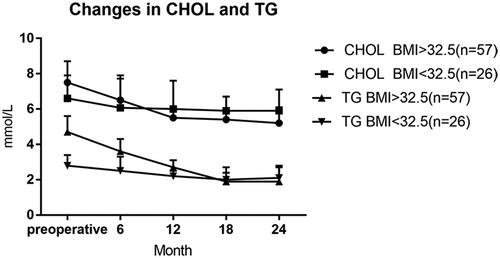
24 months after surgery, cholesterol and triglyceride showed a gradually decreasing trend, and there were statistically significant differences between the two groups before surgery (p < .05), indicating that LRYGB surgery was effective for abnormal lipid metabolism in both groups ().
Changes in leptin and serum adiponectin
The fasting leptin level in the two groups was higher than that in the normal population before the operation, and the fasting leptin level was significantly decreased 2 years after the operation. The difference in time points was statistically significant compared with that before the operation (p < .05). Preoperative fasting adiponectin level in the two groups was lower than that in the normal population, and postoperative fasting adiponectin level showed a gradually increasing trend, and the difference in time points was statistically significant compared with that before surgery (p < .05). After 2 years, adiponectin levels in both groups reached normal population levels ().
Analysis of the related factors influencing the curative effect of postoperative diabetes in the two groups
Analysis of related factors influencing postoperative diabetic efficacy in high BMI group
The patients with complete and partial remission of diabetes after surgery in the group with high BMI were defined as the group with high BMI remission, and those who met the requirements of diabetes improvement after surgery was defined as the group with high BMI improvement. There were no invalid cases in this study. The results showed that the postoperative efficacy of LRYGB in the high BMI group was not correlated with the course of diabetes (p > .05), but correlated with whether or not to use insulin before the operation. The proportion of insulin use in the remission group was lower than that in the improvement group, and the difference was statistically significant (p < .05). There was no correlation between preoperative glucose and lipid metabolism indexes and postoperative diabetes remission in the patients, and the difference was not statistically significant (p > .05). However, the changes in glucose and lipid metabolism indexes after surgery are correlated with the postoperative remission of diabetes mellitus. The glucose and lipid metabolism indexes in the remission group recover better than those in the improvement group, and there is a statistical difference between the two groups (p < .05). In addition, the change of %EWL 2 years after surgery was related to the postoperative remission of diabetes mellitus. The improvement group had a better weight loss effect than the remission group, and there was a statistical difference between the two groups (p < .05; and ; ).
Figure 6. Analysis of glucose metabolism factors influencing the effect of 2 years post-operation diabetes in the group with high BMI. p-Values for differences are <.05 after 2 years in FIn and HOMA-IR.
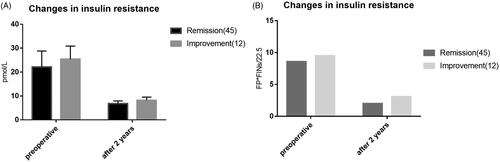
Figure 7. Analysis of lipid metabolism factors influencing the efficacy of diabetes mellitus 2 years after operation in the group with high BMI. p-Values for differences are <.05 after 2 years in TG, CHOL, Leptin, Adiponectin.
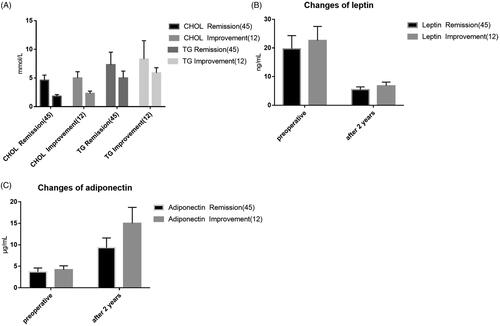
Table 4. Analysis of other factors influencing the efficacy of diabetes mellitus 2 years after operation in the high BMI group.
Analysis of factors related to the effect of low BMI group on postoperative diabetic efficacy
In the low BMI group, the patients with complete and partial remission of postoperative diabetes were defined as the low BMI remission group, and the patients with postoperative diabetes were defined as the low BMI improvement group. In this study, there were no invalid cases. The results of the study showed that the postoperative efficacy of LRYGB in the low BMI group was correlated with the preoperative course of diabetes and the proportion of insulin used before surgery. The patients with a short preoperative course of diabetes and those without insulin treatment had a high rate of postoperative diabetes remission, and the difference between the two groups was statistically significant (p < .05). Abnormal glucose and lipid metabolism indicators before the operation were correlated with postoperative efficacy, and the difference was statistically significant (p < .05). Postoperative efficacy was correlated with changes in insulin resistance, leptin, and adiponectin after surgery, with statistically significant differences (p < .05). There was no significant correlation between the changes in insulin level, total cholesterol and triglyceride after the operation, and the differences were not statistically significant (p > .05). The results indicated that the remission of diabetes after gastric bypass with low BMI was not completely correlated with the changes in glucose metabolism and lipid metabolism after surgery. In addition, the study showed that 2 years after surgery %EWL was not correlated with the remission of diabetes, and the difference was not statistically significant (p > .05), indicating that the remission of diabetes was not significantly related to the patient’s weight loss ( and ; ).
Figure 8. Analysis of glucose metabolism factors influencing the effect of 2 years post-operation diabetes in the low BMI group. p-Values for differences are <.05 after 2 years in HOMA-IR.
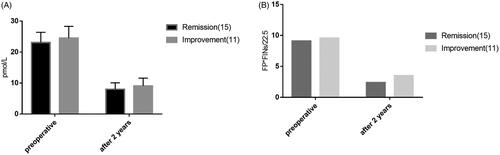
Figure 9. Analysis of lipid metabolism factors influencing the effect of 2 years of postoperative diabetes in the low BMI group. p-Values for differences are <.05 preoperative in TG, CHOL, Adiponectin, after 2 years and preoperative both <.05.
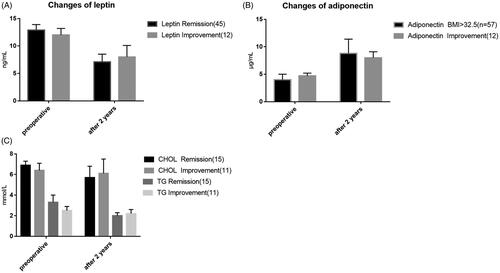
Table 5. Analysis of other factors influencing the efficacy of diabetes mellitus 2 years after operation in the low BMI group.
Discussion
The interaction effects of laparoscopic gastric bypass on lipid and glucose metabolism
Adipocytes secrete a variety of bioactive adipokines, which are involved in regulating glucose metabolism, insulin and energy metabolism. The core of weight loss after weight loss is a decrease in the total number of fat cells. In this study, it was found that during the follow-up period of 24 months after the operation of LRYGB, the patients’ BMI was significantly reduced, while TG and CH were significantly improved, indicating that the function of fat cells was significantly changed at the same time of weight loss. Leptin, a protein hormone derived from adipose tissue, has the biological effects of suppressing appetite, reducing energy intake, improving metabolism rate and reducing fat accumulation. Studies have confirmed that: in obese patients, the accumulation of fat, increased secretion of fat cells and increased levels of leptin in peripheral blood, but due to the obstruction of central signalling, the physiological dose of leptin produces physiological effects lower than normal, namely, leptin resistance [Citation17]. On the one hand, high levels of leptin interfere with muscle sensitivity to insulin by promoting the decomposition of fat cells and increasing the concentration of free fatty acids. Leptin; on the other hand, reduces the biological effects of insulin, the development of insulin resistance. In T2DM patients with obesity, insulin resistance is associated with lipid metabolism. This pattern of lipid abnormalities is thought to be secondary to insulin resistance [Citation18]. Adiponectin is a lipid-derived plasma protein that has a positive regulating effect on insulin, and high adiponectin level helps to enhance insulin sensitivity and antagonize insulin resistance [Citation19]. In the state of obesity, the fat capacity of patients increases, while the plasma concentration of adiponectin decreases, the antagonistic effect of insulin resistance decreases, and insulin sensitivity decreases. In this study, it was found that the expression of leptin after LRYGB showed the same trend with the change of insulin resistance (IR), while the expression of adiponectin showed the opposite trend with the change of IR. The gradual decrease of IR after surgery was accompanied by the return to normal fasting glucose.
Obese patients with T2DM may be associated with hyperlipidaemia. In this pathological state, the metabolic disorder of adipokine is mainly manifested by leptin resistance and down-regulation of adiponectin expression [Citation20], which is also the pathological mechanism of insulin resistance. Gastric bypass re-establishes adipokine homeostasis through weight loss or other mechanisms: reduced leptin resistance and up-regulated adiponectin expression, thereby improving insulin resistance. Its possible mechanisms of improving IR is that leptin resistance loss, central leptin receptor sensitivity to leptin, loss of appetite, promote adipose decompose, increase energy metabolism, promote the liver, skeletal muscle for glucose uptake, and weakened the control of the pancreas, reduce the action of the islet beta cells, directly promoting synthesis and secretion of insulin by the pancreas. Moreover, the effect of insulin is to promote the reduction of blood fat, leading to the reduction of insulin resistance. Adiponectin levels, inhibit liver glycogen, enhanced the output at the same time increase the body to absorb and oxidation of lipid, strengthening transshipment and fatty acid oxidation in skeletal muscle cells, increase fat burning and the release of energy, at the same time promote the lipid gathered to the adipose cell, skeletal muscle from liver lipid infiltration, reducing blood lipid levels, enhance the liver and skeletal muscle insulin sensitivity, inhibit insulin resistance [Citation21].
In summary, reduced adiponectin levels and leptin resistance are common in obese and T2DM patients. The increase of serum adiponectin level and the decrease of leptin resistance after LRYGB surgery restored the metabolic balance of leptin and adiponectin, improved insulin resistance, and thus improved blood glucose level. Therefore, LRYGB has a definite therapeutic effect on obese patients with T2DM, and elevated adiponectin and improved leptin resistance are some of the mechanisms of surgical treatment of diabetes.
The effect of different BMI after LRYGB surgery in T2DM and the change of lipid metabolism
In this study, it was found that patients with high BMI had a more significant therapeutic effect on LRYGB surgical diabetes than those with low BMI. In addition, postoperative diabetes remission rate and weight loss effects were correlated with improved lipid metabolism in patients with high BMI. The postoperative remission rate of diabetes in patients with low BMI was correlated with the presence of abnormal lipid metabolism before surgery. For patients with abnormal lipid metabolism, surgery can improve their abnormal lipid metabolism and achieve better results in the treatment of diabetes. Numerous studies have demonstrated the high levels of metabolic risk factors at relatively low levels of BMI among the Asian population because they have a significantly high level of subcutaneous and visceral fat, which corresponds to a high risk of cardiovascular and metabolic disease [Citation22,Citation23]. Therefore, BMI is regarded as an indication for the appropriateness of metabolic surgery is insufficient [Citation24]. This discovery could lead to more accurate screening of patients before surgery. It is recommended that patients with T2DM with low BMI and no obvious metabolic abnormalities should carefully consider whether to have surgery, so as to ensure a higher postoperative remission rate and cure rate of diabetes.
Analysis of the influencing factors of different BMI 2 diabetes patients with gastric bypass
This study found that after LRYGB surgery, patients with high BMI and patients with low BMI could obtain a better diabetes remission rate. The reason for the better effect may be that people with high BMI have higher insulin resistance and have a higher degree of insulin resistance relief after surgery. Lee et al. [Citation25] observed that weight loss remained the dominant influence on the remission of T2DM following metabolic surgery in non-obese patients. The Korean study also found that the remission of T2DM was related to a postsurgical weight loss of more than 12% [Citation26]. Thus, it is still controversial and more clinical trials should be necessary before a conclusion can be made in the weight loss effect on diabetes remission.
The postoperative diabetes remission rate of the two groups was negatively correlated with the preoperative use of insulin therapy. The postoperative diabetes remission rate of the patients with low BMI was correlated with the length of diabetes history of the patients, while the patients with high BMI had no correlation. Due to the fact that most patients receiving insulin therapy have impaired insulin secretion and need to be supplemented with exogenous insulin, the islet function of patients receiving insulin therapy is worse than that of those receiving pure drug therapy. Although they have undergone surgery to improve insulin resistance and increase insulin secretion through other mechanisms, the impairment of the islet function itself will affect the long-term efficacy [Citation27]. The longer the history of diabetes, the greater the chance of islet damage. However, in high BMI patients, insulin resistance caused by obesity leads to the earlier onset of diabetes, so the effect of diabetes history on islet function is secondary in high BMI cases.
The postoperative remission rate of diabetes in the high BMI group was not correlated with the preoperative lipid metabolism indexes of the patients but was positively correlated with the remission degree of postoperative lipid metabolism indexes (CHOL, TG, leptin, adiponectin). The postoperative remission rate of diabetes in the low BMI group was positively correlated with the preoperative abnormal lipid metabolism of the patients, and positively correlated with the postoperative remission of leptin and adiponectin, but was not correlated with the postoperative remission of CHOL and TG. This phenomenon indicates that for T2DM patients with low BMI, preoperative hyperlipidaemia is a favourable factor for predicting postoperative remission of diabetes. The combination of abnormal lipid metabolism may aggravate the patient’s insulin resistance, while gastric bypass metabolic surgery can relieve insulin resistance to a greater extent through some mechanisms, so the diabetes relief effect is good [Citation28,Citation29]. The postoperative remission of leptin and adiponectin was positively correlated with the remission of diabetes mellitus, suggesting that these two factors play an important role in reducing the resistance of diabetes mellitus. In addition, there was no correlation between the remission of diabetes after gastric bypass with low BMI and the remission of total cholesterol and triglyceride after the surgery, suggesting that there are still some unclear mechanisms of lipid-mediated changes in insulin resistance that need to be further explored.
The change of %EWL 2 years after surgery was correlated with the postoperative diabetes remission in the high BMI group, but not in the low BMI group. The results show that for patients with high BMI, effective weight loss is one of the important factors to achieve long-term stable improvement of blood glucose, while for patients with low BMI, the remission of diabetes has little relationship with the effect of weight loss, and more endocrine factors are needed to regulate blood glucose, with a more complex mechanism. In summary, for T2DM patients with high BMI, LRYGB can achieve a better remission rate of diabetes, but it is affected by the long-term weight loss effect and the remission of lipid metabolism indicators (total cholesterol, triglyceride, leptin, and adiponectin), indicating that postoperative management guidance plays an important role for these patients. But for patients with T2DM lower BMI, postoperative diabetes remission rate more and preoperative indicators related: history length, hyperlipidaemia, leptin and adiponectin levels, insulin resistance, etc., to evaluate this request in preoperative sufficient, grasp the more strict operation indication, in order to get better postoperative diabetes remission rate.
Ethical approval
This study was approved by the Ethics Committee of the Tianjin Nankai hospital. Participants have provided their written informed consent to participate in this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122(3):248–256.
- Li W, Zhu L, Mo Z, et al. Effect of laparoscopic Roux-en-Y gastric bypass on body composition and insulin resistance in Chinese patients with type 2 diabetes mellitus. Obes Surg. 2014;24(4):578–583.
- Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814.
- Kansou G, Lechaux D, Delarue J, et al. Laparoscopic sleeve gastrectomy versus laparoscopic mini gastric bypass: one year outcomes. Int J Surg. 2016;33:18–22.
- Benaiges D, Flores Le-Roux JA, Pedro-Botet J, et al. Sleeve gastrectomy and Roux-en-Y gastric bypass are equally effective in correcting insulin resistance. Int J Surg. 2013;11(4):309–313.
- Dodd GT, Decherf S, Loh K, et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160(1–2):88–104.
- Geloneze B, Geloneze SR, Chaim E, et al. Metabolic surgery for non-obese type 2 diabetes: incretins, adipocytokines, and insulin secretion/resistance changes in a 1-year interventional clinical controlled study. Ann Surg. 2012;256(1):72–78.
- Navarrete SA, Leyba JL, Llopis SN. Laparoscopic sleeve gastrectomy with duodenojejunal bypass for the treatment of type 2 diabetes in non-obese patients: technique and preliminary results. Obes Surg. 2011;21(5):663–667.
- Baskota A, Li S, Dhakal N, et al. Bariatric surgery for type 2 diabetes mellitus in patients with BMI <30 kg/m2: a systematic review and meta-analysis. PLoS One. 2015;10(7):e0132335.
- Reis CE, Alvarez-Leite JI, Bressan J, et al. Role of bariatric-metabolic surgery in the treatment of obese type 2 diabetes with body mass index <35 kg/m2: a literature review. Diabetes Technol Ther. 2012;14(4):365–372.
- Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959.
- Liu JG, Zheng CZ, Wang Y. Guidelines for surgical treatment of obesity and type 2 diabetes in China. Chin J Pract Surg. 2014;34(11):1005–1010.
- Zhang Y, Zhao H, Cao Z, et al. A randomized clinical trial of laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy for the treatment of morbid obesity in China: a 5-year outcome. Obes Surg. 2014;24(10):1617–1624.
- Shu C, Shangping D, Tong W, et al. Normal serum leptin and its relationship with type 2 diabetes in Chengdu, China. Chin J Endocrinol Metabol. 2000;3:57.
- Hongyan W, Zhusen L, Dajin Z. Analysis of serum adiponectin level in normal people. J Naval Gen Hospital. 2005;1:29–32.
- Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care. 2009;32(11):2133–2135.
- Cui H, López M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol. 2017;13(6):338–351.
- Haffner SM, Stern MP, Hazuda HP, et al. Cardiovascular risk factors in confirmed prediabetic individuals. Does the clock for coronary heart disease start ticking before the onset of clinical diabetes? JAMA. 1990;263(21):2893–2898.
- Farooqi IS, O’Rahilly S. 20 years of leptin: human disorders of leptin action. J Endocrinol. 2014;223(1):T63–T70.
- Engin A. Adiponectin-resistance in obesity. Adv Exp Med Biol. 2017;960:415–441.
- Shoar S, Saber AA. Long-term and midterm outcomes of laparoscopic sleeve gastrectomy versus Roux-en-Y gastric bypass: a systematic review and meta-analysis of comparative studies. Surg Obes Relat Dis. 2017;13(2):170–180.
- Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord. 1998;22(12):1164–1171.
- Ko GT, Tang JS. Waist circumference and BMI cut-off based on 10-year cardiovascular risk: evidence for “central pre-obesity”. Obesity. 2007;15(11):2832–2839.
- Cummings DE, Cohen RV. Beyond BMI: the need for new guidelines governing the use of bariatric and metabolic surgery. Lancet Diabetes Endocrinol. 2014;2(2):175–181.
- Lee WJ, Almulaifi A, Chong K. The effect and predictive score of gastric bypass and sleeve gastrectomy on type 2 diabetes mellitus patients with BMI < 30 kg/m(2). Obes Surg. 2015;25(10):1772–1778.
- Kim JW, Cheong JH, Hyung WJ, et al. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18(1):49–54.
- Aguilar D, Fernandez ML. Hypercholesterolemia induces adipose dysfunction in conditions of obesity and nonobesity. Adv Nutr. 2014;5(5):497–502.
- Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149(2):379–388.
- Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366(17):1567–1576.