?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Obesity is associated with ventricular arrhythmia and sudden cardiac death. Numerous studies have shown that obesity may have effects on the heart by affecting the ventricular re-polarisation (VR). As an effective detection method for VR the measurement of the QT interval has been extensively studied in obese patients (OP). This review aims to investigate the relationship between obesity and obesity-related diseases; including diabetes, hypertension and cardiovascular diseases (CVD). This review compares the advantages and disadvantages of different QT interval measurement methods, as well as explores the possible mechanisms of obesity leading to heart disease. Finally, it also reviews the feasibility of various weight loss methods to reverse the risk of obesity leading to heart disease is discussed.
Introduction
Globally, the prevalence of obesity is growing at an alarming rate and it is a major threat to the public health in many parts of the world. A systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants during 1980–2013 shows that in 2008, there were about 1.46 billion obese adults worldwide [Citation1], and the number of obese adults more than doubled during that 33-year period [Citation2].
Obesity often manifests and develops with a number of chronic metabolic diseases, including dyslipidemia, insulin resistance (IR), and type 2 Diabetes [Citation3]. Obesity and these obesity-related diseases can increase cardiovascular morbidity and mortality, which can lead to arrhythmia, heart failure (HF) and sudden cardiac death (SCD) [Citation4,Citation5]. Adjusting the delicate balance between food intake and energy expenditure can effectively reduce the risk of obesity-related diseases.
Comparison of different VR measurement methods
Obesity is associated with a variety of electrocardiogram (ECG) abnormalities [Citation6]. In addition to various indicators of the shape of the heart and the heart rate changes [Citation7], the most studied is ventricular electrophysiology. The indicators of ventricular electrophysiology are various (). There is no consensus on which indicators to use in ventricular electrophysiology.
Table 1. Different indicators in ventricular electrophysiology.
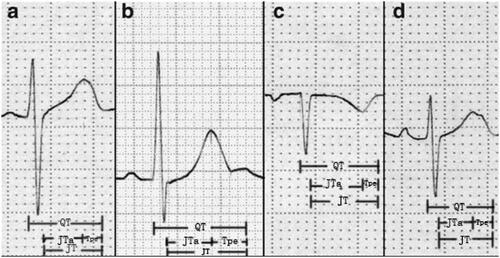
The QT interval is measured from the start of the Q wave to the end of the T wave, including the QRS wave, the ST segment, and the T wave (). When the U wave exists, the end point of QT interval measurement is the lowest point of the curve between the T wave and the U wave [Citation8] (). The normal QT interval range is from 0.35 s to 0.43 s, or 0.39 s ± 0.04 s. Since the QRS wave represents the ventricular depolarisation time and the T wave stands for the VR, the QT interval is the measure of ventricular electrical activity.
Figure 1. Measurement of RV indicators. The measurement of QT starts from the Q wave to the end of the T wave, including the QRS wave, the ST segment, and the T wave. JT is defined as the QT to remove the QRS wave portion. Tpe starts from the T peak and ends at the T wave. JTa is the interval between the J point and the apex of the T wave b. When there is a U wave, the T wave end is defined as the lowest point of the curve between the T wave and the U wave.c. In the p-wave inverted electrocardiogram, the representation of QT interval, Tpe, JT and JTa.d. In the bi-peak ECG of T wave, the representation of QT interval, Tpe, JT and JTa.
The difference in heart rate will cause measurement errors of the QT interval. When the heart rate increases, the QT interval is shortened, and when the heart rate is slowed down, it is prolonged. The corrected QT interval according to heart rate is called the QTc interval [Citation9] (). The normal QTc is defined in the range of 400 ms–440 ms. The boundary line of QTc is 431 ms–450 ms in males and 451 ms–470 ms in females. The QTc interval is often derived from ECG lead II, I or V 5 [Citation10]. There are several formulas to calculate QTc. The Bazett formula is a early correction of QT. However, it is overcorrected at high heart rate and undercorrected at low heart rate [Citation11]. Therefore, Fridericia proposed another QT interval formula for correcting heart rate, which was used by many experts [Citation12] (). Compared to other methods, Sagie et al. used the linear regression analysis to pose the QTc formula more effectively when assessing the risk of death for 30 days or 1 year [Citation13] (). Recently, a study investigated the possibility of predicting QTc values in OP by using the best-fit regression method to represent the relationship between QTc and body mass index (BMI), which allowed all the medical and paramedical personnel to establish the cardiovascular risk in the OP immediately [Citation14] ().
QT dispersion (QTd) is proposed as a marker of dispersion of ventricular repolarization and can be obtained by subtracting the minimum QT interval from the maximum QT interval [Citation15] (). QTd was originally proposed to measure the spatial dispersion of VR. It has also been reported that QTd may be a further non-invasive marker of susceptibility to ventricular arrhythmias [Citation16]. In recent years, QTd is considered to be a sign of general abnormalities in VR [Citation17].
JT or JTC intervals are obtained by subtracting the QRS from the QT or QTc. As the indicators of VR, JT and JTc are often used to evaluate the efficacy of antiarrhythmic drugs [Citation18,Citation19] (, ). JTa is the interval between the J point and the apex of the T wave. TaTe is expressed as the interval between the apex of the T wave and the end of the T wave (, ). The two are used as new repolarization parameters, which may be related to arrhythmia under clinical conditions [Citation20].
Recently, other new parameters have been used as indicators of increased risk of arrhythmia, such as T peak‐Tend (T pe), T peak‐Tend dispersion (Tpe-d) and T peak‐Tend/QT ratio (). Tpe is obtained by measuring the interval from the peak of T wave to the end of T wave on the surface ECG [Citation21] (Picture1). Current studies have shown that Tpe, Tpe-d and Tpe/QT can be used as indicators of total dispersion of repolarization (TDR) [Citation22,Citation23].
The relationship between obesity and VR
The association between obesity and VR in the surface ECG has been extensively studied (). In addition, some studies have reported that all or part of VR indicators in the OP did not change [Citation20,Citation51–56], more researches proved that the two have statistical significance. Similar conclusions have been obtained in obese animal models, and weight loss by different means could reduce the association between them [Citation24–26,Citation38,Citation44,Citation57], and as result obesity can affect the VR, thereby increasing the risk of arrhythmia and SCD. Except for cardiac electrical activity, some indicators of echocardiography in the OP were also abnormal [Citation27,Citation30,Citation39,Citation45,Citation58].
Table 2. The association of obesity and cardiac indicators.
Obesity can prolong VR, and obesity-related diseases such as hypertension, diabetes, and IR can enhance this effect. Similar conclusions can be drawn from reports on women, men, children, and animal groups.
The possible mechanisms by which obesity affects the heart
The effect of obesity on the heart is multifaceted and can be discussed from the structure, function of the heart, electrophysiological activity of the cardiomyocytes and metabolism of the body. The influences of these aspects are mutual. Obesity can not only affect the structure of the heart through fat accumulation but also causes metabolic abnormalities including dyslipidemia, increased secretion of pro-inflammatory cytokines, fibrosis [Citation59,Citation60], hyperglycemia [Citation61], High uric acid(HUA) [Citation62] and IR that can influence the electrophysiology and function of the heart.
Heart structure change
Changes in cardiac structure and function caused by obesity are mainly manifested by ventricular remodelling and ventricular hypertrophy, as well as ventricular diastolic and systolic dysfunction, which can cause heart failure when the structure and function are abnormally severe. In addition, elevation of the diaphragmatic level caused by abdominal obesity, and accumulation of fat under the skin and subepicardial-acting as an electrical insulation layer-could cause various changes in the surface ECG [Citation63].
Metabolic disorder
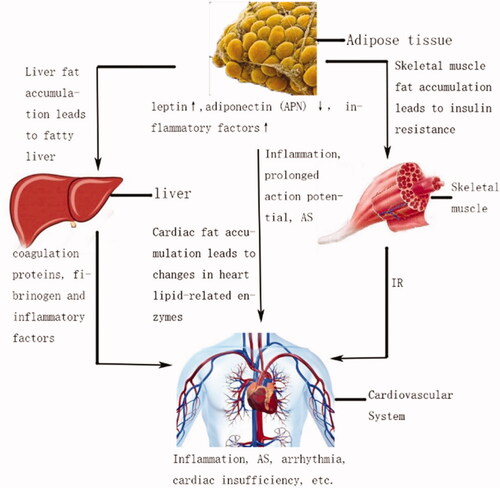
Systemic hyperlipidaemia caused by obesity, especially low-density lipoprotein (LDL) [Citation64,Citation65], or excessive accumulation of lipids in the viscera could lead to cardiovascular disease (CVD) [Citation66,Citation67]. (Picture 2) Oxidatively modified low-density lipoprotein (OX-LDL) has the biological characteristics of being rapidly phagocytosed by macrophages and smooth muscle cells, and is involved in the formation of atherosclerotic plaques. OX-LDL also has strong cytotoxicity that can change the functional state of endothelial cells, accelerating the formation of lipid fringes and arteriosclerosis.
Adipose tissue can secrete a variety of adipokines, such as leptin, adiponectin (APN) and inflammatory factors, which can affect the cardiovascular system. Leptin was discovered in 1994 [Citation68]. The initial understanding of leptin was to act on the nervous system and inhibit the feeding behaviour of the body [Citation69]. Later studies have shown that several peripheral tissues, including those around heart, also expressed leptin receptors [Citation70]. There was evidence that leptin increased the oxidation of non-esterified free fatty acids (NEFA) in peripheral tissues and prevented the accumulation of fat in peripheral organs [Citation71]. In other roles, the discovery of leptin receptors on leukocytes suggested that leptin may mediate activation of the inflammatory system. In other words, adipose tissue was a potential organ that may affect long-term inflammatory responses [Citation72]. In addition, studies by Lin YK et al. found that leptin prolonged the duration of action potential (AP) in left atrial (LA) myocytes and affected its ionic current [Citation73]. These studies indicated that leptin inhibited the accumulation of visceral fat, while affected the cardiovascular system through inflammation and myocardial electrophysiology.
APN is a protein secreted by fat cells. In general, APN has the function of anti-atherosclerosis (AS) [Citation74] that can reduce the formation of foam cells and decrease the expression of scavenger receptor A. Studies have shown that a decrease in plasma APN levels were associated with increased plaque calcification[Citation75]. APN could also enhance the oxidation of NEFA in internal organs and reduce the accumulation of fat [Citation76]. In addition, ANP inhibited the NF-κB-dependent pathway [Citation77] and reduced the expression of adhesion molecules in endothelial cells [Citation78] by which ANP played a role in anti-inflammation. APN receptors were expressed in mammalian hearts, and animal experiments have shown that APN knockout mice exhibit a tendency to left ventricular hypertrophy and myocardial infarction [Citation79]. In OP, the secretion of ANP is reduced, which is not conducive to the heart.
Obesity and obesity-related diseases can cause CVD by inflammatory factors. Adipose tissue promotes increased expression of inflammatory factors, decreased expression of anti-inflammatory factors, and extensive infiltration of adipose tissue macrophages. Inflammatory factors including interleukin-2 (IL-2), interleukin-6 (IL-6) [Citation80], interleukin-8 (IL-8) tumour necrosis factor alpha (TNF-α), etc. can cause inflammation and thrombosis in the body by injuring endothelial cells, producing monocyte tissue factor, increasing platelet activation and fibrinogen expression, thereby affects CVD [Citation81]. Among them, TNFα can also activate NF-κB, induce oxidative stress, which causes endothelial damage and AS [Citation61].
When fat exceeds the load capacity of adipose tissue, free fatty tissues accumulate in other internal organs such as the heart, the liver, the pancreas, and the skeletal muscle. Cardiac dysfunction is closely related to cardiomyocyte-specific lipid deposition. In OP, the expression of lipid metabolism-related enzymes such as cardiac-specific lipoprotein lipase (LpL), acyl-CoA synthetase [Citation82], FA transporter (FATP1) [Citation83], and fatty triglyceride lipase (ATGL) [Citation83] in the heart is higher than that of the control group, which eventually lead to heart hypertrophy and enlarged heart chamber.
Lipid deposition in skeletal muscle can lead to CVD. The skeletal muscle regulates the metabolism between glucose and lipids. In obese Individuals, the accumulation of lipids in skeletal muscle can cause mitochondrial dysfunction [Citation84]. IR in skeletal muscle can trigger systemic IR, which induces increased FFA uptake and lipid accumulation in the heart, ultimately leading to cardiovascular disease [Citation85].
Free fatty tissues accumulate in liver can cause CVD. Non-alcoholic fatty liver disease (NAFLD) is often associated with obesity and is one of the consequences of systemic visceral fat accumulation. NAFLD may induce thrombosis, cardiomyocyte hypertrophy and apoptosis through the release of coagulation proteins, fibrinogen [Citation86–88] and inflammatory factors [Citation89,Citation90], which contributes to CVD.
Adverse effects of left ventricular function
Dysfunction can be manifested as diastolic and/or systolic dysfunction, shortened ejection fraction [Citation85]. The adverse effects of obesity on cardiac function are often manifested in the left ventricle, with left ventricular remodelling, diastolic dysfunction, and reduced ejection fraction. Increased plasma volume caused by hyperlipidaemia and high blood pressure secondary to obesity can lead to increased ventricular afterload; atherosclerosis due to obesity can cause systolic and diastolic dysfunction [Citation91].
Myocardial electrophysiological changes
The resting membrane potential of the heart is maintained by the inwardly rectifying K + current (I K1). In the working cells of the heart, phase 0 depolarisation of action potential (AP) is caused by fast sodium current (I Na). Repolarization is controlled by fast transient outward potassium currents (Ito), the rapid component of the delayed rectifier K current (IKr) and the slowly activating component of the delayed rectifier (IKs). The 2 phase is the plateau, which forms a short equilibrium by the L-type Ca (ICa, L) inflow and the K outflow. In the atrium, the repolarization is largely controlled by the ultra-rapid delayed rectifier K current (I Kur) [Citation92].
Common types of arrhythmias in OP are long QT syndrome (LQTS) and atrial fibrillation (AF). LQTS is usually defined by a decrease in re-polarisation current or an increase in depolarisation current. In the case of AF, increasing the outward potassium current or reducing inward calcium current may accelerate atrial re-polarisation, resulting in shortened AP duration and atrial refractory, thereby promoting ectopic firing and single/multiple wave re-entrant mechanisms. Multiple metabolic disorders caused by obesity may affect the electrophysiology of the myocardium by changing the density of ion channels. However, in the high-fat diet-induced obese animal model, the mRNA and protein expression of various ions were inconsistent, indicating that the effects of obesity on myocardial electrophysiology are far more complicated than we think [Citation93].
The treatment of obesity
The health risks that obesity brings to human beings are far-reaching, so it is necessary to take active measures to lose weight. According to the surgical procedure, bariatric surgery can be divided into gastric bypass (Roux-en-Y gastric bypass; duodenal transposition; biliary-pancreatic shunt and Roux-en-Y gastric bypass), adjustable gastric banding, sleeve gastrectomy, and vertical occlusion gastroplasty. In the early days, most bariatric surgeries was vertical occlusion gastroplasty. However, in the United States, this procedure was associated with a number of complications, which have essentially been halted. Gastric bypass has resulted in more effective weight loss but is associated with more complications. Adjustable gastric banding is associated with lower mortality and comorbidities, while there is a higher rate of re-operation than gastric bypass and less weight loss. Sleeve gastrectomy, which is increasingly popular, reduces body weight more than adjustable gastric banding and is comparable to gastric bypass surgery [Citation94].
Epidemiological evidence suggested that performing bariatric surgery often improved the effects of obesity on the cardiovascular system and reduces the risks of CVD. In patients undergoing sleeve gastrectomy, P wave dispersion (PWD) and QTd values were shown to be weakened [Citation95], and QTc was shortened [Citation96]. Similarly, shorter QTc was shown after jejunal Roux-en-Y gastric bypass surgery [Citation97]. A decrease in heart rate variability (HRV) and QT variation index (QTVI) after laparoscopic gastric banding (LGB) and biliary-pancreatic shunt (BPD) indicated an improvement in autonomic nervous system (ANS) dysfunction in OP [Citation98]. Vertical band-shaped gastroplasty (VBG) could shorten the QTc interval [Citation99], but some studies have shown that only OP with left ventricular hypertrophy could have an improvement in VR indicators [Citation100,Citation101]. Low-calorie diet [Citation102,Citation103] and aerobic exercise are also effective weight-loss methods [Citation104]. However, it should be noted that there have been reports of SCD during a low-fat diet [Citation105,Citation106]. Therefore, when performing such treatment, it should be carried out under strict medical supervision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567.
- Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014; 384(9945):766–781.
- Schulze PC, Drosatos K, Goldberg IJ. Lipid use and misuse by the heart. Circ Res. 2016;118(11):1736–1751.
- Messerli FH, Nunez BD, Ventura HO, et al. Overweight and sudden death. Increased ventricular ectopy in cardiopathy of obesity. Arch Intern Med. 1987;147(10):1725–1728.
- Kannel WB, Plehn JF, Cupples LA. Cardiac failure and sudden death in the Framingham Study. Am Heart J. 1988; 115(4):869–875.
- Fraley MA, Birchem JA, Senkottaiyan N, et al. Obesity and the electrocardiogram. Obes Rev. 2005;6(4):275–281.
- Mutiso SK, Rono DK, Bukachi F. Relationship between anthropometric measures and early electrocardiographic changes in obese rats. BMC Res Notes. 2014;7(1):931.
- Lepeschkin E, Surawicz B. The measurement of the Q-T interval of the electrocardiogram. Circulation. 1952;6(3):378–388.
- Funck-Brentano C, Jaillon P. Rate-corrected QT interval: techniques and limitations. Am J Cardiol. 1993;72(6):17B–22B.
- Omran J, Bostick BP, Chan AK, et al. Obesity and ventricular repolarization: a comprehensive review. Prog Cardiovasc Dis. 2018;61(2):124–135.
- Ahnve S. Correction of the QT interval for heart rate: review of different formulas and the use of Bazett’s formula in myocardial infarction. Am Heart J. 1985;109(3 Pt 1):568–574.
- Davey P. How to correct the QT interval for the effects of heart rate in clinical studies. J Pharmacol Toxicol Methods. 2002;48(1):3–9.
- Sagie A, Larson MG, Goldberg RJ, et al. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70(7):797–801.
- Curione M, Tego A, Capoccia D, et al. Prediction of QTc length as function of BMI: a clinical tool to establish arrhythmias risk in obesity. Clin Ter. 2011;162(6):e155–159–e159.
- Day CP, McComb JM, Campbell RW. QT dispersion: an indication of arrhythmia risk in patients with long QT intervals. Br Heart J. 1990;63(6):342–344.
- Pye M, Quinn AC, Cobbe SM. QT interval dispersion: a non-invasive marker of susceptibility to arrhythmia in patients with sustained ventricular arrhythmias? Br Heart J. 1994;71(6):511–514.
- Malik M, Batchvarov VN. Measurement, interpretation and clinical potential of QT dispersion. J Am Coll Cardiol. 2000;36(6):1749–1766.
- Lehmann MH, Hardy S, Archibald D, et al. JTc prolongation with d,l-sotalol in women versus men. Am J Cardiol. 1999;83(3):354–359.
- Tsai SF, Houmsse M, Dakhil B, et al. QTc compared to JTc for monitoring drug-induced repolarization changes in the setting of ventricular pacing. Heart Rhythm. 2014;11(3):485–491.
- Vardar SA, Ozturk L, Altun A, et al. Ventricular repolarization in overweight and normal weight healthy young men. Anadolu Kardiyol Derg. 2008;8(1):27–31.
- Yamaguchi M, Shimizu M, Ino H, et al. T wave peak-to-end interval and QT dispersion in acquired long QT syndrome: a new index for arrhythmogenicity. Clin Sci. 2003;105(6):671–676.
- Kors JA, Ritsema van Eck HJ, van Herpen G. The meaning of the Tp-Te interval and its diagnostic value. J Electrocardiol. 2008;41(6):575–580.
- Antzelevitch C, Sicouri S, Di Diego JM, et al. Does Tpeak-Tend provide an index of transmural dispersion of repolarization? Heart Rhythm. 2007;4(8):1114–1116. author reply 1116–1119.
- Seyfeli E, Duru M, Kuvandik G, et al. Effect of weight loss on QTc dispersion in obese subjects. Anadolu Kardiyol Derg. 2006;6(2):126–129.
- Carella MJ, Mantz SL, Rovner DR, et al. Obesity, adiposity, and lengthening of the QT interval: improvement after weight loss. Int J Obes Relat Metab Disord. 1996;20(10):938–942.
- Gupta AK, Xie B, Thakur RK, et al. Effect of weight loss on QT dispersion in obesity. Indian Heart J. 2002;54(4):399–403.
- Mukerji R, Terry BE, Fresen JL, et al. Relation of left ventricular mass to QTc in normotensive severely obese patients. Obesity (Silver Spring). 2012;20(9):1950–1954.
- Mshui ME, Saikawa T, Ito K, et al. QT interval and QT dispersion before and after diet therapy in patients with simple obesity. Proc Soc Exp Biol Med. 1999;220(3):133–138.
- Braschi A, Abrignani MG, Francavilla VC, et al. Novel electrocardiographic parameters of altered repolarization in uncomplicated overweight and obesity. Obesity (Silver Spring). 2011;19(4):875–881.
- Pontiroli AE, Pizzocri P, Saibene A, et al. Left ventricular hypertrophy and QT interval in obesity and in hypertension: effects of weight loss and of normalisation of blood pressure. Int J Obes Relat Metab Disord. 2004;28(9):1118–1123.
- Guo X, Li Z, Guo L, et al. Effects of metabolically healthy and unhealthy obesity on prolongation of corrected QT interval. Am J Cardiol. 2017;119(8):1199–1204.
- Elffers TW, de Mutsert R, Lamb HJ, et al. Association of metabolic syndrome and electrocardiographic markers of subclinical cardiovascular disease. Diabetol Metab Syndr. 2017;9:40.
- Ziegler D, Zentai C, Perz S, et al. Selective contribution of diabetes and other cardiovascular risk factors to cardiac autonomic dysfunction in the general population. Exp Clin Endocrinol Diabetes. 2006;114(4):153–159.
- Takebayashi K, Aso Y, Sugita R, et al. Clinical usefulness of corrected QT intervals in diabetic autonomic neuropathy in patients with type 2 diabetes. Diabetes Metab. 2002;28(2):127–132.
- Brown DW, Giles WH, Greenlund KJ, et al. Impaired fasting glucose, diabetes mellitus, and cardiovascular disease risk factors are associated with prolonged QTc duration. Results from the Third National Health and Nutrition Examination Survey. J Cardiovasc Risk. 2001;8(4):227–233.
- Arslan E, Yiğiner Ö, Yavaşoğlu İ, et al. Effect of uncomplicated obesity on QT interval in young men. Pol Arch Med Wewn. 2010;120(6):209–213.
- Bilora F, Vettore G, Barbata A, et al. Electrocardiographic findings in obese subjects. Minerva Gastroenterol Dietol. 1999;45(3):193–197.
- Esposito K, Marfella R, Gualdiero P, et al. Sympathovagal balance, nighttime blood pressure, and QT intervals in normotensive obese women. Obes Res. 2003;11(5):653–659.
- Mizia-Stec K, Mandecki T, Zahorska-Markiewicz B, et al. The QT interval dispersion and ventricular late potential in obese women. Pol Merkur Lekarski. 2000;8(44):84–86.
- Seyfeli E, Duru M, Kuvandik G, et al. Effect of obesity on P-wave dispersion and QT dispersion in women. Int J Obes. 2006;30(6):957–961.
- Mizia-Stec K, Mandecki T, Zahorska-Markiewicz B, et al. [QT interval dispersion and the type of obesity in women]. Pol Arch Med Wewn. 1999;101(5):391–396.
- Corbi GM, Carbone S, Ziccardi P, et al. FFAs and QT intervals in obese women with visceral adiposity: effects of sustained weight loss over 1 year. J Clin Endocrinol Metab. 2002;87(5):2080–2083.
- Park JJ, Swan PD. Effect of obesity and regional adiposity on the QTc interval in women. Int J Obes. 1997;21(12):1104–1110.
- Pidlich J, Pfeffel F, Zwiauer K, et al. The effect of weight reduction on the surface electrocardiogram: a prospective trial in obese children and adolescents. Int J Obes. 1997;21(11):1018–1023.
- Olivares Lopez JL, Vazquez Olivares M, Fleta Zaragozano J, et al. [Electrocardiographic and echocardiographic findings in children with overweight and obesity]. Med Clin (Barc). 2005;125(3):93–94.
- Daar G, Serin HI, Ede H, et al. Association between the corrected QT interval, carotid artery intima-media thickness, and hepatic steatosis in obese children. Anatol J Cardiol. 2015;16(7):524–528.
- Guven A, Ozgen T, Gungor O, et al. Association between the corrected QT interval and carotid artery intima-media thickness in obese children. JCRPE. 2010;2(1):21–27.
- Lee S, Cowan PA, Wetzel GT, et al. Prediabetes and blood pressure effects on heart rate variability, QT-interval duration, and left ventricular hypertrophy in overweight-obese adolescents. J Pediatr Nurs. 2011;26(5):416–427.
- Queen SR, Smulevitz B, Rentfro AR, et al. Electrocardiographic abnormalities among Mexican Americans: correlations with diabetes, obesity, and the metabolic syndrome. World J Cardiovasc Dis. 2012;2(2):50–56.
- Durak A, Olgar Y, Tuncay E, et al. Onset of decreased heart work is correlated with increased heart rate and shortened QT interval in high-carbohydrate fed overweight rats. Can J Physiol Pharmacol. 2017;95(11):1335–1342.
- Girola A, Enrini R, Garbetta F, et al. QT dispersion in uncomplicated human obesity. Obes Res. 2001;9(2):71–77.
- Sun GZ, Li Y, Zhou XH, et al. Association between obesity and ECG variables in children and adolescents: a cross-sectional study. Exp Ther Med. 2013;6(6):1455–1462.
- Akyuz A, Alpsoy S, Akkoyun DC, et al. Effect of overweight on P-wave and QT dispersions in childhood. Turk Kardiyol Dern Ars. 2013;41(6):515–521.
- Carnethon MR, Prineas RJ, Temprosa M, et al. The association among autonomic nervous system function, incident diabetes, and intervention arm in the Diabetes Prevention Program. Diabetes Care. 2006;29(4):914–919.
- Fukushige T, Yoshinaga M, Shimago A, et al. Effect of age and overweight on the QT interval and the prevalence of long QT syndrome in children. Am J Cardiol. 2002;89(4):395–398.
- Nomura A, Zareba W, Moss AJ. Obesity does not influence electrocardiographic parameters in coronary patients. Am J Cardiol. 2000;85(1):106–108.
- Omran J, Firwana B, Koerber S, et al. Effect of obesity and weight loss on ventricular repolarization: a systematic review and meta-analysis. Obes Rev. 2016;17(6):520–530.
- Yıldırım Ş, Binnetoğlu FK, Battal F, et al. Relation between QT variables and left ventricular geometry in athletes and obese children. Acta Med Port. 2016;29(2):95–100.
- Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10(1):90–100.
- Ternacle J, Wan F, Sawaki D, et al. Short-term high-fat diet compromises myocardial function: a radial strain rate imaging study. Eur Heart J Cardiovasc Imaging. 2017;18(11):1283–1291.
- Sonnenberg GE, Krakower GR, Kissebah AH. A novel pathway to the manifestations of metabolic syndrome. Obes Res. 2004;12(2):180–186.
- Viazzi F, Piscitelli P, Giorda C, et al. Metabolic syndrome, serum uric acid and renal risk in patients with T2D. PLoS One. 2017;12(4):e0176058.
- Eisenstein I, Edelstein J, Sarma R, et al. The electrocardiogram in obesity. J Electrocardiol. 1982;15(2):115–118.
- Tribble DL, Holl LG, Wood PD, et al. Variations in oxidative susceptibility among six low density lipoprotein subfractions of differing density and particle size. Atherosclerosis. 1992;93(3):189–199.
- Shaw PX. Rethinking oxidized low-density lipoprotein, its role in atherogenesis and the immune responses associated with it. Arch Immunol Ther Exp (Warsz). 2004;52(4):225–239.
- Phillips LK, Prins JB. The link between abdominal obesity and the metabolic syndrome. Curr Hypertens Rep. 2008;10(2):156–164.
- Mathieu P, Pibarot P, Larose E, et al. Visceral obesity and the heart. Int J Biochem Cell Biol. 2008;40(5):821–836.
- Zhang Y, Proenca R, Maffei M, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432.
- Kelesidis T, Kelesidis I, Chou S, et al. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152(2):93–100.
- Lollmann B, Gruninger S, Stricker-Krongrad A, et al. Detection and quantification of the leptin receptor splice variants Ob-Ra, b, and, e in different mouse tissues. Biochem Biophys Res Commun. 1997;238(2):648–652.
- Dyck DJ, Heigenhauser GJ, Bruce CR. The role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol (Oxf). 2006;186(1):5–16.
- Peelman F, Waelput W, Iserentant H, et al. Leptin: linking adipocyte metabolism with cardiovascular and autoimmune diseases. Prog Lipid Res. 2004;43(4):283–301.
- Lin YK, Chen YC, Huang JH, et al. Leptin modulates electrophysiological characteristics and isoproterenol-induced arrhythmogenesis in atrial myocytes. J Biomed Sci. 2013;20(1):94.
- Fortuno A, Rodriguez A, Gomez-Ambrosi J, et al. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59(1):51–60.
- Maahs DM, Ogden LG, Kinney GL, et al. Low plasma adiponectin levels predict progression of coronary artery calcification. Circulation. 2005;111(6):747–753.
- Staiger H, Haring HU. Adipocytokines: fat-derived humoral mediators of metabolic homeostasis. Exp Clin Endocrinol Diabetes. 2005;113(2):67–79.
- Ouchi N, Kihara S, Arita Y, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296–1301.
- Shimabukuro M, Higa N, Asahi T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88(7):3236–3240.
- Denzel MS, Scimia MC, Zumstein PM, et al. T-cadherin is critical for adiponectin-mediated cardioprotection in mice. J Clin Invest. 2010;120(12):4342–4352.
- Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83(3):847–850.
- Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60(22):2263–2270.
- Chiu HC, Kovacs A, Blanton RM, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96(2):225–233.
- Hoy AJ, Bruce CR, Turpin SM, et al. Adipose triglyceride lipase-null mice are resistant to high-fat diet-induced insulin resistance despite reduced energy expenditure and ectopic lipid accumulation. Endocrinology. 2011;152(1):48–58.
- Chow L, From A, Seaquist E. Skeletal muscle insulin resistance: the interplay of local lipid excess and mitochondrial dysfunction. Metab Clin Exp. 2010;59(1):70–85.
- Birse RT, Bodmer R. Lipotoxicity and cardiac dysfunction in mammals and Drosophila. Crit Rev Biochem Mol Biol. 2011;46(5):376–385.
- Meade TW, Mellows S, Brozovic M, et al. Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet. 1986;2(8506):533–537.
- Kannel WB, Wolf PA, Castelli WP, et al. Fibrinogen and risk of cardiovascular disease. The Framingham Study. JAMA. 1987;258(9):1183–1186.
- Stec JJ, Silbershatz H, Tofler GH, et al. Association of fibrinogen with cardiovascular risk factors and cardiovascular disease in the Framingham Offspring Population. Circulation. 2000;102(14):1634–1638.
- Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab. 2009;94(9):3171–3182.
- Picano E, Morales MA, del Ry S, et al. Innate inflammation in myocardial perfusion and its implication for heart failure. Ann N Y Acad Sci. 2010;1207:107–115.
- Galinier M, Pathak A, Roncalli J, et al. [Obesity and cardiac failure]. Arch Mal Coeur Vaiss. 2005;98(1):39–45.
- Tian M, Dong MQ, Chiu SW, et al. Effects of the antifungal antibiotic clotrimazole on human cardiac repolarization potassium currents. Br J Pharmacol. 2006;147(3):289–297.
- Aromolaran AS, Boutjdir M. Cardiac ion channel regulation in obesity and the metabolic syndrome: relevance to long QT syndrome and atrial fibrillation. Front Physiol. 2017;8:431.
- Chang SH, Freeman NLB, Lee JA, et al. Early major complications after bariatric surgery in the USA, 2003–2014: a systematic review and meta-analysis. Obes Rev. 2018;19(4):529–537.
- Yilmaz M, Altin C, Tekin A, et al. Assessment of atrial fibrillation and ventricular arrhythmia risk after bariatric surgery by P wave/QT interval dispersion. Obes Surg. 2018;28(4):932–938.
- Al-Salameh A, Allain J, Jacques A, et al. Shortening of the QT interval is observed soon after sleeve gastrectomy in morbidly obese patients. Obes Surg. 2014;24(1):167–170.
- Grasser EK, Ernst B, Thurnheer M, et al. QT interval shortening after bariatric surgery depends on the applied heart rate correction equation. Obes Surg. 2017;27(4):973–982.
- Alam I, Lewis MJ, Lewis KE, et al. Influence of bariatric surgery on indices of cardiac autonomic control. Auton Neurosci. 2009;151(2):168–173.
- Papaioannou A, Michaloudis D, Fraidakis O, et al. Effects of weight loss on QT interval in morbidly obese patients. Obes Surg. 2003;13(6):869–873.
- Alpert MA, Nusair MB, Mukerji R, et al. Effect of weight loss on ventricular repolarization in normotensive severely obese patients with and without heart failure. Am J Med Sci. 2015;349(1):17–23.
- Mukerji R, Petruc M, Fresen JL, et al. Effect of weight loss after bariatric surgery on left ventricular mass and ventricular repolarization in normotensive morbidly obese patients. Am J Cardiol. 2012;110(3):415–419.
- Seshadri P, Samaha FF, Stern L, et al. Free fatty acids, insulin resistance, and corrected qt intervals in morbid obesity: effect of weight loss during 6 months with differing dietary interventions. Endocr Pract. 2005;11(4):234–239.
- Pietrobelli A, Rothacker D, Gallagher D, et al. Electrocardiographic QTC interval: short-term weight loss effects. Int J Obes Relat Metab Disord. 1997;21(2):110–114.
- VanHoose L, Sawers Y, Loganathan R, et al. Electrocardiographic changes with the onset of diabetes and the impact of aerobic exercise training in the Zucker Diabetic Fatty (ZDF) rat. Cardiovasc Diabetol. 2010;9(1):56.
- Surawicz B, Waller BF. The enigma of sudden cardiac death related to dieting. Can J Cardiol. 1995;11(3):228–231.
- Zuckerman E, Yeshurun D, Goldhammer E, et al. 24 h electrocardiographic monitoring in morbidly obese patients during short-term zero calorie diet. Int J Obes Relat Metab Disord. 1993;17(6):359–361.