Abstract
Rheumatoid arthritis (RA) is a chronic joint inflammatory disease that is closely associated with dysregulation of fibroblast-like synoviocytes (FLSs). Protocatechuic acid (PCA), a phenolic compound of anthocyanins, has been proven to possess anti-inflammatory activity. However, the role of PCA in RA has not been investigated. In the present study, we aimed to explore the effects of PCA on the RA-FLSs. The results showed that PCA suppressed the proliferation, invasion, and migration of RA-FLSs in a dose-dependent manner. PCA treatment also inhibited the expressions of matrix metalloproteinase (MMP)-3 and MMP-13, as well as the secretion of inflammatory cytokines including TNF-α, IL-1β, IL-6 in RA-FLSs. Moreover, cell apoptosis of RA-FLSs was significantly induced by PCA treatment. PCA was found to repress the activation of NF-κB signalling, which was evidenced by the decreased expression of p-p65 and increased expression of IκBα. Furthermore, PCA significantly decreased the phosphorylation levels of Akt and mTOR in RA-FLSs. In conclusion, the results indicated that PCA exhibited an inhibitory effect on RA-FLSs via inhibiting the NF-κB and Akt/mTOR signalling pathways. These findings supported the concept that PCA might be a therapeutic agent for RA treatment.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that affects approximately 1% of the population [Citation1,Citation2]. The clinical symptoms of RA include joint swelling, tenderness, destruction of synovial joints, and even severe disability [Citation3]. Previous studies have demonstrated that the pathogenesis of RA is complex. There are many cell types involved in the development of RA, such as T cells, B cells, and macrophages [Citation3]. In addition to these immune cells, fibroblast-like synoviocytes (FLSs), the resident mesenchymal cells of synovial joints, also play a key role in the pathogenesis of RA [Citation2,Citation4]. During the course of RA, FLSs contribute to the local production of cytokines and inflammatory mediators that perpetuate inflammation. FLSs also produce multiple proteolytic enzymes that degrade the extracellular matrix (ECM), thereby contributing to cartilage destruction [Citation4]. Besides, FLSs undergo massive hyperplasia, as well as acquire a tissue-invasive phenotype that increases invasiveness into the ECM, which further exacerbates joint damage [Citation5]. Therefore, targeting FLSs might improve clinical outcomes in RA.
Protocatechuic acid (PCA), a phenolic compound of anthocyanins, has been proven to possess various pharmacological activities, such as cardioprotective, anti-hyperglycemic, anti-tumour and anti-inflammatory properties [Citation6–9]. It has been reported that PCA has chondroprotective potential by reducing glycosaminoglycans and collagen breakdown in IL-1β-induced porcine cartilage explants [Citation10]. These findings suggest that PCA may be a potential therapeutic agent in the treatment of OA. However, the role of PCA in RA remains unknown. In the present study, we investigated the effects of PCA on the proliferation, migration, invasion and inflammatory response in RA-FLSs.
Materials and methods
Cell culture and treatment
The present study was approved by the Ethics Committee of Huaihe Hospital, Henan University (Kaifeng, China). All the procedures were performed in accordance with the guidelines and regulations. Informed consent was obtained from all subjects before the study. Human synovial membrane tissues were obtained from five RA patients who were subjected to arthroplasty or synovectomy at Huaihe Hospital, Henan University. RA-FLSs were obtained via collagenase digestion as described previously [Citation11]. Briefly, the synovial membrane tissues were fully diced and transferred into 0.2% type II collagenase solution, followed by a 6 h routine digestion. The undigested tissues were removed with a 70 μm nylon sieve, then the RA-FLSs were collected via centrifugation.
The RA-FLSs were then cultured in DMEM (Life Technologies, Grand Island, NY) supplemented with 10% FBS, 0.1% penicillin/streptomycin, and 2 mM L-glutamine. Cells in the 3–5 generations were used for subsequent experiments. For the PCA treatment group, RA-FLSs were treated with various concentrations of PCA for different hours.
Cell viability assay
RA-FLSs (1 × 104 cells/well) were seeded in 96-well culture plates and treated with serial concentrations of PCA (51,02,040 μM) for 24 or 48 h. Cell viability was determined by MTT assay. MTT reagent (5 mg/ml) was added to the cells, followed by a 4 h incubation at 37 °C. Afterwards, the medium was removed, 100 µl DMSO was added and agitated for 15 min at 37 °C to dissolve the crystal. The absorbance at 490 nm of each well was recorded using a microplate reader (Bio-Tek, Winooski, VT).
Cell proliferation assay
RA-FLSs (5 × 103 cells/well) were seeded in 96-well plates in triplicate. Cell proliferation was measured with a BrdU assay kit (Roche, Basel, Switzerland) according to the manufacturer’s protocol. Finally, the absorbance at 450 nm was measured using a multi-well microplate reader (Bio-Tek).
Transwell assay
Transwell assay was performed to evaluate cell migration and invasion using Transwell chambers with 8-μm pores. For the invasion assay, the bottom surface of each membrane was pre-coated with Matrigel. RA-FLSs were treated with PCA (51,020 μM) at a density of 2 × 105 cells in serum-free DMEM for 24 h and then seeded in the upper chamber. While 600 μl of complete medium was added to the lower chamber as a chemoattractant. After incubation for 24 h at 37 °C, the cells remained on the upper surface of each membrane was removed with a cotton swab. Cells on the bottom side were fixed with 4% paraformaldehyde for 30 min stained with crystal violet for 15 min. The numbers of the stained cells were counted (five fields/well) at 200× magnification under a microscope (Olympus, Tokyo, Japan).
Cell apoptosis assay
Cell apoptosis was assessed by flow cytometry using an Annexin V-FITC/propidium iodide (PI) assay kit (Trevigen Inc., Gaithersburg, MD). After different incubation, RA-FLSs were resuspended in binding buffer and stained with 5 µl Annexin V-FITC and 10 µl PI solution for 15 min in the dark at room temperature. Apoptotic rates were analyzed using a flow cytometer (FACSCalibur; BD Biosciences Corp., San Diego, CA).
ELISA
After incubation with PCA for 48 h, cell culture was centrifuged, and supernatants were collected for the determining the concentration of inflammatory cytokines (TNF-α, IL-1β, IL-6) and matrix metalloproteinases (MMP-3, MMP-13) using ELISA kits (R&D Systems, Minneapolis, MN, USA).
qRT-PCR
Total RNA was extracted from RA-FLSs with TRIzol reagent (Gibco Life Technologies, MD, USA). Then the cDNA was synthesized with the PrimeScript® First Strand cDNA Synthesis Kit (TaKaRa Bio, Shiga, Japan) according to the manufacturers’ instructions. Aliquots of the reaction mixture were used for PCR amplification with Taq PCR Mastermix kit (Qiagen, Hilden, Germany). The sequences of the primers are listed as follows: TNF-α Forward: 5′-GAG TGA CAA GCC TGT AGC C-3′, Reverse: 5′-GGT TGA CTT TCT CCT GGT AT-3′; IL-1β Forward: 5′-TTC GAC ACA TGG GAT AAC GAG G-3′, Reverse: 5′-TTT TTG CTG TGA GTC CCG GAG-3′; IL-6, Forward: 5′-CCC TGA GAA AGG AGA CAT GTA AC-3′, Reverse: 5′-CCT CTT TGC TGC TTT CAC ACA TG-3′; MMP-3, Forward: 5′-TTC CGC CTG TCT CAA GAT GAT AT-3′, Reverse: 5′-AAA GGA CAA AGC AGG ATC ACA GTT-3′; MMP-13 Forward: 5′-TCC TGA TGT GGG TGA ATA CAA TG-3′, Reverse: 5′-GCC ATC GTG AAG TCT GGT AAA AT-3′; β-actin Forward: 5′-CTG TCC ACC TTC CAG CAG ATG T-3′, Reverse: 5′-CGC AAC TAA GTC ATA GTC CGC C-3′.
Western blot analysis
RA-FLSs were completely lysed in ice-cold RIPA lysis buffer for 30 min. The lysates were centrifuged at 12,000 g, 4 °C for 10 min, and the supernatant was collected for the determination of protein concentration using a BCA Protein Assay kit (Pierce; Thermo Fisher Scientific, Waltham, MA). Equal amounts of proteins (40 μg) were then subjected to 10% SDS-PAGE electrophoresis and subsequently transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were then blocked in 5% non-fat dry milk in TBST buffer for 1.5 h and subsequently incubated with p65 (1:1500), p-p65 (1:2000), IκBα (1:2500), p-Akt (1:1500), Akt (1:1000), p-mTOR (1:2500), mTOR (1:3000) or β-actin (1:1500) primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4 °C. After washing three times, the membranes were incubated with an HRP-conjugated secondary antibody (1:1000; Santa Cruz) for 1 h at 37 °C. The specific immunoreactive bands were visualized using enhanced chemiluminescence reagent (Thermo Fisher Scientific).
Statistical analysis
All data were presented as the mean ± SD from at least three independent experiments. The results were analyzed using SPSS version 13.0 software (SPSS Inc., Chicago, IL) with one-way ANOVA or Student’s t-test. Differences were considered statistically significant when p-values were less than .05.
Results
PCA inhibits RA-FLSs viability and proliferation
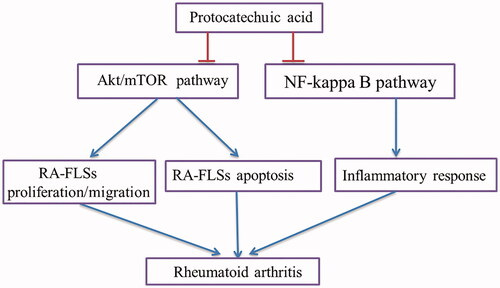
To evaluate the effect of PCA on the cell viability of RA-FLSs, the cells were treated with different doses of PCA (51,02,040 μM) for 24 or 48 h. MTT assay proved that cell viability was not affected after treatment with PCA for 24 h (). However, cell viability was reduced after treatment PCA for 48 h (). The inhibition rate of cell viability treated with 40 μM PCA for 48 h was about 53.4%. Thus, we used the incubation time of 48 h, and the concentrations of 5, 10 and 20 μM for the following experiments. Besides, BrdU incorporation assays demonstrated that PCA suppressed the cell proliferation of RA-FLSs in a dose-dependent manner ().
Figure 1. Effect of PCA on cell viability and proliferation of RA-FLSs. (A, B) MTT assay was carried out to evaluate cell viability of RA-FLSs after treatment with different doses of PCA (51,02,040 μM) for 24 or 48 h. (C) BrdU incorporation assay was performed to assess cell proliferation after treatment with 5, 10 and 20 μM of PCA for 48 h. n = 4. *p < .05.
PCA inhibits RA-FLSs migration and invasion
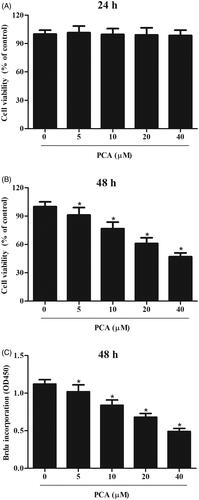
Next, we investigated the effects of PCA on migration and invasion. Transwell assay confirmed the concentration-dependent inhibitory effects of PCA on RA-FLSs migration and invasion (). It has been well known that MMPs play crucial roles in cell migration and invasion. Results of ELISA assay showed that the production of MMP-3 and MMP-13 were significantly suppressed by PCA treatment (). Additionally, qRT-PCR results also indicated that PCA markedly inhibited the expressions of MMP-3 and MMP-13 in RA-FLSs ().
Figure 2. Effect of PCA on RA-FLSs migration and invasion. RA-FLSs were treated with different doses of PCA (51,020 μM) for 24 h. (A, B) Transwell assay was conducted to examine the migration and invasion of RA-FLSs. (C–F) The production and mRNA levels of MMP-3 and MMP-13 were measured using ELISA and qRT-PCR, respectively. n = 3. *p < .05.
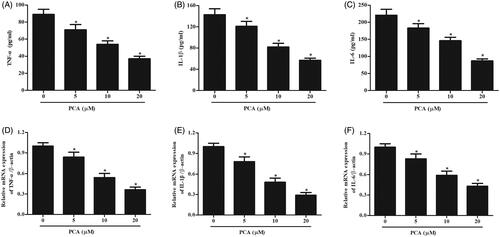
PCA suppresses inflammatory response in RA-FLSs
The mRNA levels of inflammatory cytokines including TNF-α, IL-1β, IL-6 were measured using qRT-PCR. The results showed that PCA markedly prevented the expressions of TNF-α, IL-1β, and IL-6 in RA-FLSs (). Moreover, the contents of TNF-α, IL-1β, IL-6 in cell culture were measured using ELISA. The results indicated that the secretion of TNF-α, IL-1β, and IL-6 in RA-FLS was markedly prevented by PCA ().
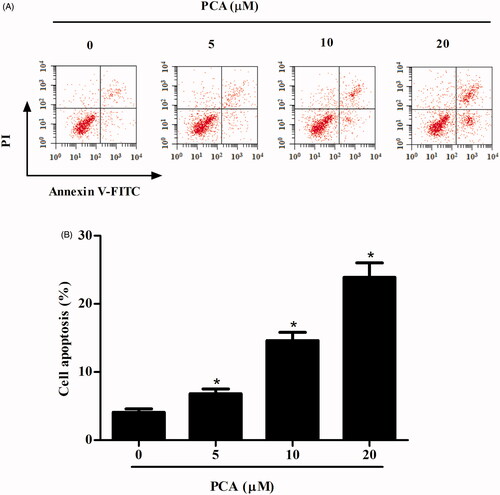
PCA induces cell apoptosis in RA-FLSs
Next, we examined the effect of PCA on RA-FLSs apoptosis. The results indicated that the percentage of apoptotic cells in PCA-treated group was obviously higher than that in the control group ().
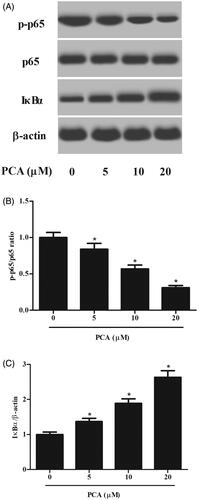
PCA inhibits the activation of the NF-κB pathway in RA-FLSs
NF-κB is an important signalling pathway involved in inflammation. To assess the effect of PCA on the NF-κB pathway, the expressions of p65, p-p65 and IκBα were detected using western blot. As shown in , the expression of p-p65 was significantly decreased, while the expression of IκBα was increased after treatment with PCA (Figure 5 and Supplemental data).
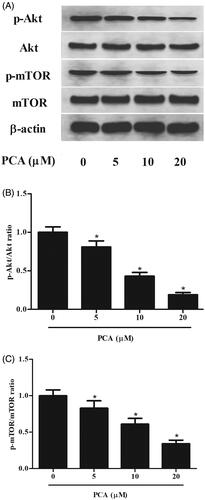
PCA inhibits the activation of Akt/mTOR pathway in RA-FLSs
Akt/mTOR pathway plays important roles in cell proliferation, survival and apoptosis in RA-FLSs. Thus, we evaluated the effect of PCA on the activation of the Akt/mTOR pathway in RA-FLSs. As shown in , as compared with the control group, PCA significantly down-regulated the phosphorylated levels of Akt and mTOR in RA-FLSs. While the total level of AKT and mTOR protein remained constant (Figure 6 and Supplemental data).
Discussion
It is evident that RA is characterized by hyperplastic synovial pannus tissue, which contributes to the destruction of cartilage and bone [Citation12]. The hyperplastic rheumatoid pannus in RA is characterized by an overabundance of FLSs, which are considered to be a key component of the invasive synovium [Citation4,Citation12]. During the course of RA, FLSs exhibit a major role in the initiation and perpetuation of destructive joint inflammation due to their capacity to express inflammatory cytokines and mediators [Citation13]. In addition, FLSs obtained from synovia of patients with RA exhibit tumour-like behaviours, including aggressive proliferation, increased migration, invasion, and reduced apoptosis [Citation14]. In this study, we found that PCA significantly inhibited RA-FLSs proliferation, migration/invasion, as well as induced cell apoptosis.
TNF-α, IL-1β, IL-6 are important mediators, which maintain the inflammatory response and, in turn, induce the activation of RA-FLSs in the development of RA [Citation15].
Additionally, other important pathologic features of FLSs in RA is abundant ECM components, increased expressions of collagenases (MMP-1, MMP-3 and MMP13), aggrecanases (ADAMTS4 and ADAMTS5) and cathepsins, which results in the imbalance between proteases and their inhibitors towards tissue destruction [Citation4]. Herein, our data showed that PCA decreased expressions of TNF-α, IL-1β, IL-6, MMP-3 and MMP-13 in RA-FLSs, implying that PCA exerts anti-RA action by repressing the production of proinflammatory cytokines and MMPs.
NF-κB is a predominant transcription factor in vertebrates, which is responsible for amplifying the inflammatory response [Citation16]. Under normal condition, NF-κB is hindered by the inhibitory proteins such as IκB family (IκBα, IκBβ, IκBε, p105, p100 and Bcl-3) [Citation17]. In response to stimuli, NF-κB translocates into the nucleus to activate the transcriptional events and induce the production of proinflammatory cytokines. Emerging evidence has proven that inhibition of NF-κB blocks the activation of proinflammatory cascades [Citation18]. Aberrant activation of NF-κB has been observed in RA synovial tissues and recognized as a pivotal regulator of inflammation in RA [Citation19–21]. However, recent documents have revealed a broad involvement of NF-κB in other aspects of RA pathology, such as abnormal apoptosis and proliferation of RA-FLSs, development of T helper 1 responses, and differentiation and activation of bone-resorbing activity of osteoclasts [Citation19]. Therefore, NF-κB is considered as a potential therapeutic target in RA [Citation22–24]. PCA has been reported to exert its anti-arthritic activity via inhibiting the activation of NF-κB signalling pathway [Citation10]. Our results demonstrated that PCA prevented the activation of the NF-κB signalling pathway in RA-FLSs. The results indicated that PCA executed its inhibitory effects on RA-FLSs via inhibiting NF-κB signalling pathway.
Akt/mTOR signalling pathway plays an important role in regulating a variety of cellular functions, such as cell proliferation, migration/invasion and apoptosis in RA-FLSs [Citation25,Citation26]. Laragione et al. reported that the mTOR inhibitor rapamycin decreases FLS invasion via the suppression of the mTOR signalling pathway [Citation27]. More importantly, PCA inhibits the activation of the Akt/mTOR pathway in lipopolysaccharide-stimulated keratinocytes [Citation28]. Our results demonstrated that PCA blocked the activation of the Akt/mTOR pathway in RA-FLSs. These results suggest that PCA suppressed the Akt/mTOR pathway for RA-FLSs proliferation, migration and apoptosis.
Conclusion
In summary, the results showed that PCA suppressed cell proliferation, invasion, migration, inflammatory response, as induces cell apoptosis in RA-FLSs. The inhibitory effects were mediated by the inhibition of NF-κB and Akt/mTOR signalling pathways (). The findings supported the concept that PCA might be a therapeutic agent for RA treatment.
western_blot.docx
Download MS Word (77.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356–361.
- Beatrix B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233(1):233–255.
- Charles J, Britt H, Pan Y. Rheumatoid arthritis. Aust Fam Physician. 2013;42(11):765.
- Nunzio B, Firestein GS. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol. 2013;9(1):24–33.
- Asif Amin M, Fox DA, Ruth JH. Synovial cellular and molecular markers in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):385–393.
- Drevensek G, Lunder M, Benkovic ET, et al. Cardioprotective effects of silver fir (Abies alba) extract in ischemic-reperfused isolated rat hearts. Food Nutr Res. 2016;60:29623.
- Harini R, Pugalendi KV. Antihyperglycemic effect of protocatechuic acid on streptozotocin-diabetic rats. J Basic Clin Physiol Pharmacol. 2011;21:79–92.
- Tanaka T, Kojima T, Suzui M, et al. Chemoprevention of colon carcinogenesis by the natural product of a simple phenolic compound protocatechuic acid: suppressing effects on tumor development and biomarkers expression of colon tumorigenesis. Cancer Res. 1993;53(17):3908–3913.
- Son JH, Kim SY, Jang HH, et al. Protective effect of protocatechuic acid against inflammatory stress induced in human dermal fibroblasts. Biomed Dermatol. 2018;2(1):9.
- Wongwichai T, Teeyakasem P, Pruksakorn D, et al. Anthocyanins and metabolites from purple rice inhibit IL-1β-induced matrix metalloproteinases expression in human articular chondrocytes through the NF-κB and ERK/MAPK pathway. Biomed Pharmacother. 2019;112:108610.
- Huh JE, Hong JM, Baek YH, et al. Anti-inflammatory and anti-nociceptive effect of Betula platyphylla var. japonica in human interleukin-1β-stimulated fibroblast-like synoviocytes and in experimental animal models. J Ethnopharmacol. 2011;135(1):126–134.
- Pap T, Müller-Ladner U, Gay RE, et al. Fibroblast biology. Role of synovial fibroblasts in the pathogenesis of rheumatoid arthritis. Arthritis Res. 2000;2(5):361–367.
- Lipsky PE, Davis LS, Cush JJ, et al. The role of cytokines in the pathogenesis of rheumatoid arthritis. Springer Semin Immunopathol. 1989;11(2):123–162.
- Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39(11):1781–1790.
- Zhu Q, Huang J, Wang SZ, et al. Cobrotoxin extracted from Naja atra venom relieves arthritis symptoms through anti-inflammation and immunosuppression effects in rat arthritis model. J Ethnopharmacol. 2016;194:1087–1095.
- Gilmore T. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684.
- Baeuerle PA. IkappaB-NF-kappaB structures: at the interface of inflammation control. Cell. 1998;95(6):729–731.
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7–11.
- Makarov SS. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001;3(4):200–206.
- Hartkamp LM, Grabiec AM, Ludikhuize J, et al. Functional interactions between PI3-kinase and NF-κB signaling pathways promote joint destruction in rheumatoid arthritis. Ann Rheum Dis. 2011;70(2):A27–A28.
- Sarmiento Salinas FL, Santillán Benítez JG, Hernández Navarro MD, et al. NF-κB1/IKKε gene expression and clinical activity in patients with rheumatoid arthritis. Lab Med. 2017;49(1):11–17.
- Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr Cartil. 2006;14(9):839–848.
- Loo GV, Beyaert R. Negative regulation of NF-κB and its involvement in rheumatoid arthritis. Arthritis Res Ther. 2011;13(3):221–231.
- Okamoto H, Yoshio T, Kaneko H, et al. Inhibition of NF-kappaB signaling by fasudil as a potential therapeutic strategy for rheumatoid arthritis. Arthritis Rheum. 2010;62(1):82–92.
- Wang J, Zhao Q. Kaempferitrin inhibits proliferation, induces apoptosis, and ameliorates inflammation in human rheumatoid arthritis fibroblast-like synoviocytes. Phytother Res. 2019;33(6):1726–1735.
- Chen K, Lin ZW, He SM, et al. Metformin inhibits the proliferation of rheumatoid arthritis fibroblast-like synoviocytes through IGF-IR/PI3K/AKT/m-TOR pathway. Biomed Pharmacother. 2019;115:108875–108882.
- Laragione T, Gulko PS. mTOR regulates the invasive properties of synovial fibroblasts in rheumatoid arthritis. Mol Med. 2010;16(9–10):352–358.
- Nam YJ, Lee CS. Protocatechuic acid inhibits Toll-like receptor-4-dependent activation of NF-κB by suppressing activation of the Akt, mTOR, JNK and p38-MAPK. Int Immunopharmacol. 2018;55:272–281.