Abstract
Many traditional procedures, including surgical methods such as microfracture of subchondral bone and soft tissue transplantation, have been widely used to treat damaged cartilage. However, there is still no definitive cure for cartilage defects. In recent decades, tissue engineering has raised hopes for the repair of defective cartilage. Different approaches are used for cartilage engineering, in which cells, scaffolds, and biological signals or growth factors may be used alone or in combination. Additionally, the imitation of the mechanical properties of the natural cartilage tissue by bioreactors is also helpful in this regard. It should be noted that in the transplantation of engineered cartilage tissue, there are challenges such as poor integration, inflammation and phenotypic instability that may lead to failure of neo-cartilage transplantation. Therefore, a comprehensive understanding of the multiple therapeutic approaches, including surgical procedures, cell-based methods and tissue engineering, should be obtained. The present review article provides this information, along with a variety of factors, including cells, materials, and biological/biomechanical factors required for the engineering of cartilage tissue, as well as the challenges ahead and their solutions.
Introduction
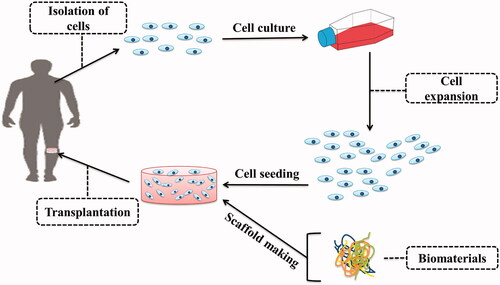
In the orthopaedic field, cartilage damage is often created due to congenital, trauma, cancer, or ageing diseases [Citation1–4]. After the injury, cartilage tissue has a minimal ability to repair [Citation5,Citation6]. Traditional methods of cartilage repair include autograft transplantation of periosteum/perichondrium [Citation7], allograft/autograft transplantation of osteochondral [Citation8,Citation9], microfracturing [Citation10] and autologous chondrocyte implantation (ACI) [Citation11]. Major limitations of these methods are morbidity of donor-site and lack of integration of transplanted tissue [Citation12–14]. The treatment used to overcome these limitations is particulated juvenile articular cartilage (PJAC) method, which is an allograft articular cartilage graft in which 1-mm cartilage cubes are taken from 13 years old donors [Citation15]. Also, Autologous matrix-induced chondrogenesis (AMIC), which combines the microfracture method with matrix-based techniques, is another procedure that has been used for repair of cartilage defects [Citation16]. As an alternative strategy, tissue engineering using stem cells and biodegradable scaffolds has raised many hopes [Citation17] ().
Cartilage engineered in tissue engineering has many uses in orthopaedic fields to repair damaged cartilage tissue [Citation14]. In cartilage tissue engineering, various three-dimensional (3 D) scaffolds have been developed to mimic native ECM. The 3 D neo-cartilage tissues are then produced by the cultivation of the chondrogenic cells on the artificial scaffolds in a medium containing biochemical and biomechanical stimuli. Besides, sometimes, scaffold-free engineered products are used to avoid the side effects of the products resulting from the destruction of scaffolds [Citation18,Citation19]. Different natural or synthetic materials [Citation20–23], various types of cells, including stem cells and primary cells [Citation24,Citation25], biochemical factors, including bone morphogenetic proteins (BMPs), Transforming growth factor-β (TGFβ) [Citation26,Citation27], fibroblast growth factors (FGFs) [Citation28,Citation29], insulin-like growth factors (IGFs) [Citation30], SRY (sex determining region Y)-box (SOX) [Citation31,Citation32], Cartilage-Derived Morphogenetic Protein I and II (CDMP I and II) [Citation33,Citation34], and biomechanical stimuli, including shear, compressive and tensile stresses [Citation35,Citation36] have been investigated for the cartilage tissue engineering.
Despite the great efforts in cartilage tissue engineering, there are several challenges, including inflammation, poor integration and phenotypic instability that need to be addressed [Citation37,Citation38]. In summary, various methods such as enzymatic treatment and therapies based on growth factors, platelet-rich plasma (PRP), glycosaminoglycan (GAG) combinations, caspase inhibitors, antioxidants and P188 surfactants have been evaluated to address these problems [Citation39–41].
The present paper provides a comprehensive understanding of the issues related to cartilage tissue engineering, cartilage damage mechanisms, cartilage response to injury and traditional methods of repairing damaged cartilage, including subchondral bone microfracture, soft tissue grafts and cell therapy. It also mentioned some of the most effective factors, including antioxidants, caspase inhibitors, and anti-inflammatory drugs that protect chondrocytes from mechanical damage. Also, the present paper focuses on recent advances in the use of scaffolds, growth factors, and stem cells in improving cartilage repair.
Articular cartilage injury and response of damaged tissue
Common causes of joint degeneration are ligament/meniscal/joint capsule tears, intra-articular fractures and joint dislocations [Citation42,Citation43]. Understanding the mechanical aspects and loading forces on the articular cartilage is necessary to understanding the mechanisms of injury [Citation44].
The extracellular matrix of articular cartilage is composed of water and macromolecules such as proteoglycans and collagens. Proteoglycans are the main cause of tissue stiffness, resilience and durability, and collagens are the main cause of tissue tensile strength and its shape. Therefore, slowly or suddenly applied loads have different effects on articular cartilage [Citation45]. When the forces are applied slowly, fluid motion deforms the cartilage and reduces the force applied to the matrix framework. On the contrary, when the forces are suddenly and rapidly applied, the matrix framework must withstand a lot of force. Following high force, damage to the matrix network, cells, and subchondral bone occurs [Citation45]. The severity and type of injury, as well as the repair and remodelling of the damaged surfaces, determine the risk of developing post-traumatic osteoarthritis [Citation46,Citation47]. Joint injuries are divided into three groups:
Chondral damage without visible disruption of articular cartilage
Chondral damage with visible mechanical disruption of cartilage
Osteochondral damage with visible mechanical disruption of cartilage and bone [Citation48–52]
In cases where there is no mechanical disruption to the articular surface, chondrocytes can repair the damage. This is if they are safe from further injury [Citation53]. While in the presence of mechanical disruption in the cartilage surface, activated chondrocytes are unable to repair the damage [Citation54,Citation55]. Also, in the presence of mechanical disruption in the subchondral bone and cartilage, the bone repair is performed, but complete cartilage repair is not done [Citation54].
Blunt trauma and its repetition over a period of time can cause changes in the cartilage matrix, including a reduction in the matrix proteoglycan and collagen disruptions [Citation56,Citation57]. In these cases, if the complication is not severe and chronic, the matrix is regenerated by chondrocytes [Citation58,Citation59]. For example, a study of the effect of blunt trauma on articular cartilage demonstrated that articular cartilage could endure impact loads of up to 25 newtons per square millimetre (25 MPa) without being damage [Citation60]. They showed that the impact loads above this level killed the chondrocyte and cleaved the cartilage. Besides, many of these acute or repetitive blunt traumas cause mechanical damage to the calcified cartilage zone-subchondral bone area, while the joint surface remains intact [Citation61–62]. Many experimental studies have also shown that repetitive impact loads split the cartilage matrix lead to progressive destruction of the cartilage [Citation63]. In this regard, the cartilage tissue after being injured and the body's response to it, is damaged and destroyed in three phases [Citation64,Citation65]. The initial phase leads to apoptosis of chondrocyte and an increase in proinflammatory cytokines such as tumour necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), matrix metalloproteinases (MMPs), nitric oxide, and free radicals [Citation66,Citation67]. An example of a treatment that can target this phase is intra-articular injection of agents that inhibit proinflammatory cytokines [Citation68]. Therapeutic procedures that target the second phase focus on the downstream effects of immediate response. Finally, the third stage involves events related to joint injury care. In general, the main goal of treatment in these cases is to reduce the inflammatory response, decrease the apoptosis of cartilage cells, reduce the destruction of the cartilage matrix and increase the construction of the new matrix [Citation69–71].
In severe mechanical injuries, in addition to articular surface cartilage damage, the subchondral is also damaged, causing haemorrhage, fibrin clot formation, and inflammatory responses [Citation51,Citation52,Citation72]. In this case, releasing various growth factors from collagen-associated platelets, including platelet-derived growth factor (PDGF), bone morphogenic protein (BMP), transforming growth factor-beta (TGF-ß) and insulin-like growth factor (IGF-I and II), may improve the repair of osteochondral damages [Citation73]. Also, by using biological interactions, cartilage damage caused by mechanical stress is reduced. For example, D’Lima and Haut showed respectively that caspase inhibitor reduces chondrocyte apoptosis [Citation74–76], and P188 surfactant decreases the chondrocyte necrosis [Citation77–79]. In addition, antioxidants also reduce the chondrocyte damage caused by mechanical stress [Citation80,Citation81].
Major procedures for repairing articular cartilage
In addition to traditional techniques such as subchondral bone microfracture, periosteum/perichondrium transplantation, and autologous chondrocyte implantation, scientists have used various techniques in recent years to repair damaged cartilage, including osteochondral allograft/autograft transplantation (OATS), autologous matrix-induced chondrogenesis (AMIC), particulated juvenile allograft chondrocytes (PJAC), and tissue engineering [Citation82–85].
Subchondral bone microfracture
Throughout the history of damaged cartilage repair, many methods have been used to stimulate the bone marrow, such as subchondral drilling and microfracturing. The method of microfracturing was developed by Steadman [Citation10,Citation86]. In marrow-stimulating procedures, penetration of the subchondral bone leads to fill of defect by marrow-derived cells and blood, and eventually, a blood clot is formed. Wound repair cascade then occurs, including acute inflammatory response and cell chemotaxis. Finally, it leads to the creation of vascularised granulation tissue and the proliferation of high-potency mesenchymal precursor cells with the capacity to differentiate into different mesenchymal cell types. In the first few days after subchondral perforations, fibrinous arcades are made at the surface of the defect that direct the growth of the mesenchymal cell along the long axes. After that, undifferentiated mesenchymal cells gradually differentiate into osteoblasts, fibroblasts, chondroblasts, and chondrocytes, which eventually lead to the formation of new bone in deeper zones and fibrocartilage in more superficial zones of the defect [Citation87–90]. Therefore, full-thickness articular cartilage defects could be improved through the subchondral bone penetration, so that after about 6 to 8 weeks, bone and fibrocartilaginous tissues are formed by MSCs in the bone and cartilage defect sites [Citation91–94]. Currently, surgeons use different methods to stimulate the formation of cartilaginous surfaces. These methods include abrasion of the articular surface, arthroscopic drilling, and making small fractures with tools such as awls [Citation95–100]. The clinical effect of treating cartilage damage with microstructure has been described in a recent case-control study [Citation101]. However, fibrocartilage is not mechanically comparable to hyaline cartilage and is easily degraded [Citation102,Citation103]. Achieving the formation of hyaline cartilage without ossified or fibrous tissue in cartilage defects is still challenging, which has ultimately led to extensive scientific efforts and researchs to find adjuvant therapies to improve repair with microfracture procedure [Citation90].
Autologous chondrocyte implantation (ACI)
Method of cell transplantation, as an alternative surgical procedure, is used for providing cell populations to the defected areas of the chondral and osteochondral. Through this method, both undifferentiated cells and chondrocytes can be used for transplantation, which ultimately leads to the production of new cartilage matrix [Citation104]. Also, autologous cell transplantation is used to treat cartilage defects [Citation105,Citation106]. Autologous chondrocyte implantation (ACI), introduced by Brittberg, is a two-step technique in which the chondrocytes are first harvested from a non-weight bearing part of the body, and then expanded in the laboratory. In the second stage, the chondrocytes are implanted at the site of the defect and protected by a periosteal flap (ACI-P) [Citation11,Citation107]. Because ACI is a two-step procedure and has a long recovery rate, it may not be ideal for the elderly population [Citation108,Citation109]. In many studies, the periosteum has been used as a cover for ACI (ACI-P) due to its chondrogenic properties. Subsequent studies in this area led to the development of absorbable covers such as porcine-derived type I/type III collagen (ACI-C) [Citation110–112].
Soft tissue grafts
Providing a new cell source with a natural and organic matrix, as well as protecting host cells and grafts from overload, are important benefits of the soft tissue grafts. Based on animal experiments and clinical studies, the researchers concluded that new cartilage could be created by transplanting soft tissues such as the periosteum or perichondrium [Citation95,Citation113,Citation114]. An important factor in soft tissue transplantation is the patient's age, which has a negative impact on the outcome [Citation115–117]. Studies have shown that patients over 40 years old did not have a good results after arthroplasty [Citation118,Citation119]. Therefore, soft tissue grafts have a favourable outcome in young patients. Besides, transplant hypertrophy is another limitation that can be caused by the use of periosteum as a covering material [Citation120]. Also, the harvesting of periosteum prolongs the time of operation and needs greater surgical exposure [Citation110]. Therefore, periosteotomy causes a lot of pain and arthrofibrosis. Later, to overcome these limitations, the use of matrix-induced autologous chondrocyte implantation (MACI) using a collagen bilayer seeded with chondrocytes, and porcine-derived type I/type III collagen as a cover (ACI-C) were developed, which are variations of the original periosteum-cover method [Citation121].
Osteochondral allograft/autograft transplantation (OATS)
Transplantation of osteochondral grafts from a non-weight bearing donor site is a different method, performed in a one-step procedure [Citation109,Citation122]. Hangody and Karpati first introduced this procedure in the 1990s, which is still a popular technique [Citation123,Citation124]. This procedure can be done both in the form of autograft and allograft [Citation125]. There is no risk of graft rejection and disease transmission in osteochondral autograft transplantation (OAT). However, since there are potential complications associated with autograft resection, resection is limited to smaller lesions [Citation126]. On the other hand, osteochondral allograft transplantation (OCA) circumvents the size limitation and complications associated with autograft resection, which is an advantage of allograft transplantation. However, allografts have the potential to transmit disease, and their availability is limited [Citation127].
Autologous matrix-induced chondrogenesis (AMIC)
Autologous matrix chondrogenesis (AMIC) was first introduced by Behrens, and its role in stimulating marrow was later confirmed by Steinwachs et al. [Citation103,Citation128]. AMIC is a one-step procedure that the combines standard procedure of microfracturing with the collagen I/III matrix. This matrix covers the blood clot and mesenchymal stem cells (MSCs) in it, while allowing MSCs to differentiate into chondrocytes [Citation129]. The major advantages of the AMIC method are that it is a single-step procedure and does not require cartilage removal leading to donor site complications, and is also a cost-effective method in which the cells do not need to expand in vitro [Citation130]. On the other hand, the limitations of this procedure are that it should not be performed when there are inflammatory diseases (e.g. rheumatoid arthritis), kissing lesions (2 defects on opposite sides), tumours, associated fractures, and osteoporosis [Citation130].
Particulated juvenile allograft chondrocytes (PJAC)
In recent years, there have been many advances in cartilage repair techniques that have led to the use of particulated juvenile allograft chondrocytes at the lesion site to produce the native cartilaginous surfaces [Citation131]. The DeNovo NT Natural Tissue Graft (Zimmer, Warsaw, IN, USA) is the graft material accessible for this procedure (133). This method is based on the ability of juvenile cartilage cells to migrate from cartilage graft after fixation at the site of injury [Citation132]. This allograft substance is composed of immature live chondrocytes in their native extracellular matrix. Donors in this treatment are usually younger than 13 years old, and any of the packages taken from them can be used to cover a 2.0 cm2 defect as a one-step method for focal defects [Citation133,Citation134]. Fibrin glue is also used to fix these cartilaginous fragments at the site of the lesion [Citation135,Citation136].
ECM analog and tissue engineering
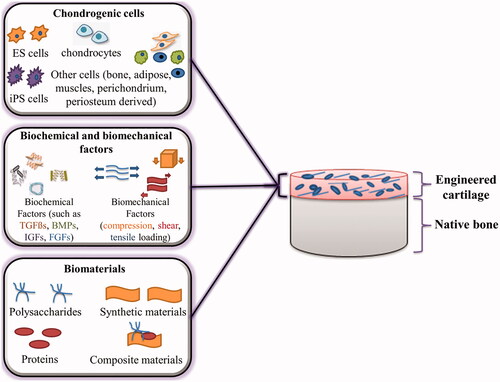
One way to deliver and protect cells in the treatment of chondral defects is to use an artificial matrix. These synthetic matrices also allow and stimulate the growth of host cells, increase the formation of new matrix, and improve the attachment of new cells and fresh matrix to each other and the host tissue [Citation137,Citation138]. Tissue engineering has proven to be very useful and promising in this regard. The primary purpose of cartilage tissue engineering is to repair diseased and damaged cartilage to restore its normal function. Tissue engineering has three main components, including cells, bio-functional factors and biomaterials, which are known as the tissue engineering triad (). Much progress has been made in the use of various cells, factors and materials in cartilage tissue engineering, which are discussed below.
Figure 2. Cartilage tissue engineering triad, including chondrogenic cells, biochemical/biomechanical factors and biomaterials.
Biomaterial engineering for construction of scaffolds
In cartilage tissue engineering, synthetic and natural materials are used to make scaffolds that mimic the natural environment of chondrocytes [Citation22,Citation139–141]. Biomaterials used must be biocompatible and do not cause immune and inflammatory reactions [Citation23]. They should be able to create a three dimensional (3-D) environment for binding, growth and differentiation of cells [Citation142,Citation143]. The final 3-D structure must also allow the transfer of nutrients, growth factors, gases, ions, etc. and be able to be transmitted to the body with minimal invasive properties [Citation144,Citation145]. In addition, biodegradability is also an important factor in tissue engineering that should be consistent with tissue remodelling [Citation142]. The various techniques that have previously been used to build multi-material scaffolds for articular cartilage tissue engineering are as follows:
Embedded solid structures [Citation146]: composite scaffolds are obtained by combining hydrogels containing multiple cells with rigid polymers. The purpose of using hydrogels in this method is to strengthen cell seeding in hard scaffolds [Citation147–150].
Embedded textiles and fibres for reinforcement [Citation146]: composite scaffolds are obtained by embedding the non-woven fibres in the hydrogel. The main advantage of this method is the good mechanical properties of the final scaffold [Citation140,Citation151–153].
Multilayered designs include multilayered cartilage constructs, layered osteochondral constructs and multilayered constructs from homogenous scaffolds. These composite scaffolds can create adhesions between the cartilage layer and its underlying bone layer [Citation146,Citation154–156].
3-D woven composite materials [Citation146]: composite scaffolds are obtained by combining the 3D woven porous fibres with the hydrogel. Scaffolds made in this way show mechanical properties, including compressive, shear and tensile forces, similar to native cartilage [Citation157].
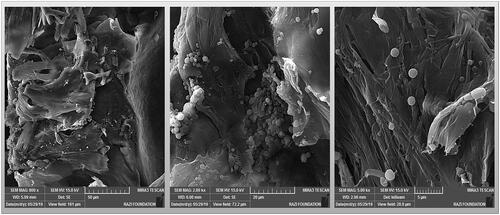
Some researchers have used solid scaffolds for cartilage tissue engineering for large defects [Citation158]. Among the solid scaffolds, collagen sponge was widely used to the cartilage engineering [Citation159,Citation160] ().
Figure 3. Collagen sponge containing nano- and micro-particles of poly caprolactone for use in cartilage tissue engineering.
On the other hand, among the various types of 3 D scaffolds, hydrogels, three dimensional and hydrophilic polymeric networks, have found more application in cartilage tissue engineering due to their ability to absorb water and biological fluids [Citation161]. For example, hydrogels made from polyethylene glycol were used in cartilage tissue engineering due to their high water absorption, biocompatibility, biodegradability, elasticity, and nutrient transport [Citation162–164]. In recent years, various types of hydrogels, including thermos-responsive hydrogels [Citation165,Citation166], photosensitive hydrogels [Citation19,Citation167,Citation168] and hydrogels loaded with the chondroinductive [Citation171] or chondroprotective factors [Citation170,Citation171], have been studied. It should be noted that in the design of hydrogels, there must be a balance between different characteristics, including chondroinductive properties, electrical conductivity, mechanical properties, biocompatibility and degradation rate [Citation158,Citation172].
Among the various types of hydrogels, Cell-laden hydrogels were widely used as bioink in 3 D bioprinting approach for cartilage tissue engineering [Citation21,Citation173–175]. In recent years, 3 D bioprinting technology in the field of tissue engineering, especially cartilage, has developed rapidly [Citation140,Citation176,Citation177]. The purpose of 3 D bioprinting is to provide structures using automated systems and the characteristics of cells/tissues to deliver living cells, scaffold materials, growth factors and nutrients among other substances [Citation140]. In this system, cells and bioactive agents are usually deposited by hydrogels in layers, causing biopaper or bioink [Citation178]. In recent years, many advances have been made in this field, which has led to the introduction of new methods in the field of cartilage tissue engineering. Robotic-assisted 3 D bioprinting is one of these methods that has had promising results in the reconstruction of damaged cartilage tissue in vivo [Citation179]. These types of robotic devices are minimally invasive surgical techniques that offer many additional benefits, including better safety, faster healing, and shorter hospital stays – the result of less traumatic surgery [Citation177,Citation180].
Various materials are used to make different scaffolds in cartilage tissue engineering. The most common biomaterials used in cartilage tissue engineering are as follows:
Proteins, including collagen (Maix®, MACI®, MaioRege®, Atelocollagen®), fibrin (Tissucol kit®), silk and gelatine [Citation20,Citation181–184].
Polysaccharides, including Chitosan (BST-CarGel®), Hyaluronic acid (HYAFF-11®), Cellulose, Alginate [Citation185–187].
Synthetic materials such as Polyethylene glycol, Polylactic acid, Poly(lactic-co-glycolic acid) (Bio-Seed®-C) [Citation188]
Different combinations of natural materials and synthetic polymers (composite materials) such as MaioRegen® [Citation189,Citation190].
MACI®, Chondro-Gide® and Maix® are the protein-based products that are clinically used to transplant autologous chondrocytes [Citation181,Citation182]. Also, Atelocollagen® is a type I collagen gel used for three-dimensional culture and in vivo transplantation of autologous chondrocytes [Citation183] or mesenchymal stem cells (MSCs) [Citation184].
Hyalograft® (HYAFF-11®) is a hyaluronic acid matrix with autologous chondrocytes [Citation185], which has led to clinical success in human articular cartilage [Citation186]. These highly porous sponges can enhance chondrogenic differentiation [Citation191]. Also, BST-CarGel® scaffold is another polysaccharide-based matrix, which is made of ß-glycerophosphate and chitosan, and has been clinically successful [Citation187].
Bio-Seed®-C is a porous structure matrix based on synthetic materials including polylactic acid (PLA), polyglycolic acid (PGA), and polydioxanone, and is clinically capable of forming hyaline cartilage [Citation188]. Besides, the combination of the ceramics with natural and synthetic polymers was used as a scaffold in cartilage tissue engineering [Citation189]. For example, MaioRegen®, which was made of type I collagen and hydroxyapatite, presented promising results [Citation190]. The chondrogenic differentiation of mesenchymal stem cells and phenotypic stability of chondrocytes on these materials have been demonstrated in various experiments.
In addition to the materials mentioned earlier, the decellularized matrix, as a natural material, has been used in many cartilage tissue engineering studies and has yielded promising results [Citation192,Citation193]. Because the structural and mechanical properties of these types of biocompatible scaffolds are similar to native tissue, cellular responses are well performed on them [Citation22,Citation196]. In a study, decellularized cartilage particles (DCC) were chemically constructed, and then cartilage-forming was investigated [Citation195]. In this study, it was found that DCC significantly increased the chondroinduction of rat bone marrow-derived mesenchymal stem cells (rBMSCs). On the other hand, another advantage of this type of material is that it can be used as a xenograft to regenerate cartilage tissue [Citation196].
Another item used in cartilage reconstruction is sheet technologies, including cell sheet, electro-spun sheet, and the previously described acellular matrix sheet. Because the use of synthetic materials causes complications such as inflammation and other immune responses, the researchers created a cell sheet method that is often developed at temperature-sensitive cultivation dishes coated with Poly N-isopropyl acrylamide (PIPAAm) [Citation197,Citation198]. However, because it is difficult to manipulate stacked cell sheets, the researchers created acellular matrix sheet technology [Citation199]. With a better understanding of native extracellular matrix nanostructures, electro-spun sheet technologies have also been developed that are more manipulative and controllable than acellular matrix sheets [Citation200]. In a study conducted by Xue, electro-spun fibrous sheets made of gelatine/polycaprolactone (GT/PCL) were used to reconstitute cartilage [Citation201]. They used the electro-spun fibres for 3 D cartilage engineering with chondrocytes through a sandwich model. Finally, they achieved promising results. They found that these types of electro-spun sheets could provide a biomimetic microenvironment, while also having little degradation and inflammation [Citation201].
Chondrogenic cells
One of the main components of cartilage tissue engineering is cells [Citation202]. In cartilage injuries, to produce the hyaline tissue, appropriate cells that can produce hyaline or hyaline-like tissue should be used [Citation20,Citation203]. In general, chondrocytes, stem cells, genetically modified cells and fibroblasts have been tested in cartilage tissue engineering. However, stem cells and chondrocytes are the main chondrogenic cells in cartilage regeneration and engineering. Chondrocytes can be obtained from a variety of sources, such as nasal septum, auricular cartilage, costal cartilage, and articular cartilage [Citation204]. Each of these chondrocytes with different sources is tissue-specific and creates tissue with the original characteristics [Citation204]. Carticel® and Celect® are procedures containing articular autologous chondrocytes that have been marketed [Citation205]. In order to autologous chondrocyte implantation for the repair of femoral condyle cartilage defects, Carticel® is used, which is a method for extraction and in vitro expansion of autologous chondrocytes [Citation206]. Another method used for transplantation of the autologous chondrocytes is Celect®, which is used only for the isolation and proliferation of chondrocytes containing a specific marker [Citation207].
On the other hand, MSCs as multipotent cells, can be differentiated into various cell types, including osteoblasts, adipocytes, chondrocytes, as well as neuronal and myogenic cells [Citation208]. MSCs were isolated from muscles, adipose tissue, bone marrow, perichondrium and periosteum [Citation209]. Among them, MSCs separated from fat, bone marrow, synovium and muscle were considered as the chondrogenic cells [Citation210]. MSCs are very promising in tissue engineering because they modulate the immune system and do not express the major histocompatibility complex (MHC) class II, which is responsible for the immune rejection of the transplant [Citation211]. Therefore, it is suggested that these cells be used as allogeneic cells. For example, Cartistem®, recently approved by Korean Food and Drug Administration, is made of allogeneic stem cells isolated from umbilical cord blood and used to treat osteoarthritis (OA) [Citation212]. Human induced pluripotent stem cells (hiPSCs) are another type of cells for cartilage tissue regeneration that are obtained from somatic cells by reprogramming transcription factors (Sox2, Oct4, Myc and Klf4) [Citation213]. Recently, the development of efficient chondrocytes from hiPSCs has been reported [Citation214]. Because these cells are patient-specific cells and are unlikely to be rejected by the immune system, they are very promising in cartilage tissue engineering and regenerative medicine. Another type of pluripotent cells that can be useful in cartilage tissue engineering is embryonic stem cells (ESCs), which first form the embryoid bodies that ultimately differentiate into chondrocyte cells [Citation215]. Embryonic stem cells (ESCs) have a great advantage due to their pluripotency and unlimited self-renewal, and are widely used [Citation216]. Because these cells are immortal, they can potentially provide an unlimited supply of chondrogenic cells, which is why they are important in regenerating or replacing damaged cartilage tissue [Citation215,Citation217]. Despite their usefulness in cartilage tissue engineering, the extraction of these cells is associated with ethical and political issues that limit their use [Citation218]. Also, undifferentiated ESCs can cause tumours and increase the risk of developing teratomas in the body, which is another limitation of the use of these potent cells [Citation219].
Bio-functional factors and chondrogenic differentiation
In addition to scaffolds and suitable cells, appropriate stimuli should be used to differentiate the cells into the desired chondrocytes and form an extracellular matrix [Citation220]. Numerous studies have shown that multiple factors, such as Transforming Growth Factor β (TGFβ) [Citation209,Citation221], Bone Morphogenic Proteins (BMPs) [Citation222,Citation223], Cartilage-Derived Morphogenetic Protein I and II (CDMP I and II) [Citation224,Citation225], Insulin-Like Growth Factor (IGF-1) [Citation221], SRY (Sex determining Region Y)-box (SOX) [Citation218,Citation226] and Fibroblastic Growth Factor (FGF) [Citation218,Citation227,Citation228] are effective in achieving this goal (). Treatments based on combining growth factors with other approaches such as transplantation of cell and scaffold have been developed in recent years.
Moreover, various aspects of the environment, including mechanical stimuli (shear, compressive and tensile stresses) [Citation229], low oxygen tension [Citation230–233] and 3 D culture () [Citation234,Citation235], are also effective in the chondrogenic differentiation of MSCs.
Table 1. Principal growth factors used in cartilage tissue engineering.
Table 2. Effective environmental factors in chondrogenic differentiation of MSCs.
The main function of cartilage tissue is mechanical resistance to mechanical stresses including compressive, shear and tensile forces, and creating an environment with a minimum coefficient of friction (0.03–0.06) [Citation247]. Compression, shear and tensile modulus for cartilage tissues are estimated to be from 0.08 to 2 MPa, 0.05 to 0.25 MPa and 5 to 25 MPa, respectively [Citation248]. These unique mechanical properties of cartilage tissue are due to the presence of abundant water, collagen, glycosaminoglycans and their specific organisation. Therefore, the creation of neo-cartilages with acceptable mechanical properties that have mechanical resistance in the environment inside the body is one of the main criteria in the engineering of cartilage tissue. Various studies have shown that imitation of the mechanical conditions of cartilage tissue by bioreactors increases the chondrogenic differentiation [Citation249,Citation250]. Bioreactors create different physicochemical conditions of native cartilage tissue in the laboratory and can control environmental factors such as temperature, pH, O2 stress, nutrient supply, and waste disposal [Citation251]. Various bioreactors have been used in this regard, such as rotating bioreactors [Citation252], wavy-walled bioreactors [Citation253] and perfused vessels [Citation254], all of which, along with growth factors, affect cell proliferation, morphology, and ECM deposition [Citation255,Citation256].
Challenges in cartilage tissue engineering
Despite many advances in engineering cartilage tissue in vitro, challenges remain in the successful clinical transfer of engineered cartilage tissue. These problems, which often arise after transplantation of engineered tissue into the body, include phenotypic instability, poor integration, and inflammation ().
Figure 4. Three major challenges in cartilage tissue engineering: poor integration, inflammation and phenotypic instability.
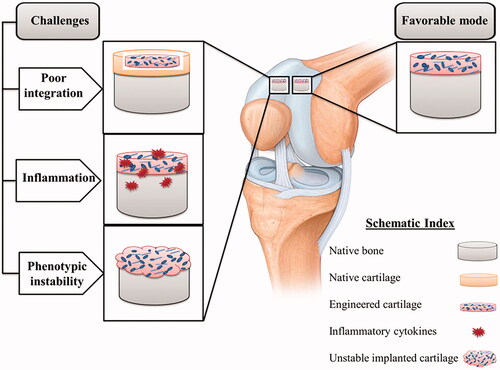
A basic problem in cartilage tissue engineering is the poor integration of engineered tissue with the surrounding environment. Factors influencing tissue integration include cell phenotype in transplanted tissue, donor age, cell death at the edge of the wound, and the maturity degree of engineered structures [Citation37,Citation257]. The extracellular matrix components of native cartilage tissue, which include collagen and glycosaminoglycans (GAGs), can interfere with integration [Citation258]. Native tissue matrix can impede the integration of engineered tissue by preventing adhesion and diffusion of matrix proteins and cells [Citation258]. Interestingly, traditionally in tissue engineering, researchers are also looking to stimulate increased production of these components at levels similar to native tissue, which could be an intervening factor for integration. Enzymatic treatment is one of the ways to deal with this problem, which enhances the integration by disrupting specific molecules of the matrix [Citation259]. Hyaluronidase and collagenase improve integration with implanted cartilage by increasing cell density at the wound site [Citation259]. It has also been shown that temporary reduction of GAG at the cartilage surface by chondroitinase-ABC (c-ABC) and trypsin, respectively, improves coverage by repair cells and integration of restored tissue [Citation218]. In order to better integrate the implanted tissue, in addition to enzymes, a series of factors that disrupt the formation of cartilage matrices, such as β-aminopropionitrile (BAPN), Insulin-like Growth Factor 1 (IGF-1) and para-nitrophenyl-β-D-xyloside, are used, which disrupt the formation of proteoglycans and lead to better integration [Citation260]. Although the temporary absence of matrix components such as GAG and collagen in engineered cartilage provides a strong integration, their presence is also essential for stress resistance in the body. Therefore, bioactive agents such as c-ABC and lysyl oxidase-like 2 (LOXL2), which play a dual role and are effective in improving integration as well as increasing collagen production and traction, should be used in tissue engineering to address this challenge.
Another challenge that may arise in cartilage tissue engineering is inflammation at the transplant site. Although the exact mechanisms of action of inflammatory cytokines in cartilage problems, especially osteoarthritis, need further clarification, inflammation is now recognised as a major factor in the development and progression of osteoarthritis [Citation38]. Severe inflammatory reactions are observed in joint injuries that cause post-traumatic osteoarthritis [Citation261]. Joint injuries increase the inflammatory cytokines in the articular cavity, which ultimately increase the cartilage destruction. Inflammatory cytokines in arthritic joints include Interleukin-1β (IL-1β) and tumour factor necrosis factor-α (TNF-α), which are involved in the progression of cartilage destruction [Citation38]. Also, several in vitro studies have shown that the destructive effects of an inflammatory environment can affect the engineered cartilage tissue inside the body. For example, it has been shown that both the use of an osteoarthritic synovium-derived medium and the activation of the nuclear factor kappa B (NF-κB) pathway using IL-1β and TNF-α inhibit chondrogenesis of MSCs [Citation262,Citation263]. In addition, inflammation in the arthritic joint can prevent the integration of neo-cartilage tissue into the joint [Citation37]. Therefore, for better cartilage repair, an environment should be provided to control the inflammation. For this purpose, growth factors that suppress IL-1β such as IGF-1, platelet-derived growth factor (PDGF)-bb and bone morphogenetic proteins (BMP-2 and BMP-9 [Citation40,Citation41], GAG compounds such as glucosamine, hyaluronic acid and chondroitin sulphate, which have anti-inflammatory properties [Citation264,Citation265], and platelet-rich plasma (PRP) [Citation39,Citation266], are used. PRP includes various levels of chemokines/cytokines, adhesive proteins, and growth factors that restore tissue function and reduce pain in osteoarthritic joints [Citation266,Citation267].
Another common challenge for the cartilage tissue is phenotypic instability, which creates fibrous cartilage that does not have the function of hyaline cartilage [Citation268]. Stem cells chondrogenesis is mostly associated with increased collagen expression (type X) and hypertrophic differentiation. These complications are especially common in osteoarthritis and are stimulated by some growth factors, ECM degradation products, and cytokines [Citation269,Citation270]. Therefore, the osteoarthritis environment is likely to cause hypertrophic differentiation of transplanted cells in cartilage tissue engineering. On the other hand, the development of hypertrophic phenotype in engineered tissue increases mineralisation [Citation268]. To overcome the challenge of phenotypic instability in engineered tissue, much information needs to be obtained about signalling pathways and molecules associated with hypertrophy processes. For example, a combination of SOX-5/-6/-9 is able to suppress hypertrophic markers and osteogenic markers in human MSCs [Citation271,Citation272]. Also, Nkx3.2, which is involved in the development of cartilage as a transcription factor, demonstrates the potential to prevent hypertrophy by inhibiting runt-related transcription factor-2 (RUNX-2) function [Citation273,Citation274]. Besides, studies have shown that parathyroid hormone (PTH), parathyroid hormone-related peptide (PTHrP), and BMP-7 molecules, while inducing chondrogenic differentiation, inhibit type X collagen in MSCs, thereby inhibiting hypertrophic differentiation [Citation275–277].
Conclusions
Tissue engineering, which is continually evolving as an interdisciplinary field of research, could be a permanent solution to many incurable diseases, especially cartilage damage, which is often complicated and not completely curable. Previously, surgical procedures such as subchondral bone microfracture and soft tissue transplantation were the main methods of treating damaged cartilage. Later, cell therapy and now tissue engineering became more suitable options for this purpose. Therefore, it seems that the engineering of cartilage tissue and the production of neo-cartilages from natural or synthetic biomaterials that have the ability to release effective factors in the treatment of cartilage damage is a more effective therapeutic approach for cartilage diseases, especially OA. However, before using this new treatment strategy, it is necessary to evaluate a large number of compounds of biological substances, cells, biomaterials and various agents such as antioxidants, anti-inflammatory agents, etc.
Authors’ contributions
Azizeh Rahmani Del Bakhshayesh conceived the study and participated in its design and coordination. All authors helped in drafting the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Tissue Engineering, Faculty of Advanced Medical Science of Tabriz University for all supports provided.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Fathi Karkan S, Mohammadhosseini M, Panahi Y, et al. Magnetic nanoparticles in cancer diagnosis and treatment: a review. Artif Cells Nanomed Biotechnol. 2017;45(1):1–5.
- Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3(5):257–264.
- Jørgensen AEM, Kjær M, Heinemeier KM. The effect of aging and mechanical loading on the metabolism of articular cartilage. J Rheumatol. 2017;44(4):410–417.
- Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23(11):1966–1971.
- Bernhard JC, Vunjak-Novakovic G. Should we use cells, biomaterials, or tissue engineering for cartilage regeneration? Stem Cell Res Ther. 2016;7(1):56.
- Reinholz G, Lu L, Saris D, et al. Animal models for cartilage reconstruction. Biomaterials. 2004;25(9):1511–1521.
- Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. JBJS. 2003;85:25–32.
- Hangody L, Vásárhelyi G, Hangody LR, et al. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(1):32–39.
- Hangody L, Kish G, Kárpáti Z, et al. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics. 1998;21(7):751–756.
- Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369.
- Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889–895.
- Spiller KL, Maher SA, Lowman AM. Hydrogels for the repair of articular cartilage defects. Tissue Eng B Rev. 2011;17(4):281–299.
- Moran CJ, Pascual-Garrido C, Chubinskaya S, et al. Restoration of articular cartilage. JBJS. 2014;96(4):336–344.
- Huey DJ, Hu JC, Athanasiou KA. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338(6109):917–921.
- Ao Y, Li Z, You Q, et al. The use of particulated juvenile allograft cartilage for the repair of porcine articular cartilage defects. Am J Sports Med. 2019;47(10):2308–2315.
- Panni ASD, Regno C, Mazzitelli G, et al. Good clinical results with autologous matrix-induced chondrogenesis (Amic) technique in large knee chondral defects. Knee Surg Sports Traumatol Arthrosc. 2018;26(4):1130–1136.
- Borenstein JT, King KR, Terai H, et al. Multilayer device for tissue engineering. Google Patents; 2008.
- Makris EA, Gomoll AH, Malizos KN, et al. Repair and tissue engineering techniques for articular cartilage. Nat Rev Rheumatol. 2015;11(1):21–34.
- Abbadessa A, Blokzijl M, Mouser V, et al. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr Polym. 2016;149:163–174.
- Asadi N, Alizadeh E, Rahmani Del Bakhshayesh A, et al. Fabrication and in vitro evaluation of nanocomposite hydrogel scaffolds based on gelatin/PCL–PEG–PCL for cartilage tissue engineering. ACS Omega. 2019;4(1):449–457.
- Taghipour YD, Hokmabad VR, Bakhshayesh D, et al. The application of hydrogels based on natural polymers for tissue engineering. CMC. 2020;27(16):2658–2680.
- Del Bakhshayesh AR, Asadi N, Alihemmati A, et al. An overview of advanced biocompatible and biomimetic materials for creation of replacement structures in the musculoskeletal systems: focusing on cartilage tissue engineering. J Biol Eng. 2019;13(1):85.
- Asadi N, Del Bakhshayesh AR, Davaran S, et al. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater Chem Phys. 2019;:122528.
- Panadero J, Lanceros-Mendez S, Ribelles JG. Differentiation of mesenchymal stem cells for cartilage tissue engineering: individual and synergetic effects of three-dimensional environment and mechanical loading. Acta Biomater. 2016;33:1–12.
- Xue K, Zhang X, Gao Z, et al. Cartilage progenitor cells combined with PHBV in cartilage tissue engineering. J Transl Med. 2019;17(1):104.
- Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells. 2016;8(1):1–12.
- Mendes LF, Tam WL, Chai YC, et al. Combinatorial analysis of growth factors reveals the contribution of bone morphogenetic proteins to chondrogenic differentiation of human periosteal cells. Tissue Eng C Methods. 2016;22(5):473–486.
- Chen T-M, Chen Y-H, Sun HS, et al. Fibroblast growth factors: potential novel targets for regenerative therapy of osteoarthritis. Chin J Physiol. 2019;62(1):2–10.
- Qasim M, Chae DS, Lee NY. Bioengineering strategies for bone and cartilage tissue regeneration using growth factors and stem cells. J Biomed Mater Res A. 2020;108(3):394–411.
- Patil AS, Sable RB, Kothari RM. Role of insulin-like growth factors (IGFs), their receptors and genetic regulation in the chondrogenesis and growth of the mandibular condylar cartilage. J Cell Physiol. 2012;227(5):1796–1804.
- Needham CJ, Shah SR, Dahlin RL, et al. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014;10(10):4103–4112.
- Cha B-H, Kim J-H, Kang S-W, et al. Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector. Cell Transplant. 2013;22(9):1519–1528.
- Tian H, Yang S, Xu L, et al. Chondrogenic differentiation of mouse bone marrow mesenchymal stem cells induced by cartilage-derived morphogenetic protein-2 in vitro. J Huazhong Univ Sci Technol Med Sci. 2007;27(4):429–432.
- Wu G, Cui Y, Ma L, et al. Repairing cartilage defects with bone marrow mesenchymal stem cells induced by CDMP and TGF-β1. Cell Tissue Bank. 2014;15(1):51–57.
- Fahy N, Alini M, Stoddart MJ. Mechanical stimulation of mesenchymal stem cells: implications for cartilage tissue engineering. J Orthop Res. 2018;36(1):52–63.
- Krase A, Abedian R, Steck E, et al. BMP activation and Wnt-signalling affect biochemistry and functional biomechanical properties of cartilage tissue engineering constructs. Osteoarthr Cartil. 2014;22(2):284–292.
- Khan I, Gilbert S, Singhrao S, et al. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur Cell Mater. 2008;16(2008):26–39.
- Rainbow R, Ren W, Zeng L. Inflammation and joint tissue interactions in OA: implications for potential therapeutic approaches. Arthritis. 2012;2012:741582.
- Zhu Y, Yuan M, Meng H, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil. 2013;21(11):1627–1637.
- Montaseri A, Busch F, Mobasheri A, et al. IGF-1 and PDGF-bb suppress IL-1β-induced cartilage degradation through down-regulation of NF-κB signaling: involvement of Src/PI-3K/AKT pathway. PLoS One. 2011;6(12):e28663.
- Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol. 2001;189(3):275–284.
- Gelber AC, Hochberg MC, Mead LA, et al. Joint injury in young adults and risk for subsequent knee and hip osteoarthritis. Ann Intern Med. 2000;133(5):321–328.
- Nelson F, Billinghurst R, Pidoux I, et al. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthr Cartil. 2006;14(2):114–119.
- Martin JA, Brown T, Heiner A, et al. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41(3–4):479–491.
- Woo SLY, Buckwalter JA. AAOS/NIH/ORS workshop. Injury and repair of the musculoskeletal soft tissues. Savannah, Georgia, June 18–20, 1987. J Orthop Res. 1988;6(6):907–931.
- Buckwalter J, Martin J, Mankin H. Synovial joint degeneration and the syndrome of osteoarthritis. Instr Course Lect. 2000;49:481–489.
- Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480.
- Buckwalter J, Mankin H. Articular cartilage: tissue design and chondrocyte-matrix interactions. Instr Course Lect. 1998;47:477–486.
- Simon TM, Jackson DW. Articular cartilage: injury pathways and treatment options. Sports Med Arthrosc Rev. 2018;26(1):31–39.
- Buckwalter J. Articular cartilage: injury and repair. In: Woo SLY, Buckwalter JA, editors. Injury and repair of the musculoskeletal soft tissues. Park Ridge (IL): American Academy of Orthopaedic Surgeon; 1987. p. 465–482.
- Buckwalter J. Articular cartilage: composition, structure, response to injury, and methods of facilating repair. In: Ewing JW, editor. Articular cartilage and knee joint function: basic science and arthroscopy. New York (NY): Raven Press; 1990. p. 19–56.
- Buckwalter J, Martin J, Olmstead M, et al. Osteochondral repair of primate knee femoral and patellar articular surfaces: implications for preventing post-traumatic osteoarthritis. Iowa Orthop J. 2003;23:66–74.
- Tiderius CJ, Olsson LE, Nyquist F, et al. Cartilage glycosaminoglycan loss in the acute phase after an anterior cruciate ligament injury: delayed gadolinium-enhanced magnetic resonance imaging of cartilage and synovial fluid analysis. Arthritis Rheum. 2005;52(1):120–127.
- Goldring MB, Culley KL, Otero M. Pathogenesis of osteoarthritis in general. In: Grässel S, Aszódi A, editors. Cartilage. Cham: Springer; 2017. p. 1–25.
- Karuppal R. Current concepts in the articular cartilage repair and regeneration. J Orthop. 2017;14(2):A1–A3.
- Neuman P, Dahlberg L, Englund M, et al. Concentrations of synovial fluid biomarkers and the prediction of knee osteoarthritis 16 years after anterior cruciate ligament injury. Osteoarthr Cartil. 2017;25(4):492–498.
- Farnham MS, Larson RE, Burris DL, et al. Effects of mechanical injury on the tribological rehydration and lubrication of articular cartilage. J Mech Behav Biomed Mater. 2020;101:103422.
- Tjörnstrand J, Neuman P, Svensson J, et al. Osteoarthritis development related to cartilage quality-the prognostic value of dGEMRIC after anterior cruciate ligament injury. Osteoarthr Cartil. 2019;27(11):1647–1652.
- Palmieri-Smith RM, Wojtys EM, Potter HG. Early cartilage changes after anterior cruciate ligament injury: evaluation with imaging and serum biomarkers-a pilot study. Arthroscopy. 2016;32(7):1309–1318.
- Repo R, Finlay J. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am. 1977;59(8):1068–1076.
- Saitowitz SN. Tracking cells in osteochondral explants: a study on cartilage remodeling. Zurich: University of Zurich; 2018.
- Backus JD, Furman BD, Swimmer T, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. J Orthop Res. 2011;29(4):501–510.
- Sadeghi H, Espino D, Shepherd D. Fatigue strength of bovine articular cartilage-on-bone under three-point bending: the effect of loading frequency. BMC Musculoskelet Disord. 2017;18(1):142.
- Olson SA, Horne P, Furman B, et al. The role of cytokines in posttraumatic arthritis. JAAOS. 2014;22(1):29–37.
- Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29(6):802–809.
- Lewis J, Hembree WC, Furman BD, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthr Cartil. 2011;19(7):864–873.
- Eskelinen A, Orozco G, Tanska P, et al., editors. Combining mechanical stimulus and cytokine-mediated degradation mechanisms in injured cartilage. ORS Annual Meeting, Austin, Texas; 2019.
- Lawrence JTR, Birmingham J, Toth AP. Emerging ideas: prevention of posttraumatic arthritis through interleukin-1 and tumor necrosis factor-alpha inhibition. Clin Orthop Relat Res. 2011;469(12):3522–3526.
- Moussa M, Lajeunesse D, Hilal G, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146–156.
- Zhang W, Cheng P, Hu W, et al. Inhibition of microRNA-384-5p alleviates osteoarthritis through its effects on inhibiting apoptosis of cartilage cells via the NF-κB signaling pathway by targeting SOX9. Cancer Gene Ther. 2018;25(11–12):326–338.
- Jiang X, Liu J, Liu Q, et al. Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater Sci. 2018;6(6):1616–1626.
- Buckwalter J, Mow V. Cartilage repair in osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, et al., editors. Osteoarthritis, diagnosis and medical/surgical management. Philadelphia: Saunders; 1992. p. 71–107.
- Whitney KE, Bolia I, Chahla J, et al. Physiology and homeostasis of musculoskeletal structures, injury response, healing process, and regenerative medicine approaches. In: Gobbi A, Espregueira-Mendes J, Lane J, et al., editors. Bio-orthopaedics. Berlin: Springer; 2017. p. 71–85.
- D'lima D, Hashimoto S, Chen P, et al. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthr Cartil. 2001;9(8):712–719.
- D'Lima DD, Hashimoto S, Chen PC, et al. Impact of mechanical trauma on matrix and cells. Clin Orthop Relat Res. 2001;391:S90–S99.
- D'lima DD, Hashimoto S, Chen PC, et al. Prevention of chondrocyte apoptosis. JBJS. 2001;83:25–26.
- Phillips DM, Haut RC. The use of a non-ionic surfactant (P188) to save chondrocytes from necrosis following impact loading of chondral explants. J Orthop Res. 2004;22(5):1135–1142.
- Rundell S, Baars D, Phillips D, et al. The limitation of acute necrosis in retro-patellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23(6):1363–1369.
- Bajaj S, Shoemaker T, Hakimiyan AA, et al. Protective effect of P188 in the model of acute trauma to human ankle cartilage: the mechanism of action. J Orthop Trauma. 2010;24(9):571–576.
- Kurz B, Lemke A, Kehn M, et al. Influence of tissue maturation and antioxidants on the apoptotic response of articular cartilage after injurious compression. Arthritis Rheum. 2004;50(1):123–130.
- Beecher B, Martin J, Heiner A, et al, editors. Vitamin E blocks shear stress-induced chondrocyte death in articular cartilage. Trans 52nd Annual Meeting Orthopaedic Research Society: abstract 1517; 2006.
- Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health. 2009;1(6):461–468.
- Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. The Lancet. 2011;377(9783):2115–2126.
- Bhosale AM, Richardson JB. Articular cartilage: structure, injuries and review of management. Br Med Bull. 2008;87(1):77–95.
- Buckwalter J. Cartilage researchers tell progress: technologies hold promise, but caution urged. Am Acad Orthop Surg Bull. 1996;44(2):24–26.
- Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19(5):477–484.
- Frisbie DD, Oxford JT, Southwood L, et al. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003;407:215–227.
- Frisbie D, Trotter G, Powers B, et al. Arthroscopic subchondral bone plate microfracture technique augments healing of large chondral defects in the radial carpal bone and medial femoral condyle of horses. Vet Surg. 1999;28(4):242–255.
- Wall A, Board T. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. In: Banaszkiewicz PA, Kader DF, editors. Classic papers in orthopaedics. Cham: Springer; 2014. p. 437–439.
- Schizas N, Savvidou O, Triantafyllopoulos I, et al. Adjuvant therapies for the enhancement of microfracture technique in cartilage repair. Orthop Rev. 2019;11(3):7950.
- Johnson L. The sclerotic lesion: pathology and the clinical response to arthroscopic abrasion arthroplasty. In: Ewing JW, editor. Articular cartilage and knee joint function: basic science and arthroscopy. New York (NY): Raven Press; 1990. p. 319–333.
- Gao L, Goebel LK, Orth P, et al. Subchondral drilling for articular cartilage repair: a systematic review of translational research. Dis Model Mech. 2018;11(6):dmm034280.
- El-Jawhari JJ, Brockett CL, Ktistakis I, et al. The regenerative therapies of the ankle degeneration: a focus on multipotential mesenchymal stromal cells. Regener Med. 2018;13(2):175–188.
- Beck A, Murphy DJ, Carey-Smith R, et al. Treatment of articular cartilage defects with microfracture and autologous matrix-induced chondrogenesis leads to extensive subchondral bone cyst formation in a sheep model. Am J Sports Med. 2016;44(10):2629–2643.
- Buckwalter JA, Lohmander S. Current Concepts Review. Operative Treatment of Osteoarthrosis. Current Practice and Future Development. JBJS. 1994;76(9):1405–1418.
- Nousiainen MT, Mironova P, Hynes M, Glover Takahashi S, Reznick R, Kraemer W, et al. Eight-year outcomes of a competency-based residency training program in orthopedic surgery. Medical teacher. 2018;40(10):1042-54.
- Marcacci M, Filardo G, Kon E. Treatment of cartilage lesions: what works and why? Injury. 2013;44:S11-S5.
- Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2(1):54–69.
- Steadman JR, Rodkey WG, Singleton SB, et al. Microfracture technique forfull-thickness chondral defects: technique and clinical results. Oper Techn Orthop. 1997;7(4):300–304.
- Chu CR, Fortier LA, Williams A, Payne KA, McCarrel TM, Bowers ME, et al. Minimally manipulated bone marrow concentrate compared with microfracture treatment of full-thickness chondral defects: a one-year study in an equine model. The Journal of bone and joint surgery American volume. 2018;100(2):138.
- Weber AE, Locker PH, Mayer EN, et al. Clinical outcomes after microfracture of the knee: midterm follow-up. Orthop J Sports Med. 2018;6(2):232596711775357. .
- Gilbert JE. Current treatment options for the restoration of articular cartilage. Am J Knee Surg. 1998;11(1):42–46.
- Steinwachs M, Guggi T, Kreuz P. Marrow stimulation techniques. Injury. 2008;39(1):26–31.
- Wakitani S, Okabe T, Horibe S, et al. Safety of autologous bone marrow-derived mesenchymal stem cell transplantation for cartilage repair in 41 patients with 45 joints followed for up to 11 years and 5 months. J Tissue Eng Regen Med. 2011;5(2):146–150.
- Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthr Cartil. 2002;10(3):199–206.
- Horas U, Pelinkovic D, Herr G, et al. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint: a prospective, comparative trial. JBJS. 2003;85(2):185–192.
- Brittberg M. Autologous chondrocyte implantation—technique and long-term follow-up. Injury. 2008;39(1):40–49.
- Kon E, Filardo G, Condello V, et al. Second-generation autologous chondrocyte implantation: results in patients older than 40 years. Am J Sports Med. 2011;39(8):1668–1676.
- Degen RM, Coleman NW, Chang B, et al. Outcomes following structural grafting of distal femoral osteochondral injuries in patients aged 40 years and older. J Knee Surg. 2016;30(03):244–251.
- Park D, Krishnan S, Skinner J, et al., editors. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee. Minimum 2 year follow-up results. Orthopaedic Proceedings. The British Editorial Society of Bone & Joint Surgery; 2012.
- McCarthy H, Roberts S. A histological comparison of the repair tissue formed when using either Chondrogide(®) or periosteum during autologous chondrocyte implantation. Osteoarthr Cartil. 2013;21(12):2048–2057.
- Samuelson EM, Brown DE. Cost-effectiveness analysis of autologous chondrocyte implantation: a comparison of periosteal patch versus type I/III collagen membrane. Am J Sports Med. 2012;40(6):1252–1258.
- Ewing J. Arthroscopic treatment of degenerative meniscal lesions and early degenerative arthritis of the knee. In: Ewing JW, editor. Articular cartilage and knee joint function: basic science and arthroscopy. New York (NY): Raven Press; 1990. p. 137–145.
- Khan M, Evaniew N, Bedi A, et al. Arthroscopic surgery for degenerative tears of the meniscus: a systematic review and meta-analysis. Can Med Assoc J. 2014;186(14):1057–1064.
- Seradge H, Kutz J, Kleinert H, et al. Perichondrial resurfacing arthroplasty in the hand. J Hand Surg Am. 1984;9(6):880–886.
- Von Keudell A, Sodha S, Collins J, et al. Patient satisfaction after primary total and unicompartmental knee arthroplasty: an age-dependent analysis. The Knee. 2014;21(1):180–184.
- Griffin JW, Hadeed MM, Novicoff WM, et al. Patient age is a factor in early outcomes after shoulder arthroplasty. J Shoulder Elbow Surg. 2014;23(12):1867–1871.
- Lange JK, Lee Y-Y, Spiro SK, et al. Satisfaction rates and quality of life changes following total knee arthroplasty in age-differentiated cohorts. J Arthroplasty. 2018;33(5):1373–1378.
- Wagner ER, Houdek MT, Schleck CD, et al. The role age plays in the outcomes and complications of shoulder arthroplasty. J Shoulder Elbow Surg. 2017;26(9):1573–1580.
- Henderson I, Gui J, Lavigne P. Autologous chondrocyte implantation: natural history of postimplantation periosteal hypertrophy and effects of repair-site debridement on outcome. Arthroscopy. 2006;22(12):1318–1324. e1.
- Bartlett W, Skinner J, Gooding C, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87(5):640–645.
- Emre T, Atbasi Z, Demircioglu D, et al. Autologous osteochondral transplantation (mosaicplasty) in articular cartilage defects of the patellofemoral joint: retrospective analysis of 33 cases. Musculoskelet Surg. 2017;101(2):133–138.
- Kish G, Módis L, Hangody L. Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete: rationale, indications, techniques, and results. Clin Sports Med. 1999;18(1):45–66.
- Tschon M, Veronesi F, Giannini S, et al. Fresh osteochondral allotransplants: outcomes, failures and future developments. Injury. 2017;48(7):1287–1295.
- Glenn RE, McCarty EC, Potter HG, et al. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34(7):1084–1093.
- Shimozono Y, Hurley ET, Nguyen JT, et al. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. JBJS. 2018;100(21):1838–1844.
- McCarty EC, Fader RR, Mitchell JJ, et al. Fresh osteochondral allograft versus autograft: twelve-month results in isolated canine knee defects. Am J Sports Med. 2016;44(9):2354–2365.
- Behrens P, Bosch U, Bruns J, et al. Indications and implementation of recommendations of the working group “Tissue Regeneration and Tissue Substitutes” for autologous chondrocyte transplantation (ACT). Z Orthop Ihre Grenzgeb. 2004;142(5):529–539.
- Benthien JP, Behrens P. The treatment of chondral and osteochondral defects of the knee with autologous matrix-induced chondrogenesis (AMIC): method description and recent developments. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1316–1319.
- Benthien JP, Behrens P. Autologous matrix-induced chondrogenesis (AMIC). A one-step procedure for retropatellar articular resurfacing. Acta Orthop Belg. 2010;76(2):260–263.
- Giza E, Delman C, Coetzee JC, et al. Arthroscopic treatment of talus osteochondral lesions with particulated juvenile allograft cartilage. Foot Ankle Int. 2014;35(10):1087–1094.
- Yanke AB, Tilton AK, Wetters NG, et al. DeNovo NT particulated juvenile cartilage implant. Sports Med Arthrosc Rev. 2015;23(3):125–129.
- Hatic SO, Berlet GC. Particulated juvenile articular cartilage graft (DeNovo NT Graft) for treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2010;3(6):361–364.
- Giza E, Howell S. Allograft juvenile articular cartilage transplantation for treatment of talus osteochondral defects. Foot Ankle Spec. 2013;6(2):141–144.
- Hinckel BB, Gomoll AH. Patellofemoral cartilage restoration: indications, techniques, and outcomes of autologous chondrocytes implantation, matrix-induced chondrocyte implantation, and particulated juvenile allograft cartilage. J Knee Surg. 2018;31(3):212–226.
- Christensen BB, Lind M, Foldager CB. Particulated cartilage auto-and allograft. In: Farr J, Gomoll AH, editors. Cartilage restoration. Cham: Springer. 2018. p. 287–296.
- Hoemann CD, Hurtig M, Rossomacha E, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. JBJS. 2005;87(12):2671–2686.
- Zadeh L G, A C, Mb H, et al. Freeze-dried chitosan-PRP injectable surgical implants for meniscus repair: pilot feasibility studies in ovine models. Regen Med Ther. 2017;1(1):16–29.
- Vinatier C, Bouffi C, Merceron C, et al. Cartilage tissue engineering: towards a biomaterial-assisted mesenchymal stem cell therapy. Curr Stem Cell Res Ther. 2009;4(4):318–329.
- Saghati S, Akbarzadeh A, Del Bakhshayesh A, et al. Electrospinning and 3D printing: prospects for market opportunity. Electrospinning. 2018; :136–155.
- Amani H, Mostafavi E, Arzaghi H, et al. Three-dimensional graphene foams: synthesis, properties, biocompatibility, biodegradability, and applications in tissue engineering. ACS Biomater Sci Eng. 2019;5(1):193–214.
- Rahmani Del Bakhshayesh A, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells Nanomed Biotechnol. 2018;46(4):691–705.
- Rahmani Del Bakhshayesh A, Mostafavi E, Alizadeh E, et al. Fabrication of three-dimensional scaffolds based on nano-biomimetic collagen hybrid constructs for skin tissue engineering. ACS Omega. 2018;3(8):8605–8611.
- Vinatier C, Guicheux J. Cartilage tissue engineering: from biomaterials and stem cells to osteoarthritis treatments. Ann Phys Rehabil Med. 2016;59(3):139–144.
- Zamani R, Aval SF, Pilehvar-Soltanahmadi Y, et al. Recent advances in cell electrospining of natural and synthetic nanofibers for regenerative medicine. Drug Res. 2018;68(8):425–435.
- Moutos FT, Guilak F. Composite scaffolds for cartilage tissue engineering. Biorheology. 2008;45(3–4):501–512.
- Caterson EJ, Nesti LJ, Li WJ, et al. Three‐dimensional cartilage formation by bone marrow‐derived cells seeded in polylactide/alginate amalgam. J Biomed Mater Res. 2001;57(3):394–403.
- Wayne JS, McDowell CL, Shields KJ, et al. In vivo response of polylactic acid-alginate scaffolds and bone marrow-derived cells for cartilage tissue engineering. Tissue Eng. 2005;11(5–6):953–963.
- Taboas J, Maddox R, Krebsbach P, et al. Indirect solid free form fabrication of local and global porous, biomimetic and composite 3D polymer-ceramic scaffolds. Biomaterials. 2003;24(1):181–194.
- Liao E, Yaszemski M, Krebsbach P, et al. Tissue-engineered cartilage constructs using composite hyaluronic acid/collagen I hydrogels and designed poly(propylene fumarate) scaffolds. Tissue Eng. 2007;13(3):537–550.
- Marijnissen WJ, van Osch GJ, Aigner J, et al. Alginate as a chondrocyte-delivery substance in combination with a non-woven scaffold for cartilage tissue engineering. Biomaterials. 2002;23(6):1511–1517.
- Chen G, Sato T, Ushida T, et al. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. J Biomed Mater Res A. 2003;67(4):1170–1180.
- Slivka MA, Leatherbury NC, Kieswetter K, et al. Porous, resorbable, fiber-reinforced scaffolds tailored for articular cartilage repair. Tissue Eng. 2001;7(6):767–780.
- Ng KW, Wang CCB, Mauck RL, et al. A layered agarose approach to fabricate depth-dependent inhomogeneity in chondrocyte-seeded constructs . J Orthop Res. 2005;23(1):134–141.
- Schaefer D, Martin I, Jundt G, et al. Tissue-engineered composites for the repair of large osteochondral defects. Arthritis Rheum. 2002;46(9):2524–2534.
- Tognana E, Chen F, Padera R, et al. Adjacent tissues (cartilage, bone) affect the functional integration of engineered calf cartilage in vitro. Osteoarthr Cartil. 2005;13(2):129–138.
- Moutos FT, Freed LE, Guilak F. A biomimetic three-dimensional woven composite scaffold for functional tissue engineering of cartilage. Nat Mater. 2007;6(2):162–167.
- Liu Y, Zhou G, Cao Y. Recent progress in cartilage tissue engineering—our experience and future directions. Engineering. 2017;3(1):28–35.
- Liu W, Cao Y. Application of scaffold materials in tissue reconstruction in immunocompetent mammals: our experience and future requirements. Biomaterials. 2007;28(34):5078–5086.
- Rahmani Del Bakhshayesh A, Akbarzadeh A, Alihemmati A, et al. Preparation and characterization of novel anti-inflammatory biological agents based on piroxicam-loaded poly-ε-caprolactone nano-particles for sustained NSAID delivery. Drug Deliv. 2020;27(1):269–282.
- Celik C, Mogal VT, Hui JHP, et al. Injectable hydrogels for cartilage regeneration. In: Vijay Kumar T, Manju Kumari T, editors. Hydrogels. Cham: Springer. 2018. p. 315–337.
- Moore E, West J. Bioactive poly (ethylene glycol) acrylate hydrogels for regenerative engineering. Regen Eng Transl Med. 2019;5(2):113–167.
- Kudva AK, Luyten FP, Patterson J. RGD-functionalized polyethylene glycol hydrogels support proliferation and in vitro chondrogenesis of human periosteum-derived cells. J Biomed Mater Res A. 2018;106(1):33–42.
- Gao G, Hubbell K, Schilling AF, et al. Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel. In: Koledova Z, editor. 3D cell culture. Cham: Springer; 2017. p. 391–398.
- Mellati A, Fan CM, Tamayol A, et al. Microengineered 3D cell-laden thermoresponsive hydrogels for mimicking cell morphology and orientation in cartilage tissue engineering. Biotechnol Bioeng. 2017;114(1):217–231.
- Liu H, Liu J, Qi C, et al. Thermosensitive injectable in-situ forming carboxymethyl chitin hydrogel for three-dimensional cell culture. Acta Biomater. 2016;35:228–237.
- Lee H, Park TG. Photo-crosslinkable, biomimetic, and thermo-sensitive pluronic grafted hyaluronic acid copolymers for injectable delivery of chondrocytes. J Biomed Mater Res A. 2009;88(3):797–806.
- Fedorovich NE, Oudshoorn MH, van Geemen D, et al. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials. 2009;30(3):344–353.
- Florine EM, Miller RE, Liebesny PH, et al. Delivering heparin-binding insulin-like growth factor 1 with self-assembling peptide hydrogels. Tissue Eng A. 2015;21(3–4):637–646.
- Roach BL, Kelmendi-Doko A, Balutis EC, et al. Dexamethasone release from within engineered cartilage as a chondroprotective strategy against interleukin-1α. Tissue Eng A. 2016;22(7–8):621–632.
- Florine EM, Miller RE, Porter RM, et al. Effects of dexamethasone on mesenchymal stromal cell chondrogenesis and aggrecanase activity: comparison of agarose and self-assembling peptide scaffolds. Cartilage. 2013;4(1):63–74.
- Annabi N, Tamayol A, Uquillas JA, et al. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv Mater Weinheim. 2014;26(1):85–124.
- Kesti M, Müller M, Becher J, et al. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015;11:162–172.
- Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496.
- Rhee S, Puetzer JL, Mason BN, et al. 3D bioprinting of spatially heterogeneous collagen constructs for cartilage tissue engineering. ACS Biomater Sci Eng. 2016;2(10):1800–1805.
- Hong H, Seo YB, Lee JS, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679.
- Lipskas J, Deep K, Yao W. Robotic-assisted 3D bio-printing for repairing bone and cartilage defects through a minimally invasive approach. Sci Rep. 2019;9(1):1–9.
- Biazar E, Najafi S M, Heidari K S, et al. 3D bio-printing technology for body tissues and organs regeneration. J Med Eng Technol. 2018;42(3):187–202.
- Ma K, Zhao T, Yang L, et al. Application of robotic-assisted in situ 3D printing in cartilage regeneration with HAMA hydrogel: an in vivo study. J Adv Res. 2020;23:123–132.
- King JC, Manner PA, Stamper DL, et al. Is minimally invasive total knee arthroplasty associated with lower costs than traditional TKA? Clin Orthop Relat Res. 2011;469(6):1716–1720.
- Cherubino P, Grassi F, Bulgheroni P, et al. Autologous chondrocyte implantation using a bilayer collagen membrane: a preliminary report. J Orthop Surg. 2003;11(1):10–15.
- Gigante A, Enea D, Greco F, et al. Distal realignment and patellar autologous chondrocyte implantation: mid-term results in a selected population. Knee Surg Sports Traumatol Arthrosc. 2009;17(1):2–10.
- Ochi M, Uchio Y, Kawasaki K, et al. Transplantation of cartilage-like tissue made by tissue engineering in the treatment of cartilage defects of the knee. J Bone Joint Surg Br. 2002;84(4):571–578.
- Kuroda R, Ishida K, Matsumoto T, et al. Treatment of a full-thickness articular cartilage defect in the femoral condyle of an athlete with autologous bone-marrow stromal cells. Osteoarthr Cartil. 2007;15(2):226–231.
- Brix MO, Stelzeneder D, Trattnig S, et al. Cartilage repair of the knee with Hyalograft C:® magnetic resonance imaging assessment of the glycosaminoglycan content at midterm. Int Orthop. 2013;37(1):39–43.
- Nehrer S, Domayer S, Dorotka R, et al. Three-year clinical outcome after chondrocyte transplantation using a hyaluronan matrix for cartilage repair. Eur J Radiol. 2006;57(1):3–8.
- Steinwachs MR, Waibl B, Mumme M. Arthroscopic treatment of cartilage lesions with microfracture and BST-CarGel. Arthrosc Tech. 2014;3(3):e399–e402.
- Ossendorf C, Kaps C, Kreuz PC, et al. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9(2):R41.
- Seo S-J, Mahapatra C, Singh RK, et al. Strategies for osteochondral repair: focus on scaffolds. J Tissue Eng. 2014;5:2041731414541850.
- Filardo G, Kon E, Perdisa F, et al. Osteochondral scaffold reconstruction for complex knee lesions: a comparative evaluation. The Knee. 2013;20(6):570–576.
- Solchaga LA, Tognana E, Penick K, et al. A rapid seeding technique for the assembly of large cell/scaffold composite constructs. Tissue Eng. 2006;12(7):1851–1863.
- Sun Y, Yan L, Chen S, et al. Functionality of decellularized matrix in cartilage regeneration: a comparison of tissue versus cell sources. Acta Biomater. 2018;74:56–73.
- Rowland CR, Colucci LA, Guilak F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials. 2016;91:57–72.
- Sasikumar S, Chameettachal S, Cromer B, et al. Decellularized extracellular matrix hydrogels–cell behavior as function of matrix stiffness. Curr Opin Biomed Eng. 2019;10:123–133.
- Sutherland AJ, Beck EC, Dennis SC, et al. Decellularized cartilage may be a chondroinductive material for osteochondral tissue engineering. PLoS One. 2015;10(5):e0121966.
- Das P, Singh YP, Joardar SN, et al. Decellularized caprine conchal cartilage toward repair and regeneration of damaged cartilage. ACS Appl Bio Mater. 2019;2(5):2037–2049.
- Yamada N, Okano T, Sakai H, et al. Thermo‐responsive polymeric surfaces; control of attachment and detachment of cultured cells. Makromol Chem, Rapid Commun. 1990;11(11):571–576.
- Nguyen LT, Odeleye AO, Chui CY, et al. Development of thermo-responsive polycaprolactone macrocarriers conjugated with Poly (N-isopropyl acrylamide) for cell culture. Sci Rep. 2019;9(1):1–11.
- Meng Q, Hu X, Huang H, et al. Microfracture combined with functional pig peritoneum-derived acellular matrix for cartilage repair in rabbit models. Acta Biomater. 2017;53:279–292.
- Ge Y, Gong YY, Xu Z, et al. The application of sheet technology in cartilage tissue engineering. Tissue Eng B Rev. 2016;22(2):114–124.
- Xue J, Feng B, Zheng R, et al. Engineering ear-shaped cartilage using electrospun fibrous membranes of gelatin/polycaprolactone. Biomaterials. 2013;34(11):2624–2631.
- Nguyen D, Hägg DA, Forsman A, et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci Rep. 2017;7(1):1–10.
- Lin L, Xu Y, Li Y, et al. Nanofibrous Wharton's jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater Des. 2020;186:108216.
- Isogai N, Kusuhara H, Ikada Y, et al. Comparison of different chondrocytes for use in tissue engineering of cartilage model structures. Tissue Eng. 2006;12(4):691–703.
- Negoro T, Takagaki Y, Okura H, et al. Trends in clinical trials for articular cartilage repair by cell therapy. NPJ Regen Med. 2018;3(1):1–10.
- Fakunle ES, Lane JG. Cell culture approaches for articular cartilage: repair and regeneration. In: Gobbi A, Espregueira-Mendes J, Lane J, et al., editors. Bio-orthopaedics. Berlin: Springer; 2017. p. 161–172.
- Darling EM, Athanasiou KA. Rapid phenotypic changes in passaged articular chondrocyte subpopulations. J Orthop Res. 2005;23(2):425–432.
- Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–147.
- Beane OS, Darling EM. Isolation, characterization, and differentiation of stem cells for cartilage regeneration. Ann Biomed Eng. 2012;40(10):2079–2097.
- Vinatier C, Mrugala D, Jorgensen C, et al. Cartilage engineering: a crucial combination of cells, biomaterials and biofactors. Trends Biotechnol. 2009;27(5):307–314.
- Noël D, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma. 2007;48(7):1283–1289.
- Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–260.
- Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872.
- Craft AM, Rockel JS, Nartiss Y, et al. Generation of articular chondrocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(6):638–645.
- Toh WS, Lee EH, Cao T. Potential of human embryonic stem cells in cartilage tissue engineering and regenerative medicine. Stem Cell Rev Rep. 2011;7(3):544–559.
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147.
- Hwang NS, Varghese S, Zhang Z, et al. Chondrogenic differentiation of human embryonic stem cell-derived cells in arginine-glycine-aspartate-modified hydrogels. Tissue Eng. 2006;12(9):2695–2706.
- Kwon H, Paschos NK, Hu JC, et al. Articular cartilage tissue engineering: the role of signaling molecules. Cell Mol Life Sci. 2016;73(6):1173–1194.
- Rao M. Tumorigenesis and embryonic stem cell-derived therapy. Stem Cells Dev. 2007;16(6):903–904.
- Mauck RL, Nicoll SB, Seyhan SL, et al. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9(4):597–611.
- Fukumoto T, Sperling J, Sanyal A, et al. Combined effects of insulin-like growth factor-1 and transforming growth factor-beta1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr Cartil. 2003;11(1):55–64.
- Reddi A, Morphogenesis IK. Bone morphogenetic proteins, and regeneration of bone and articular cartilage. In: Atala A, Lanza R, Mikos T, et al., editors. Principles of regenerative medicine. London: Elsevier; 2019. p. 405–416.
- Ray A, Singh PNP, Sohaskey ML, et al. Precise spatial restriction of BMP signaling is essential for articular cartilage differentiation. Development. 2015;142(6):1169–1179.
- Storm EE, Huynh TV, Copeland NG, et al. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368(6472):639–643.
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, et al. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144(1):161–173.
- Furumatsu T, Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta Med Okayama. 2010;64(6):351–357.
- Murakami S, Kan M, McKeehan WL, et al. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proc Natl Acad Sci USA. 2000;97(3):1113–1118.
- Cleary MA, van Osch GJM, Brama PA, et al. FGF, TGFβ and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med. 2015;9(4):332–342.
- Huang AH, Farrell MJ, Mauck RL. Mechanics and mechanobiology of mesenchymal stem cell-based engineered cartilage. J Biomech. 2010;43(1):128–136.
- Merceron C, Vinatier C, Portron S, et al. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am J Physiol, Cell Physiol. 2010;298(2):C355–C364.
- Portron S, Merceron C, Gauthier O, et al. Effects of in vitro low oxygen tension preconditioning of adipose stromal cells on their in vivo chondrogenic potential: application in cartilage tissue repair. PLoS One. 2013;8(4):e62368.
- Lafont JE, Talma S, Hopfgarten C, et al. Hypoxia promotes the differentiated human articular chondrocyte phenotype through SOX9-dependent and -independent pathways. J Biol Chem. 2008;283(8):4778–4786.
- Portron S, Hivernaud V, Merceron C, et al. Inverse regulation of early and late chondrogenic differentiation by oxygen tension provides cues for stem cell-based cartilage tissue engineering. Cell Physiol Biochem. 2015;35(3):841–857.
- Vinatier C, Magne D, Weiss P, et al. A silanized hydroxypropyl methylcellulose hydrogel for the three-dimensional culture of chondrocytes. Biomaterials. 2005;26(33):6643–6651.
- Merceron C, Portron S, Masson M, et al. The effect of two- and three-dimensional cell culture on the chondrogenic potential of human adipose-derived mesenchymal stem cells after subcutaneous transplantation with an injectable hydrogel. Cell Transplant. 2011;20(10):1575–1588.
- McMahon LA, Reid AJ, Campbell VA, et al. Regulatory effects of mechanical strain on the chondrogenic differentiation of MSCs in a collagen-GAG scaffold: experimental and computational analysis. Ann Biomed Eng. 2008;36(2):185–194.
- Uzieliene I, Bernotas P, Mobasheri A, et al. The role of physical stimuli on calcium channels in chondrogenic differentiation of mesenchymal stem cells. IJMS. 2018;19(10):2998.
- Xie Y, Liu X, Wang S, et al. Proper mechanical stimulation improve the chondrogenic differentiation of mesenchymal stem cells: improve the viscoelasticity and chondrogenic phenotype. Biomed Pharmacother. 2019;115:108935.
- Drobnic M, Perdisa F, Kon E, et al. Implant strategy affects scaffold stability and integrity in cartilage treatment. Knee Surg Sports Traumatol Arthrosc. 2018;26(9):2774–2783.
- Hua S, Dias TH. Hypoxia-inducible factor (HIF) as a target for novel therapies in rheumatoid arthritis. Front Pharmacol. 2016;7:184.
- Murphy CL, Thoms BL, Vaghjiani RJ, et al. Hypoxia. HIF-mediated articular chondrocyte function: prospects for cartilage repair. Arthritis Res Ther. 2009;11(1):213.
- Yasui Y, Chijimatsu R, Hart DA, et al. Preparation of scaffold-free tissue-engineered constructs derived from human synovial mesenchymal stem cells under low oxygen tension enhances their chondrogenic differentiation capacity. Tissue Eng A. 2016;22(5–6):490–500.
- Henrionnet C, Liang G, Roeder E, et al. Hypoxia for mesenchymal stem cell expansion and differentiation: the best way for enhancing TGFß-induced chondrogenesis and preventing calcifications in alginate beads. Tissue Eng A. 2017;23(17–18):913–922.
- Bhardwaj N, Kundu SC. Chondrogenic differentiation of rat MSCs on porous scaffolds of silk fibroin/chitosan blends. Biomaterials. 2012;33(10):2848–2857.
- Zhou M, Lozano N, Wychowaniec JK, et al. Graphene oxide: a growth factor delivery carrier to enhance chondrogenic differentiation of human mesenchymal stem cells in 3D hydrogels. Acta Biomater. 2019;96:271–280.
- Costa E, González-García C, Gómez Ribelles JL, et al. Maintenance of chondrocyte phenotype during expansion on PLLA microtopographies. J Tissue Eng. 2018;9:2041731418789829.
- DuRaine G, Neu CP, Chan SM, et al. Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. J Orthop Res. 2009;27(2):249–256.
- Athanasiou KA, Darling EM, Hu JC, et al. Articular cartilage. Portland: CRC Press; 2013.
- Freed L, Hollander A, Martin I, et al. Chondrogenesis in a cell-polymer-bioreactor system. Exp Cell Res. 1998;240(1):58–65.
- Li K, Zhang C, Qiu L, et al. Advances in application of mechanical stimuli in bioreactors for cartilage tissue engineering. Tissue Eng B Rev. 2017;23(4):399–411.
- Daly AC, Sathy BN, Kelly DJ. Engineering large cartilage tissues using dynamic bioreactor culture at defined oxygen conditions. Journal of tissue engineering. 2018;9:2041731417753718.
- Marolt D, Augst A, Freed LE, et al. Bone and cartilage tissue constructs grown using human bone marrow stromal cells, silk scaffolds and rotating bioreactors. Biomaterials. 2006;27(36):6138–6149.
- Bueno EM, Bilgen B, Barabino GA. Wavy-walled bioreactor supports increased cell proliferation and matrix deposition in engineered cartilage constructs. Tissue Eng. 2005;11(11–12):1699–1709.
- Pörtner R, Nagel-Heyer S, Goepfert C, et al. Bioreactor design for tissue engineering. J Biosci Bioeng. 2005;100(3):235–245.
- Valonen PK, Moutos FT, Kusanagi A, et al. In vitro generation of mechanically functional cartilage grafts based on adult human stem cells and 3D-woven poly (ɛ-caprolactone) scaffolds. Biomaterials. 2010;31(8):2193–2200.
- Schulz RM, Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;36(4–5):539–568.
- Obradovic B, Martin I, Padera R, et al. Integration of engineered cartilage. J Orthop Res. 2001;19(6):1089–1097.
- Hunziker EB, Kapfinger E, Müller M. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. J Bone Joint Surg Br. 1998;80(1):144–150.
- van de Breevaart Bravenboer J, der Maur CDI, Bos PK, et al. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res Ther. 2004;6(5):R469.
- Bastiaansen‐Jenniskens Y, Koevoet W, Feijt C, et al. Proteoglycan production is required in initial stages of new cartilage matrix formation but inhibits integrative cartilage repair. J Tissue Eng Regen Med. 2009;3(2):117–123.
- Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12(3):211.
- Wehling N, Palmer G, Pilapil C, et al. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis Rheum. 2009;60(3):801–812.
- Heldens GT, Blaney Davidson EN, Vitters EL, et al. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng A. 2012;18(1–2):45–54.
- Henrotin Y, Lambert C, Richette P. Importance of synovitis in osteoarthritis: evidence for the use of glycosaminoglycans against synovial inflammation. Semin Arthritis Rheum. 2014;43(5):579–587.
- Chen W-H, Lo W-C, Hsu W-C, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35(36):9599–9607.
- Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721–730.
- Prodromos CC, Finkle S, Dawes A, et al. editors. Intra-articular laser treatment plus Platelet Rich Plasma (PRP) significantly reduces pain in many patients who had failed prior PRP treatment. Mechanisms of Photobiomodulation Therapy XIII. International Society for Optics and Photonics; 2018.
- Vinardell T, Sheehy EJ, Buckley CT, et al. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue Eng A. 2012;18(11–12):1161–1170.
- Von der Mark K, Kirsch T, Nerlich A, et al. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35(7):806–811.
- Pitsillides AA, Beier F. Cartilage biology in osteoarthritis-lessons from developmental biology. Nat Rev Rheumatol. 2011;7(11):654–663.
- Ikeda T, Kamekura S, Mabuchi A, et al. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum. 2004;50(11):3561–3573.
- Lefebvre V, Angelozzi M, Haseeb A. SOX9 in cartilage development and disease. Curr Opin Cell Biol. 2019;61:39–47.
- Jeong DU, Choi JY, Kim DW. Cartilage-specific and cre-dependent Nkx3.2 Overexpression in vivo causes skeletal dwarfism by delaying cartilage hypertrophy. J Cell Physiol. 2017;232(1):78–90.
- Lengner CJ, Hassan MQ, Serra RW, et al. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J Biol Chem. 2005;280(16):15872–15879.
- Caron M, Emans P, Cremers A, et al. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthr Cartil. 2013;21(4):604–613.
- Kim Y-J, Kim H-J, Im G-I. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373(1):104–108.
- Mwale F, Yao G, Ouellet JA, et al. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue Eng A. 2010;16(11):3449–3455.